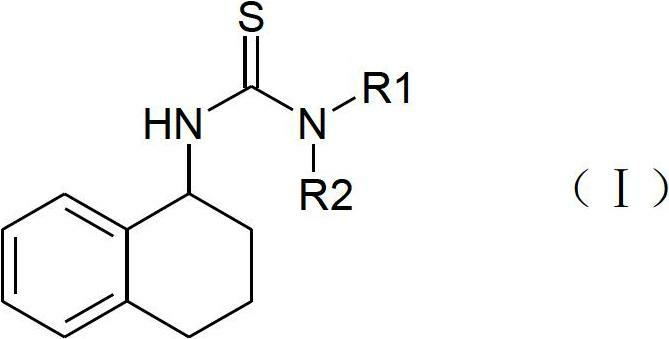
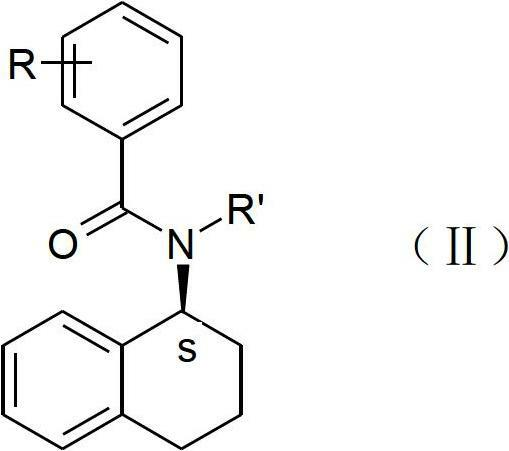
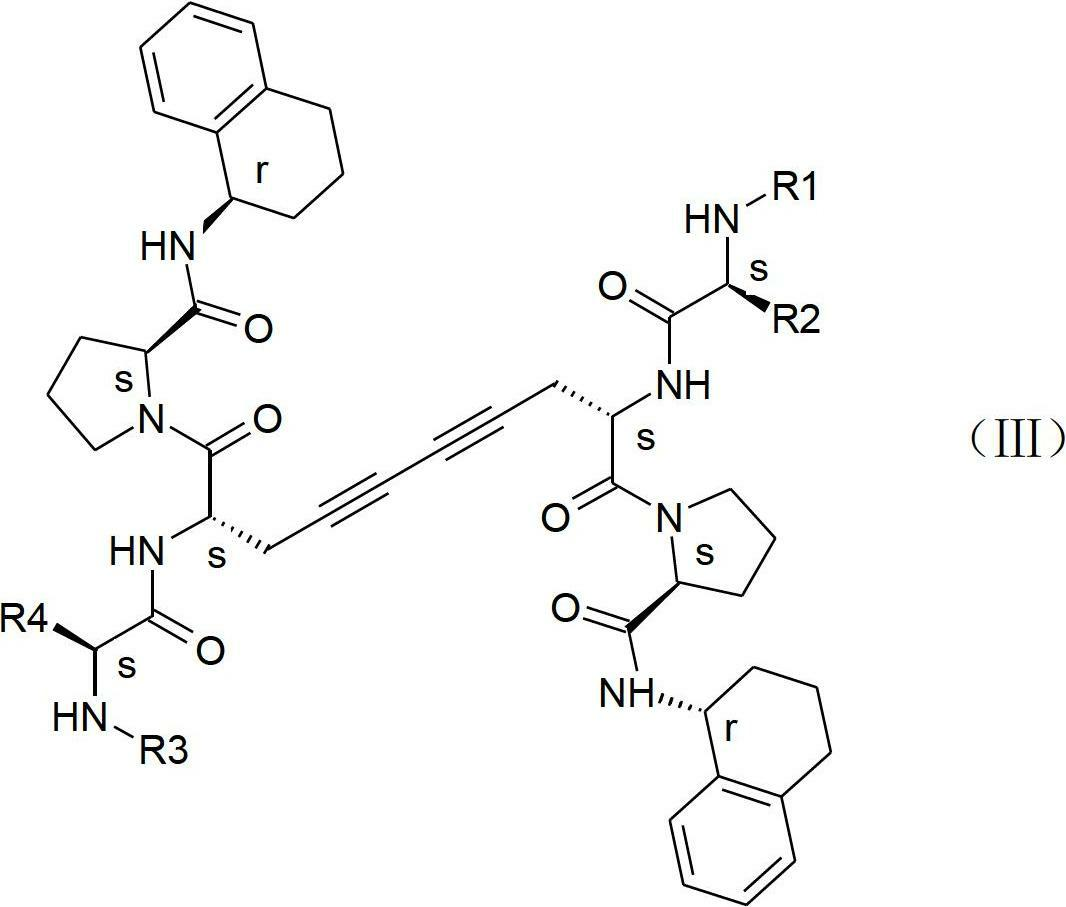
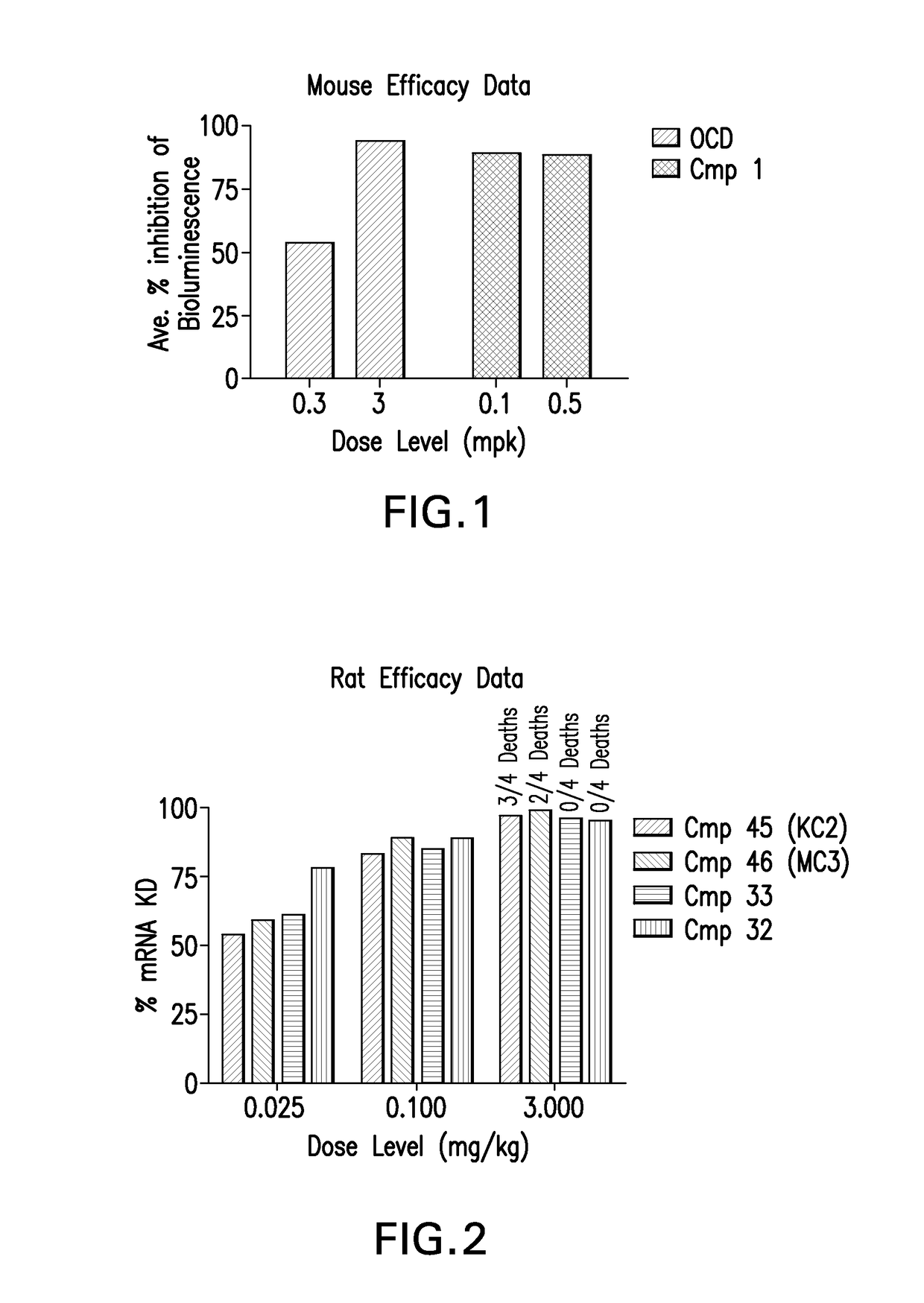
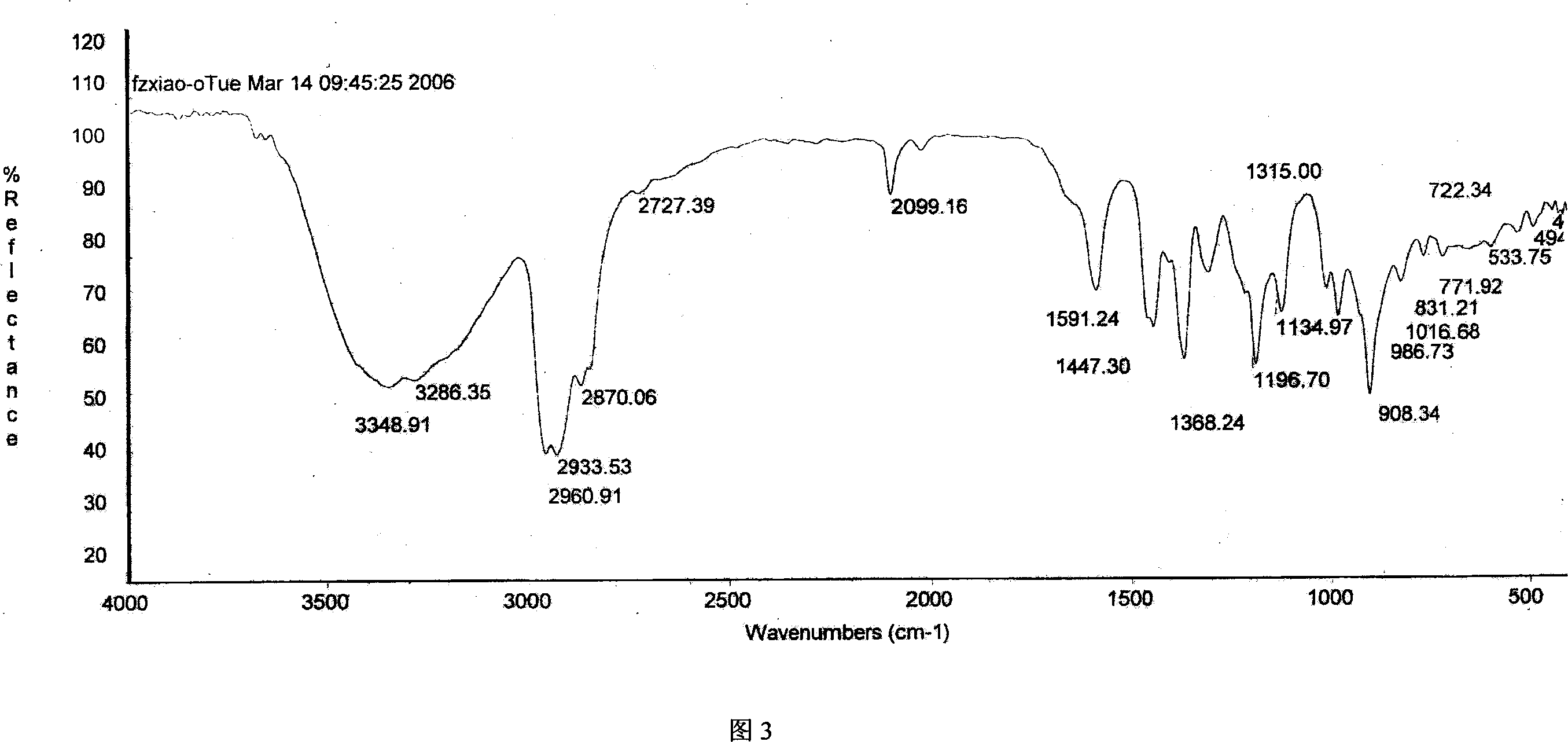
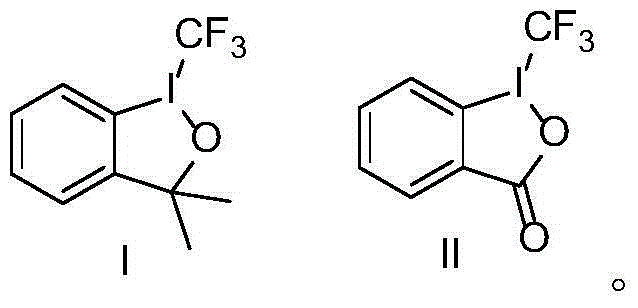
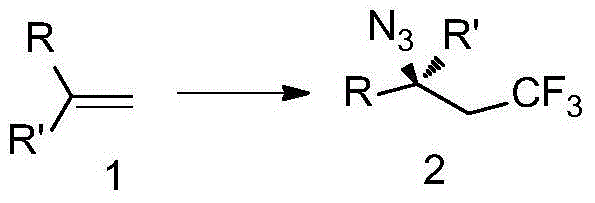
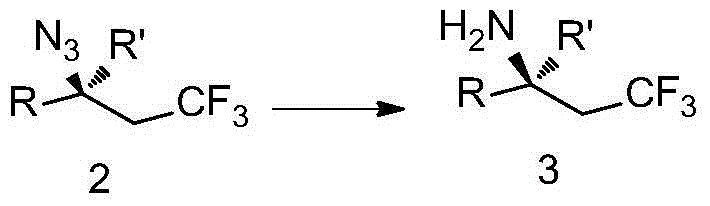
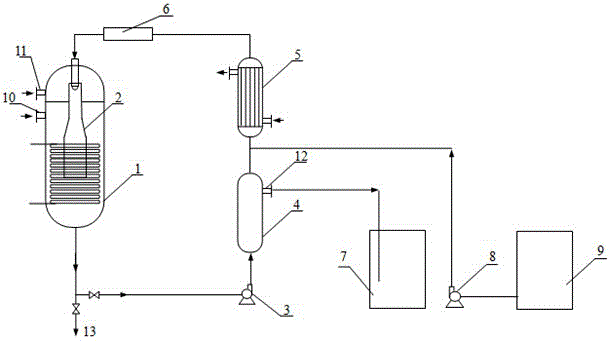
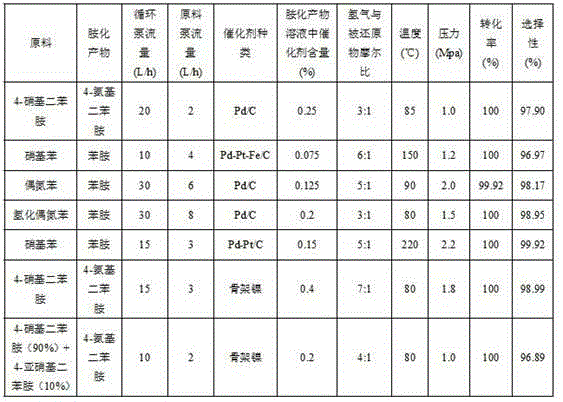
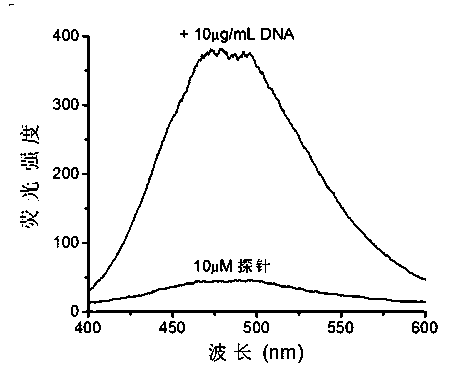
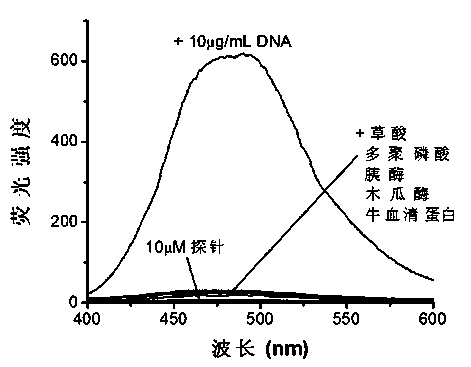
Patents
Literature
126results about "Preparation by N-O/N-N bonds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
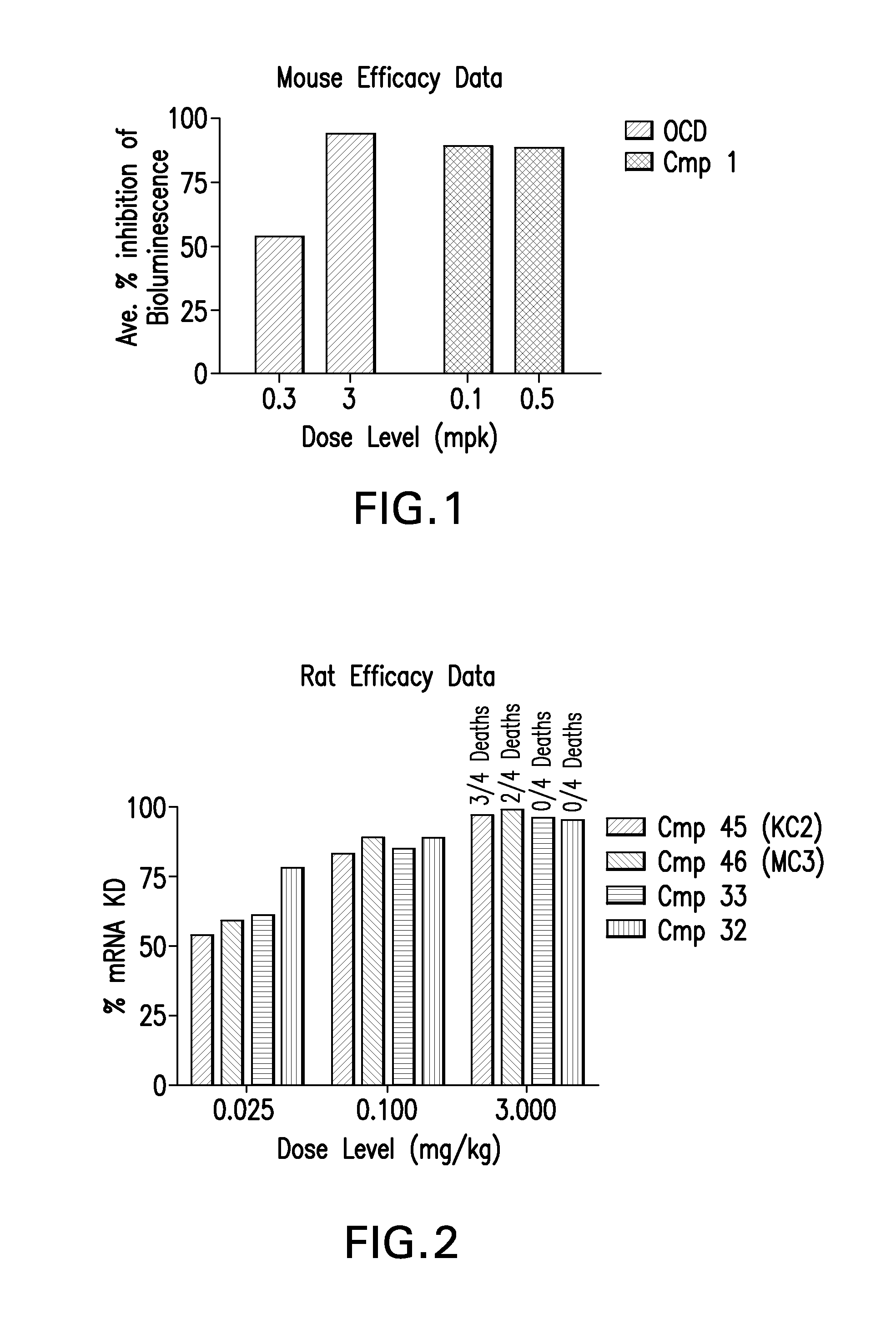
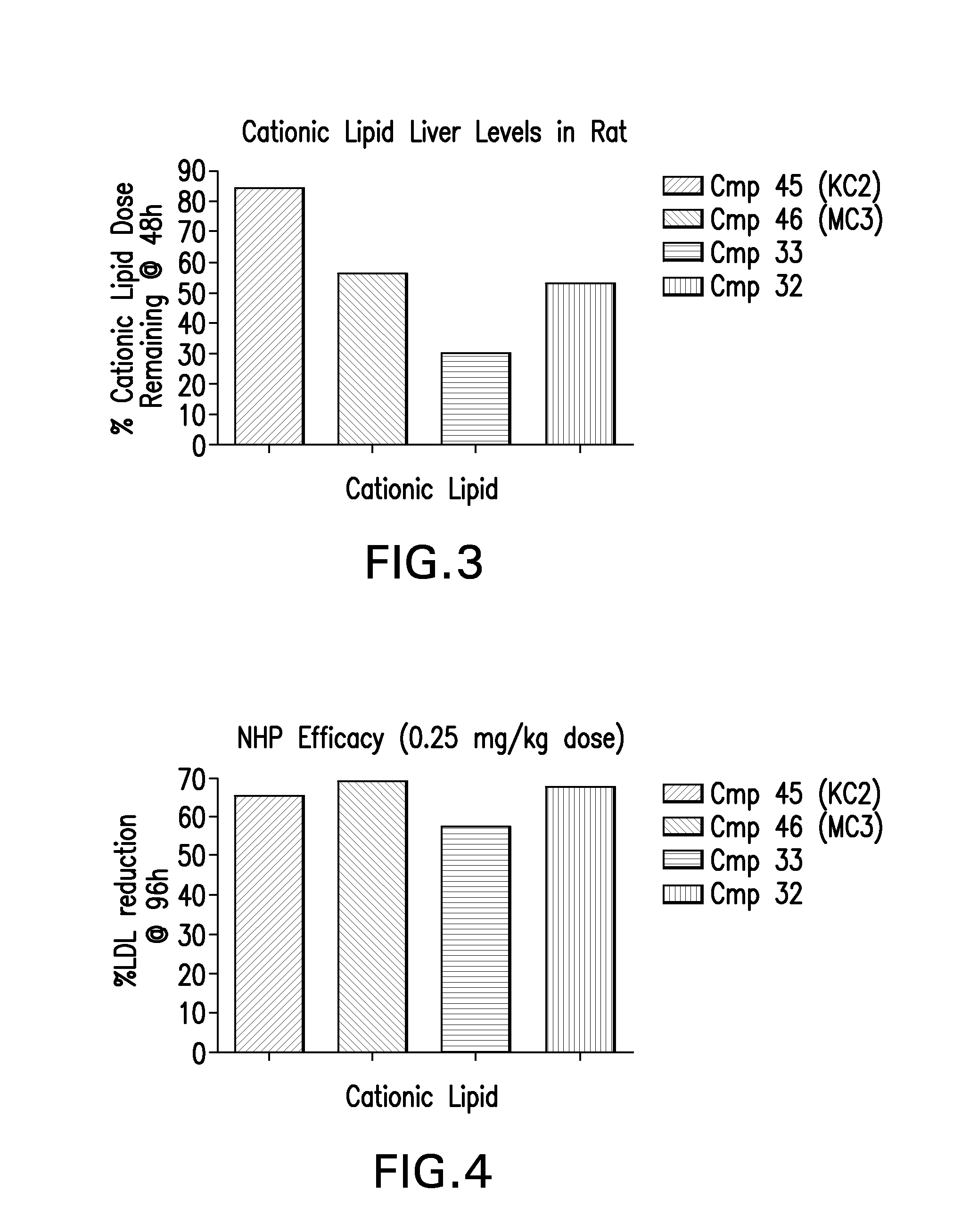
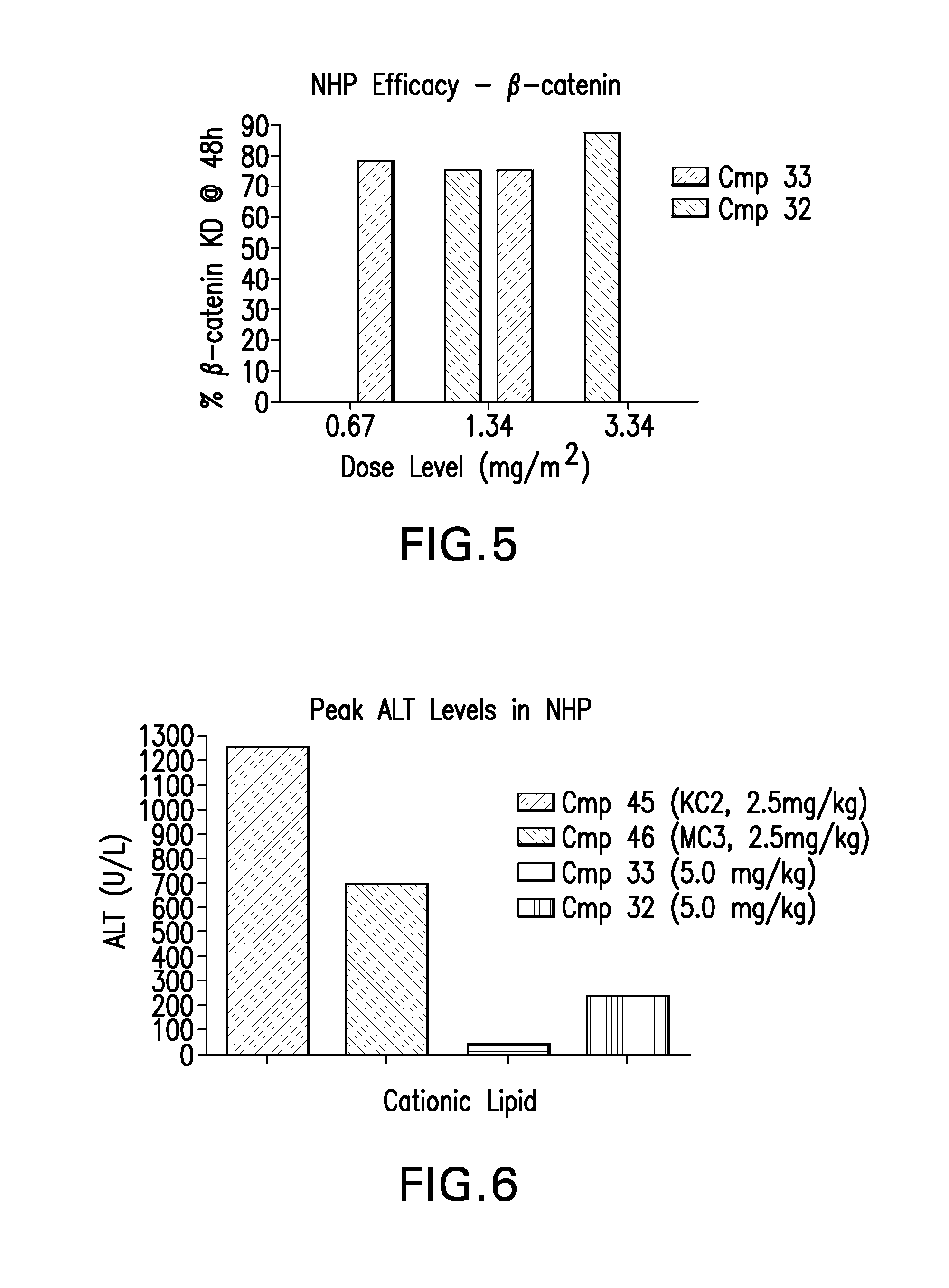
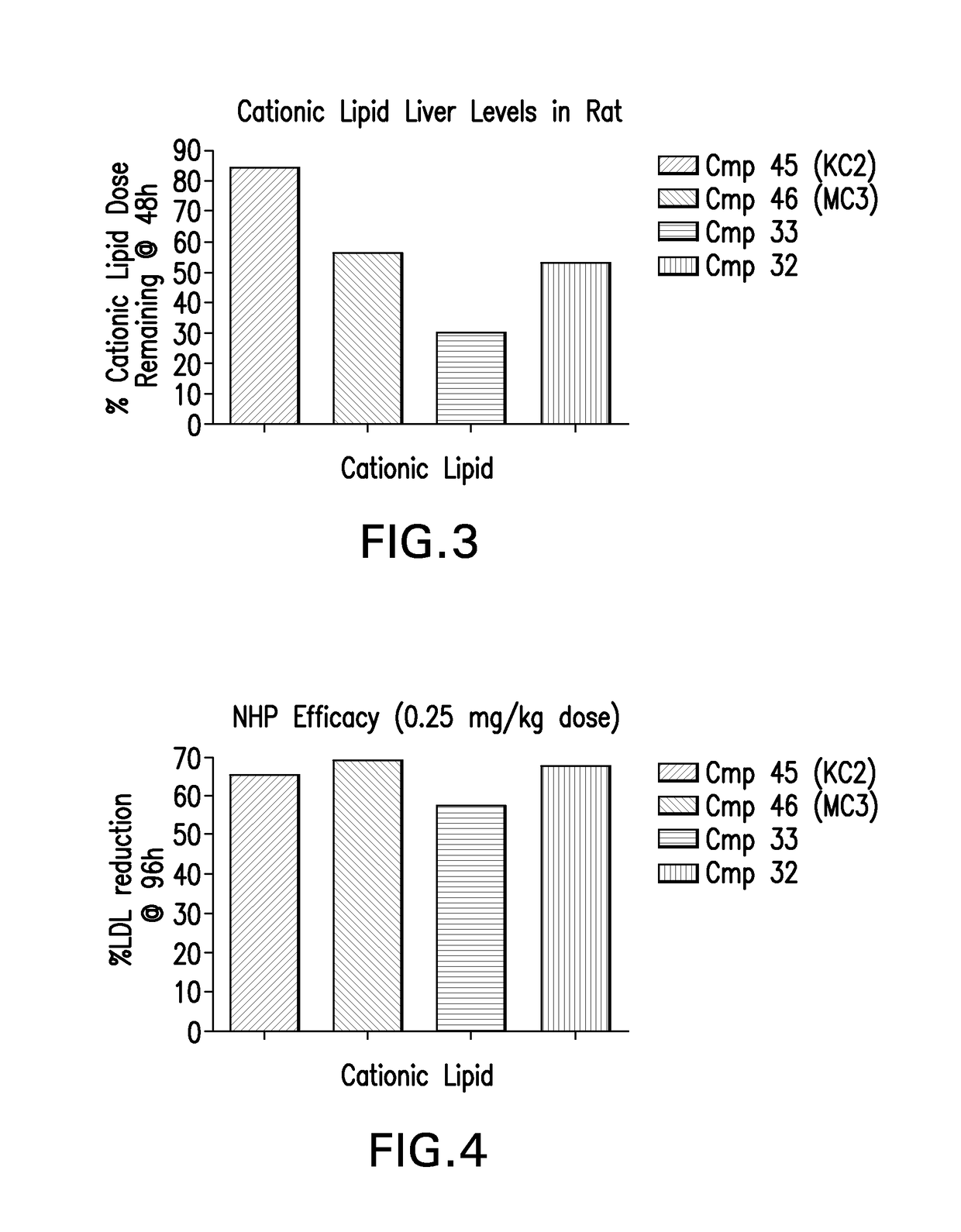
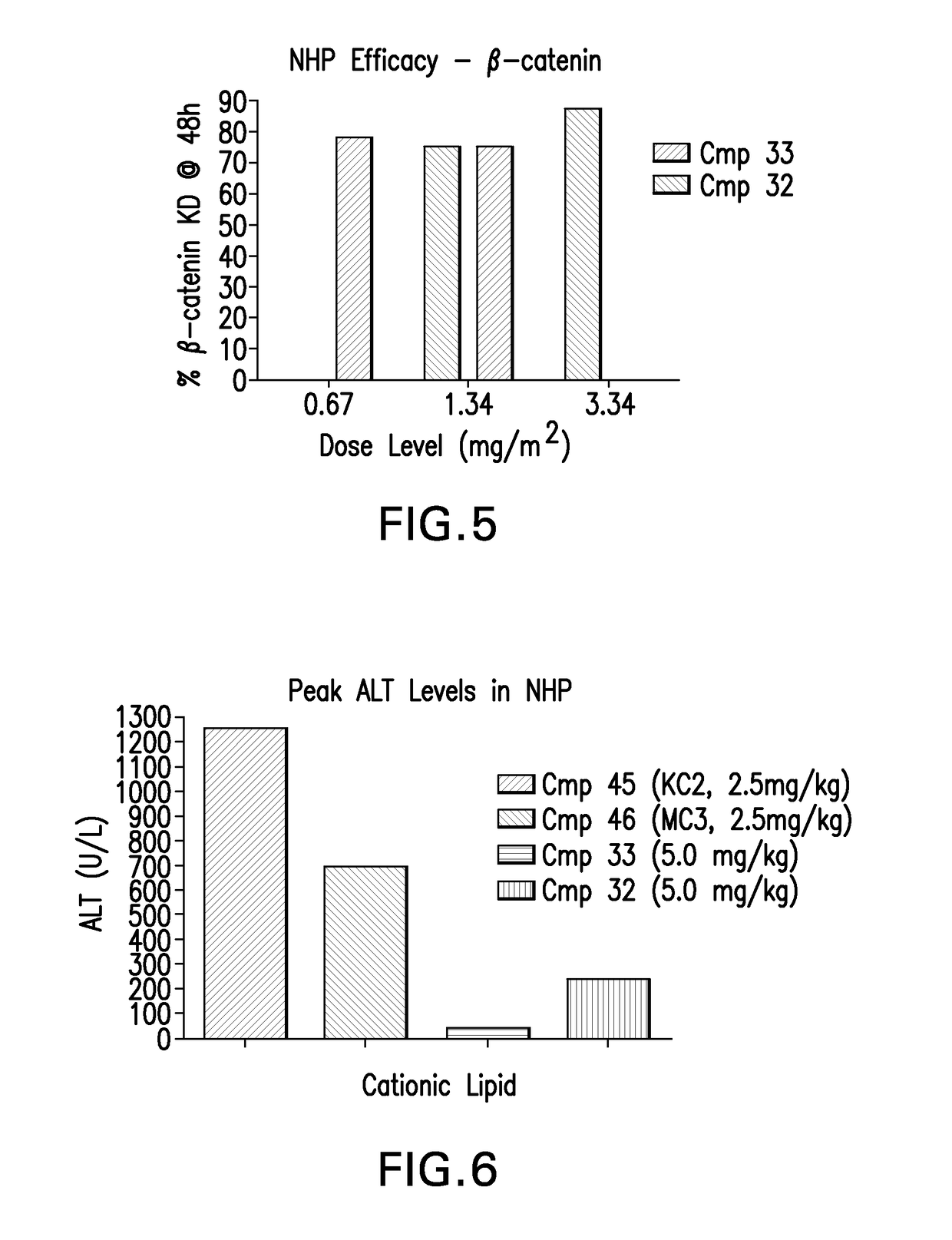
Novel low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS20130178541A1Good curative effectReduce liver toxicityBiocideMicroencapsulation basedTolerabilityNanoparticle
The instant invention provides for novel catiomc lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
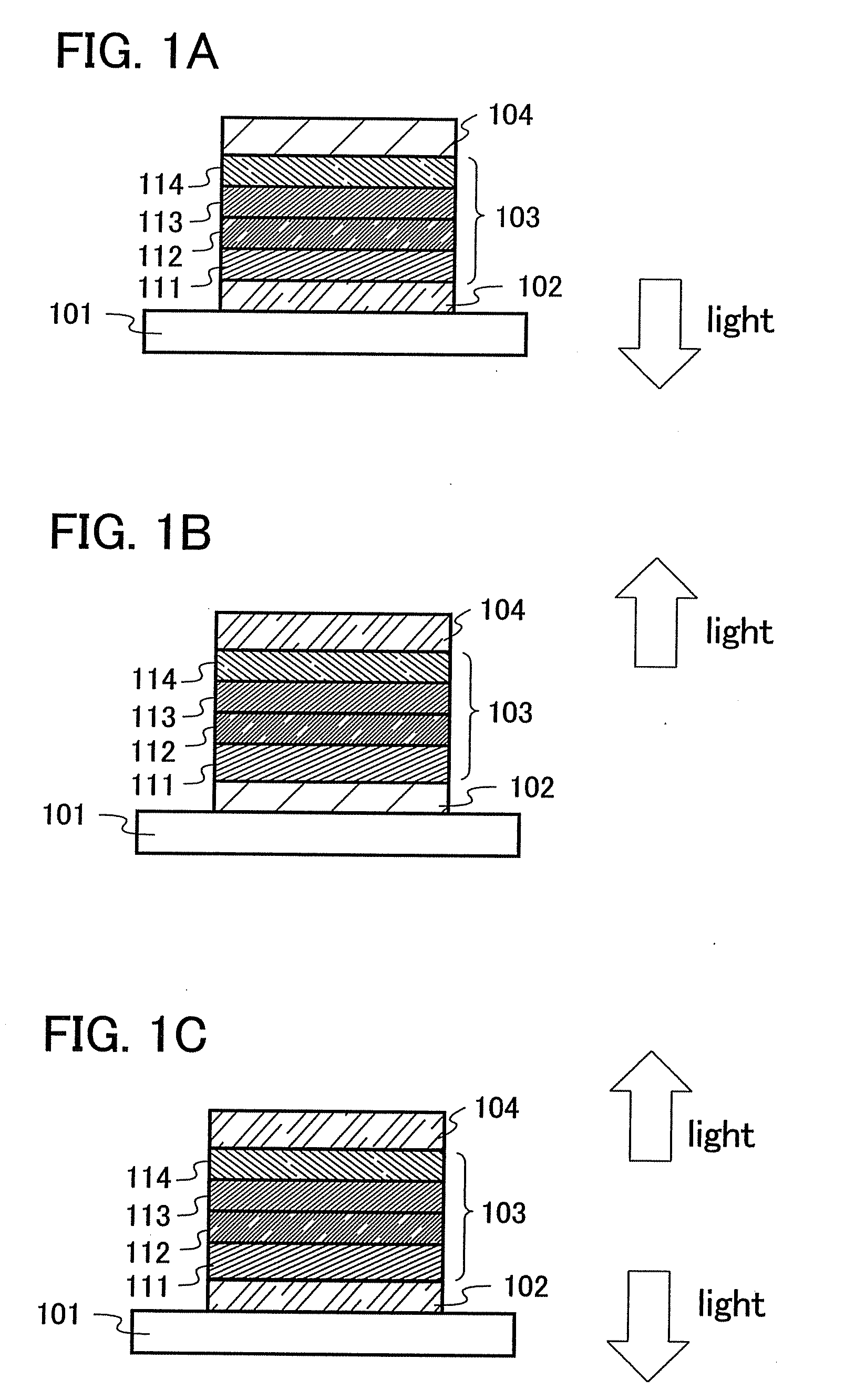
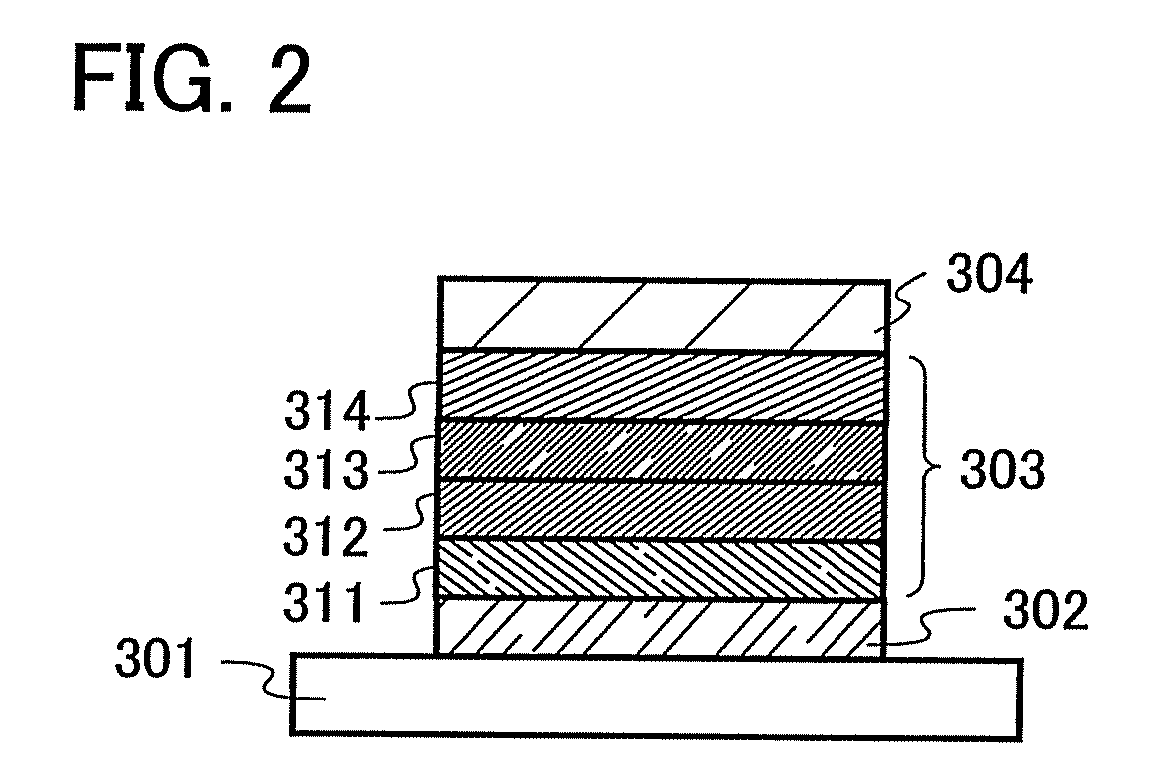
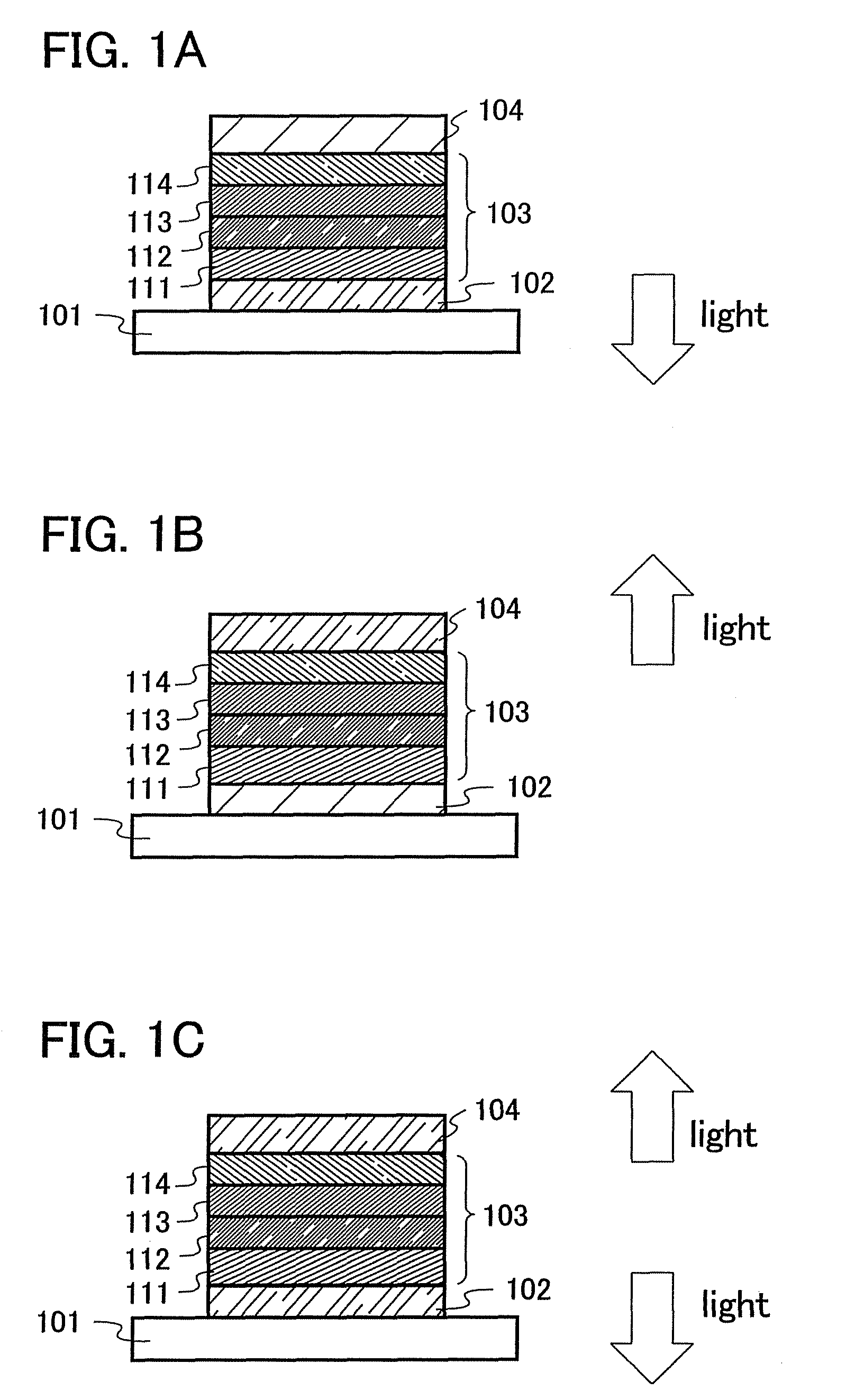
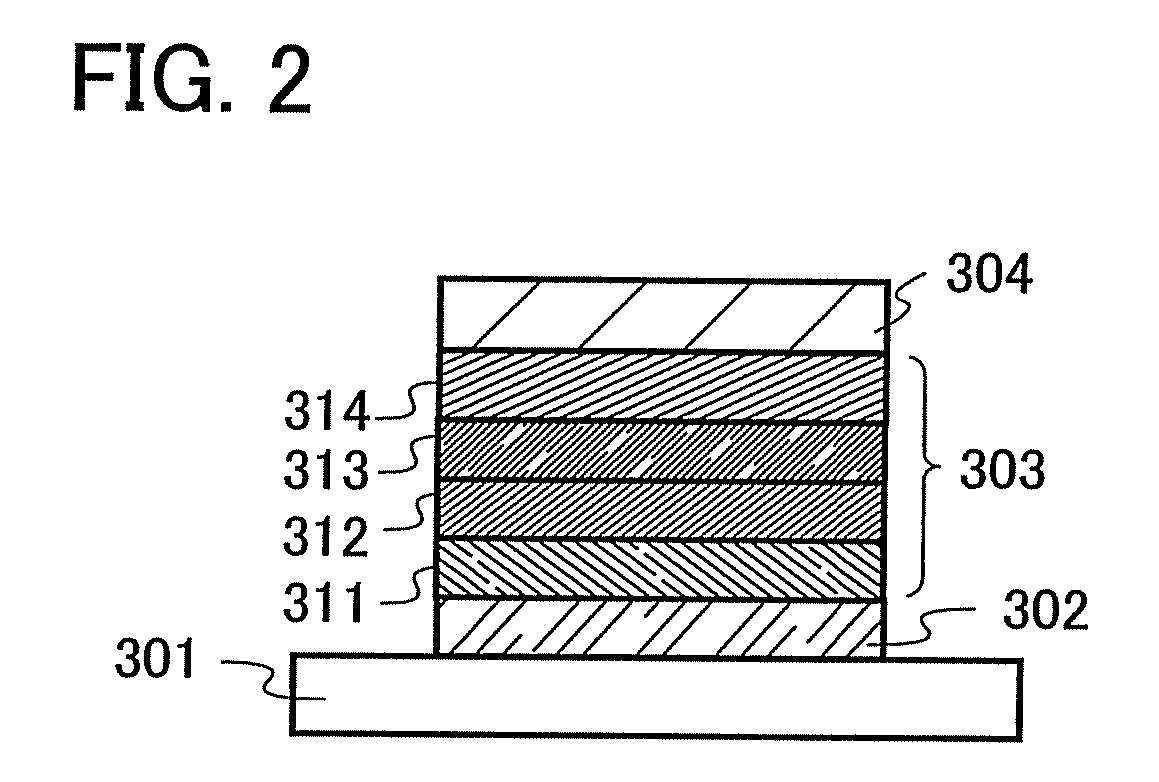
Anthracene Derivatives and Light-Emitting Devices Using the Anthracene Derivatives
ActiveUS20090004506A1Solve low luminous efficiencyLong life-timeOrganic compound preparationElectroluminescent light sourcesAnthraceneLight emitting device
The present invention provides novel anthracene derivatives. In particular, the present invention provides light-emitting elements with high luminous efficiency, and light-emitting elements with long lifetime. Further, the present invention provides light-emitting devices and electronic devices having long lifetime by using these light-emitting elements. An anthracene derivative represented by the general formula (1) is provided. In addition, since the anthracene derivative represented by the general formula (1) has high luminous efficiency, a light-emitting element using the anthracene derivative represented by the general formula (1) can also have high luminous efficiency. By using the anthracene derivative represented by the general formula (1), light-emitting elements with long lifetime can be provided.
Owner:SEMICON ENERGY LAB CO LTD
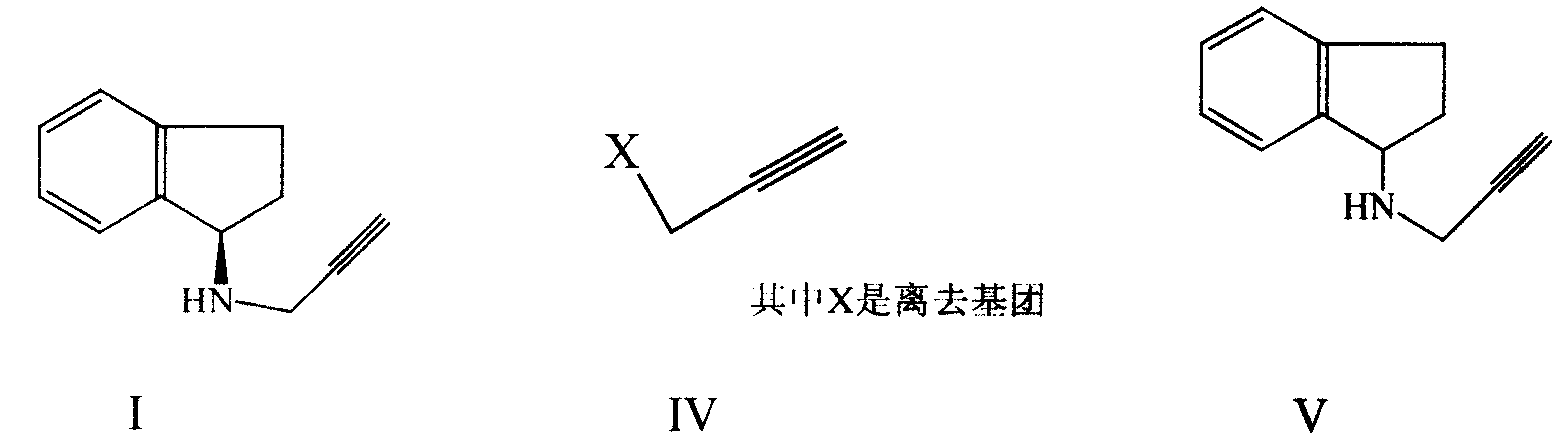
Improved process for preparing 2,3-dihydro-1H-indenes-1-amine and derivative thereof
InactiveCN101062897AEase of industrial productionEasy to operatePreparation by N-O/N-N bondsAmino preparation by functional substitutionHydrogenAntiparkinsonian drugs
The invention discloses an improved preparing method of anti-parkinson medicine detonate sand java blue important intermediate 2, 3- di-hydrogen- 1H-indenes-1-amine, which is characterized by the following: reacting 2, 3- di-hydrogen- 1H-indenes-1-ketoximes and alumino-nickel in queous alkali getting the product. This invention also discloses a 'one pot boiling' preparing method of 2, 3- di-hydrogen- 1H-indenes-1-amine with 2, 3- di-hydrogen- 1H-indenes-1- ketone. This invention also discloses a 'two steps one pot boiling' preparing method and 'three steps one pot boiling' preparing method of detonate sand java blue with 2, 3- di-hydrogen- 1H-indenes-1-ketoxime and 2, 3- di-hydrogen- 1H-indenes-1- ketone. This invention possesses simple operation, low cost and high productive efficiency, which is fit for industrial production.
Owner:CHONGQING PHARMA RES INST +1
Bifurcate alkyl chain and preparation and application thereof in organic conjugated molecules
ActiveCN102775273AEasy to convertAdjusted π-π stackingOrganic compound preparationSolid-state devicesOrganic solar cellOrganic field-effect transistor
The invention discloses a bifurcate alkyl chain and a preparation and an application thereof in organic conjugated molecules. The bifurcate alkyl chain serving as a solubilization group is applied in preparing organic conjugated molecules (particularly organic conjugated polymers), the interval number m of a formed alkyl side chain and main methylene is larger than one, capable of reducing effects of the alkyl chain on main Pi accumulation, so that the solubility of the organic conjugated molecules is guaranteed, and the mobility ratio of current carriers is improved greatly, the bifurcate alkyl chain is suitable for being used as organic semiconductor materials in photoelectric devices of organic field effect transistors, organic solar cells, organic light emitting diodes and the like.
Owner:PEKING UNIV +1
Diamine Derivative, Process of Preparation Thereof, and Fungicide Comprising Diamine Derivative as an Active Ingredient
InactiveUS20070244153A1Resistant problemBroad spectrumBiocideCarbamic acid derivatives preparationBULK ACTIVE INGREDIENTAmine derivatives
Owner:MITSUI CHEM INC
Processes for preparing pesticidal intermediates
InactiveUS6410737B1Efficient preparationOrganic compound preparationOrganic chemistry methodsMedicinal chemistryPyrazole Compound
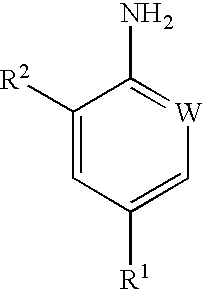
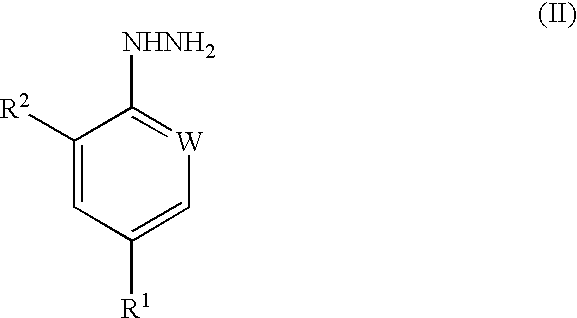
Compounds of formula (I) are prepared by hydrogenolysis of a compound of formula (II):where R1 is haloalkyl, haloalkoxy or -SF5; W is N or CR3; and R2 and R3 are H or Cl. The compounds of formula (I) can be used as intermediates in the preparation of pesticidally active arylpyrazole compounds.
Owner:AVENTIS CROPSCIENCE SA +1
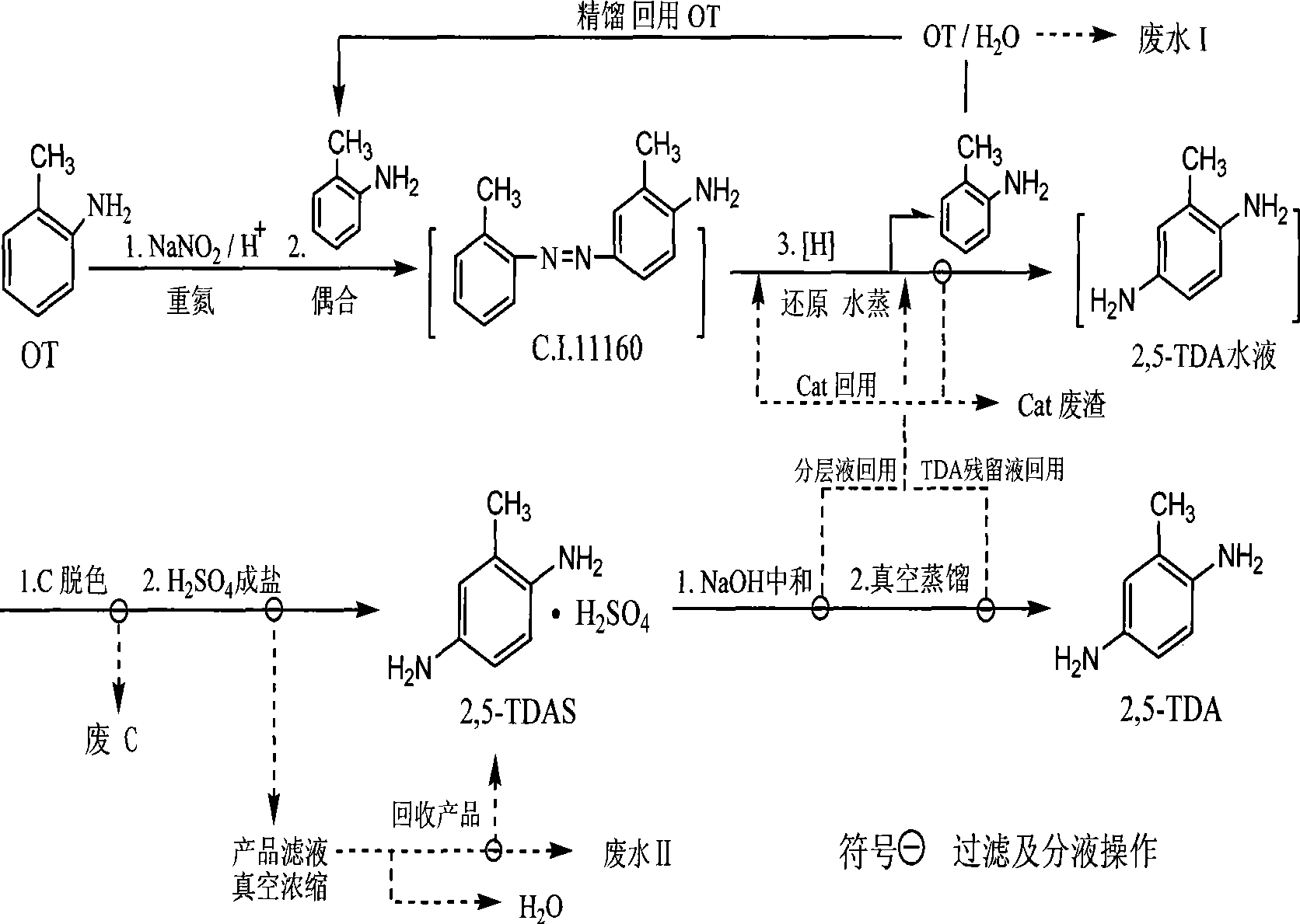
Synthetic method of 2,5-diaminotoluene and sulphate thereof
ActiveCN101450904AThe pre-processing process is simpleReduce unit consumptionPreparation by N-O/N-N bondsWater dischargeToluidine
Owner:宁夏瑞鼎科技有限公司
Resolving and racemization method for 1-amino-1,2,3,4-tetrahydronaphthalene
InactiveCN102675123AAvoid reduction reactionAvoid high pressure hydrogenationAmino compound purification/separationPreparation by N-O/N-N bondsTetralinDocument preparation
The invention provides a resolving and racemization method for 1-amino-1,2,3,4-tetrahydronaphthalene. The method comprises the following steps of: reducing 3,4-dihydro-1-(2H)-naphthalene ketoxime into DL-1-amino-1,2,3,4-tetrahydronaphthalene; and resolving DL-1-amino-1,2,3,4-tetrahydronaphthalene into optically pure (S)-1-amino-1,2,3,4-tetrahydronaphthalene and (R)-1-amino-1,2,3,4-tetrahydronaphthalene by using an acidic resolving agent, or oxidizing chiral secondary amine into corresponding imine or derivatives thereof, reducing into DL-secondary amine and continuously resolving to obtain a single optical product. The enantiomeric excess (ee) value of the obtained product is more than 99.5 percent; and the yield of one-time resolving exceeds 30 percent, and the total yield of the route reaches 46 to 54 percent. Compared with the methods reported by various documents and patents at present, the method has obvious technological and economical advantages.
Owner:SHANGHAI LANGTZE BIOMEDICAL TECH
Low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS9669097B2Good curative effectReduce liver toxicityBiocideMicroencapsulation basedTolerabilityNanoparticle
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
Method for preparing 4-amino-alpha, alpha,4-trimethyl-cyclohexanemethanamine from 1,8- terpinum
The invention relates to a method for preparing alkyl-diamine with 1, 8- terpin. It comprises following steps: preparing sulphuric acid solution with mass concentration being 10- 75% at closed condition, adding NaN3 at 10- 15 Deg. C, stirring thoroughly to disslove it in said sulphuric acid solution, then adding 1, 8- terpin, controlling temperature to be between 15-100 Deg. C, stewing, separating and drying after reaction finished and getting diazanyl. The molar ratio between NaN3 and 1, 8- terpin is (2.0- 3.0): 1, the invention employs 5%Pd-C or Lindlar as catalyst to reduce diazanyl and gets alkyl-diamine mixture, distilling, rectifying and getting final product alkyl-diamine. The invention employs NaN3 as raw material, which reduces raw material toxicity, it employs sulfuric acid solution as reaction medium and catalyst, which can be reused when the concentration is moderated, the cost is saved and pollution to enviroment is decreased.
Owner:湖南松本林业科技股份有限公司
Method for preparing 3-aminomethyl-3,5,5-trimethyl cyclohexylamine
ActiveCN101417952AReduce usageOrganic compound preparationPreparation by N-O/N-N bondsMethyl groupPhotochemistry
The invention discloses a method for preparing 3-aminomethyl-3, 5, 5-trimethyl cyclohexylamine (isophorone diamine, IPDA) by 3-cyano-3, 5, 5-trimethyl cyclohexanone (IPN, isophorone nitrile), which comprises the following steps: a) 3-cyano-3, 5, 5-trimethyl cyclohexanone and oxyammonia are arranged in an organic solvent for reaction under a temperature ranging from 40 DEG C to 80 DEG C, and 3-cyano-3, 5, 5-trimethyl cyclohexanone oxime is generated; b) 3-cyano-3, 5, 5-trimethyl cyclohexanone oxime, hydrogen and liquid ammonia are arranged in an organic solvent for reaction under a temperature ranging from 50 DEG C to 120 DEG C, a total pressure ranging from 5 MPa to 15 MPa and the effect of a hydrogenation catalyst, and 3-aminomethyl-3, 5, 5-trimethyl cyclohexylamine is obtained. The method has the advantages that, the production of a by-product 3-aminomethyl-3, 5, 5-trimethyl cyclohexanol (IPAA) which is hard to be separated from IPDA is avoided; simultaneously, the amount of liquid ammonia is largely decreased.
Owner:WANHUA CHEMICAL (NINGBO) CO LTD
Trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof
ActiveCN104649857AMild reaction conditionsHigh selectivityCarbamic acid derivatives preparationSugar derivativesTrifluoromethylationAzidotrimethylsilane
The invention discloses trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof. The preparing method of the trifluoromethyl-substituted azide compounds includes following steps of: subjecting a trifluoromethylation agent, azidotrimethylsilane and a carbon-carbon double bond of an olefin to addition in an organic solvent under the existence of a catalyst to obtain a compound in which one carbon in the carbon-carbon double bond of the olefin has trifluoromethyl and the other carbon has an azide group. The preparing methods utilize the trifluoromethylation agent which is mild relatively, directly form a carbon-nitrogen bond and a carbon-carbon bond by double-functionalization of olefins, and efficiently synthesize the trifluoromethyl-substituted azide, amine and heterocycle compounds with high selectivity. The preparing methods are easily available in raw materials, mild in reaction conditions, good in atom economy, high in selectivity, simple in after-treatment, environmental friendly, high in yields and suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method used for reductive amination using jet reactor
InactiveCN105237409AStrong entrainmentImprove mixing efficiencyOrganic compound preparationPreparation by N-O/N-N bondsAzoxyNitroso
The invention discloses a method used for reductive amination using a jet reactor, and belongs to the field of chemical technology. According to the method, the jet reactor is adopted. The jet reactor comprises a reactor, an ejector, a circulating pump, a concentration device, a heat exchanger, and a mixer. The jet reactor is used for chemical product hydrogenation reduction. According to the method, a mixture containing a catalyst, raw materials, and a target amination product is taken as a dynamic fluid, high speed liquid flow is obtained via injection, and stable turbulent flow is formed at a nozzle, and in addition, negative pressure is formed around the nozzle, entrainment of hydrogen is realized, and gas-liquid-solid complete mixing reaction is realized. After reaction, and obtained product is subjected to condensation separation so as to obtain partially aminated products; a concentrate containing the catalyst and a reduced material are subjected to preheating using the heat exchanger, and mixing using the mixer, and then are delivered into the ejector for circular reaction. The method can be used for obtaining amines via reduction of nitro compounds, nitroso compounds, azo-compounds, azoxy compounds, and hydride azo compound.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nucleic acid fluorescence probe and preparation method thereof
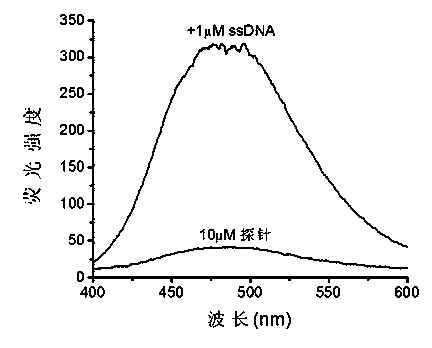
InactiveCN103772203AGood choiceLow costOrganic compound preparationMicrobiological testing/measurementNucleic acid detectionMolecular rotation
The invention discloses a novel nucleic acid fluorescence probe. Amido is introduced into tetraphenyl ethylene, amido is a basic group and can be combined with nucleic acid, so that molecular rotation is limited, nonradiative excited-state relaxation is inhibited and fluorescence is obviously enhanced, and thus nucleic acid detection is realized. The novel nucleic acid fluorescence probe has high sensitivity to double-stranded DNA and single-stranded DNA, and the probe has good application prospect to gel electrophoresis or detection of nucleic acid in biological samples.
Owner:WUHAN UNIV
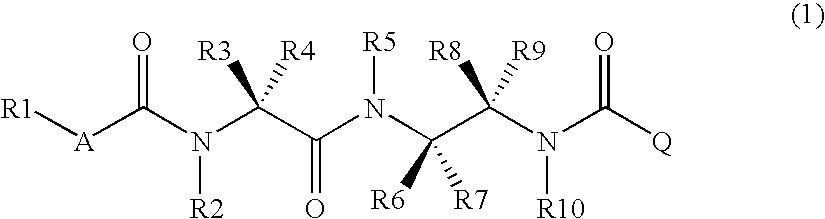
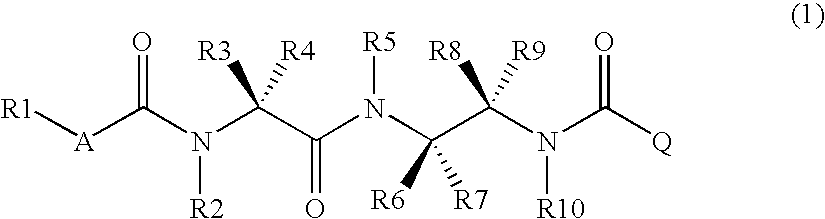
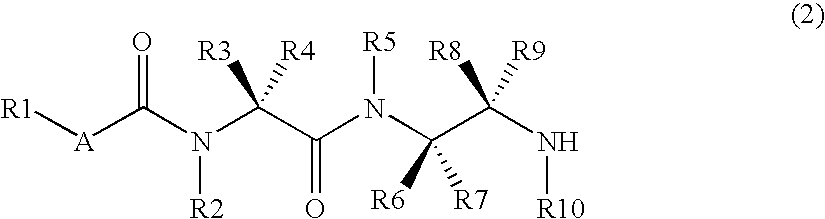
Diamine derivative, process for producing the same and fungicide containing the derivative as active ingredient
InactiveCN1984880ABroad spectrumHigh bactericidal activityBiocideCarbamic acid derivatives preparationFungicideAryl
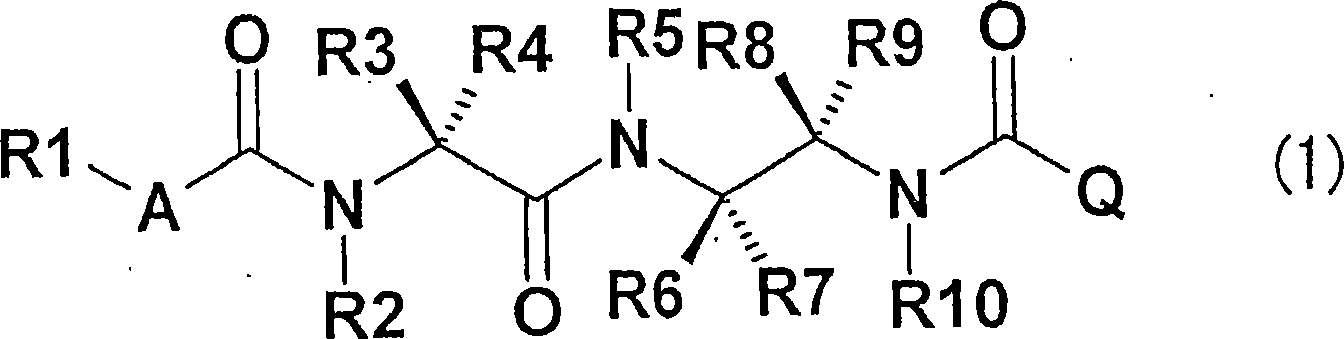
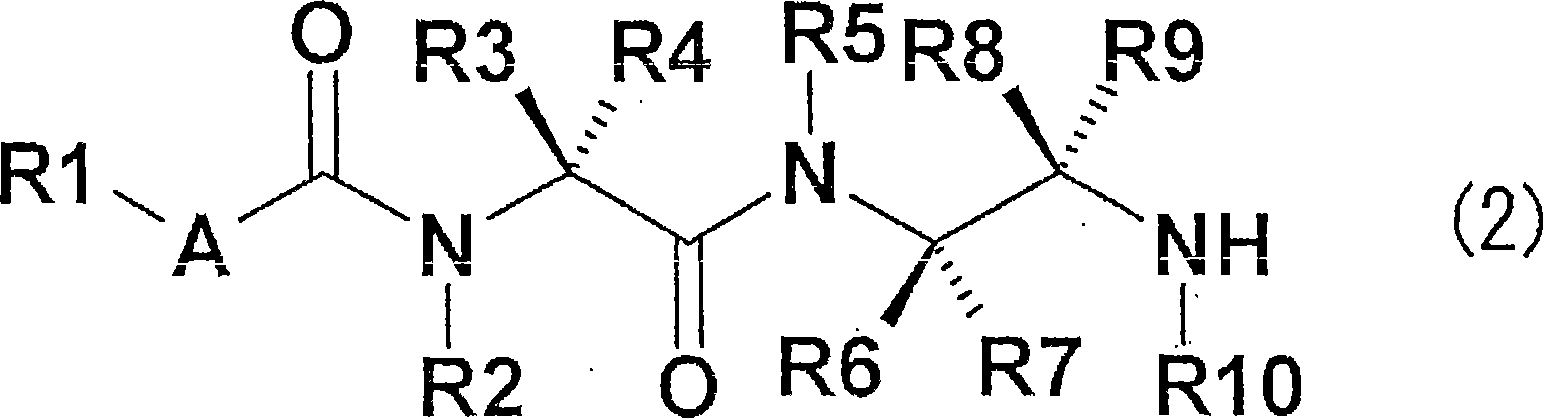
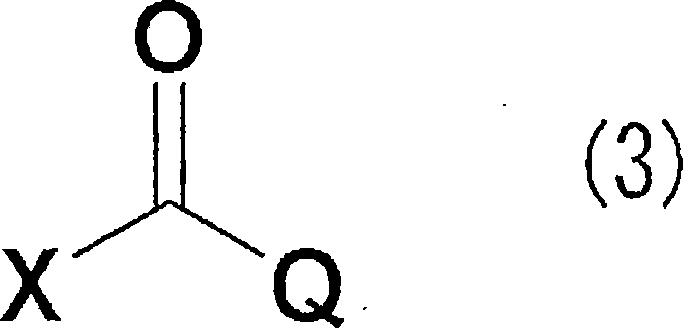
It is an object of the invention to provide a novel fungicide which exhibits a wide controlling spectrum against pathogens of various crops, and solves the toleration problem. The diamine derivative represented by the formula (1) and a process for preparation of the same, fungicides comprising the same as an active ingredient are disclosed: [wherein R1 is substituents such as an alkyl group having 1 to 6 carbon atoms and the like, R2 and R5 are each independently substituents such as hydrogen atom, an alkyl group having 1 to 6 carbon atoms and the like, R3 and R4 are each independently substituents such as hydrogen atom, an alkyl group having 1 to 6 carbon atoms and the like, or R3 and R4 may be bonded to each other to form a hydrocarbon ring having 3 to 6 carbon atoms, R6, R7, R8 and R9 are each independently substituents such as hydrogen atom, an alkyl group having 1 to 6 carbon atoms and the like, R10 is a substituent such as hydrogen atom, an alkyl group having 1 to 6 carbon atoms and the like, A is an oxygen atom or a sulfur atom, and Q is an aryl group or a heterocycle].
Owner:MITSUI CHEM INC
Method for absorbing nitrogen oxide tail gas and generating by-product p-phenylenediamine by using aniline
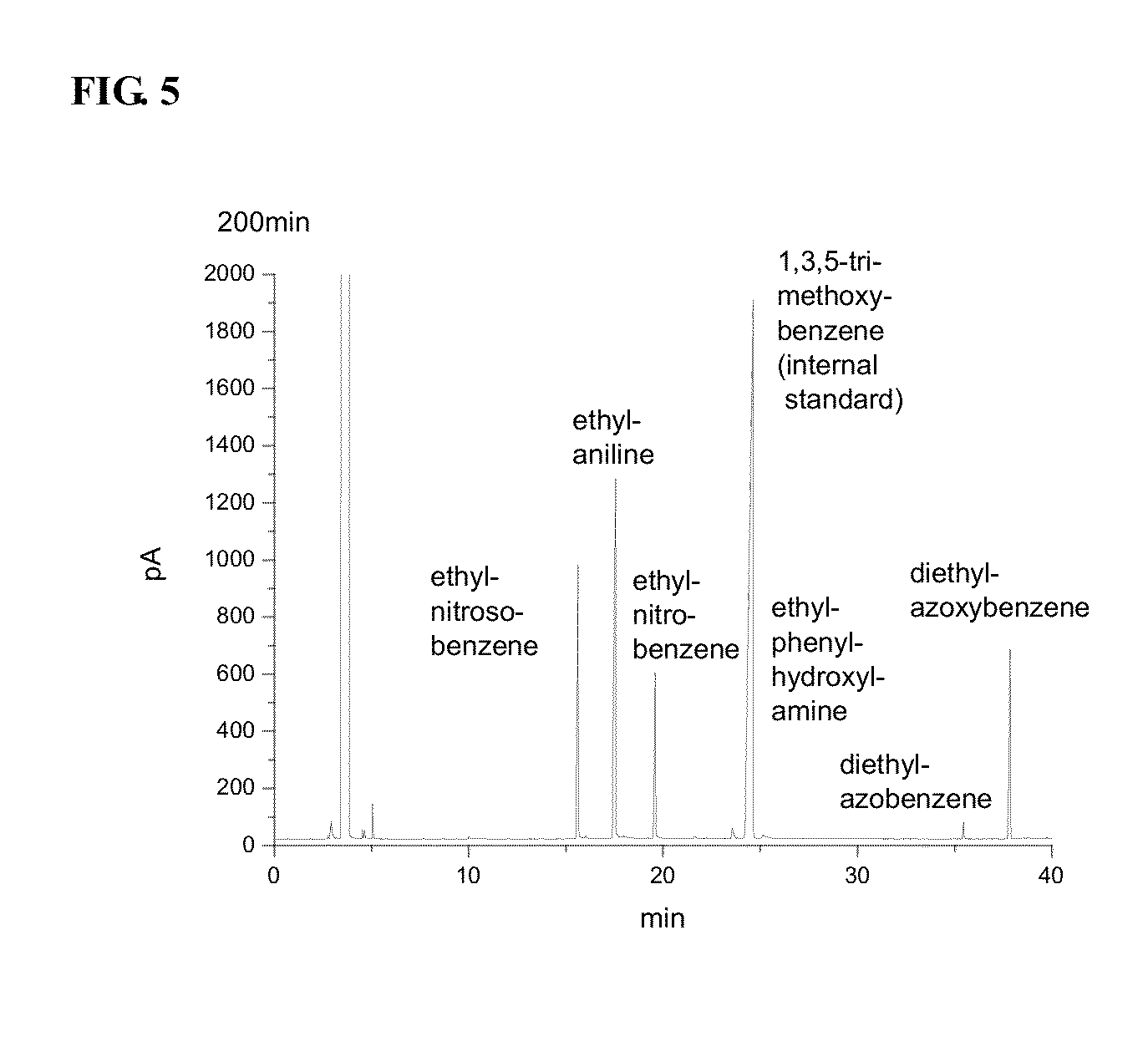
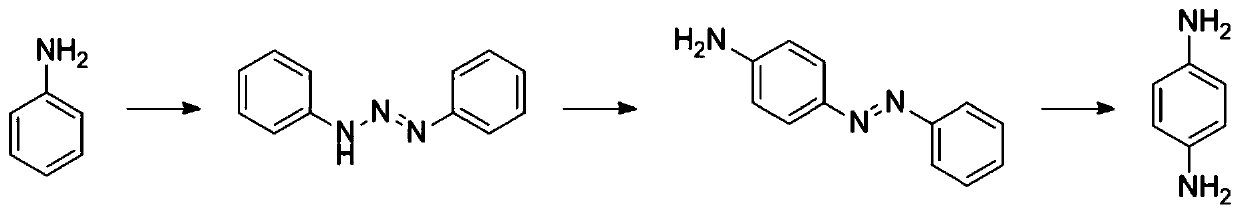
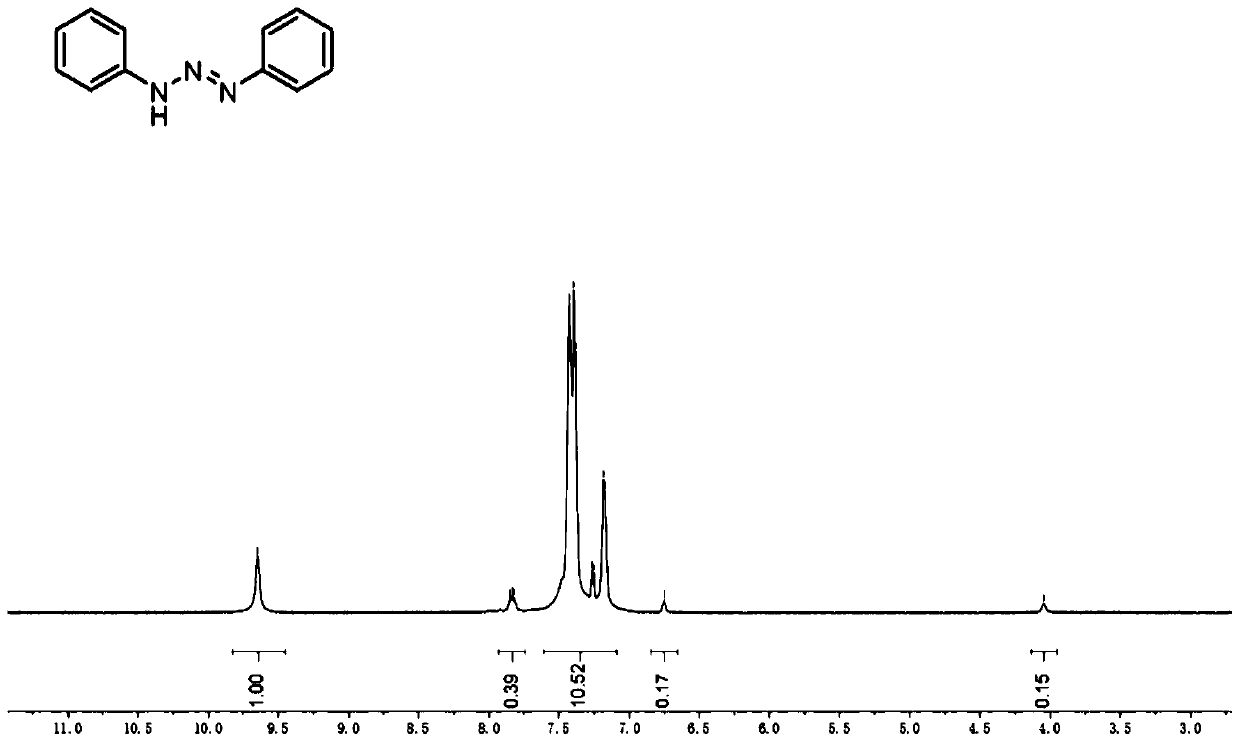
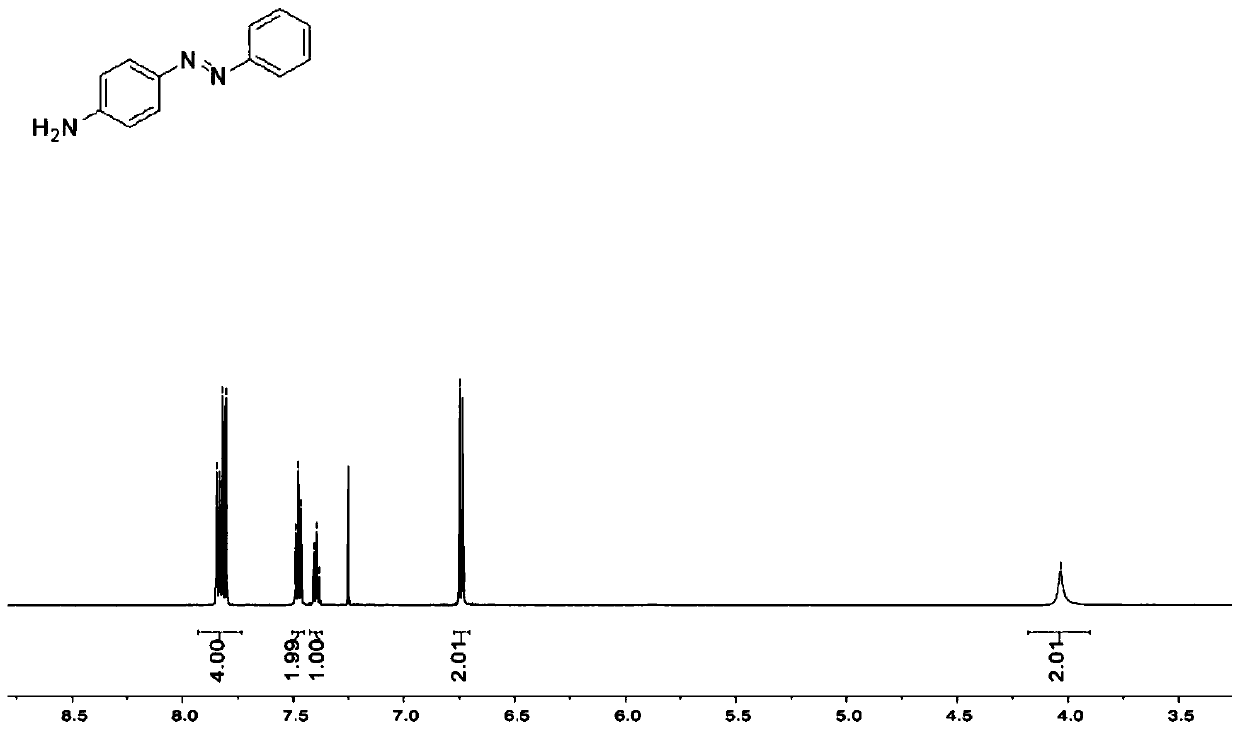
ActiveCN102731320AReduce manufacturing costReduce harmPreparation by N-O/N-N bondsP-NitroanilineP-Aminoazobenzene
The invention discloses a method for absorbing nitrogen oxide tail gas and generating by-product p-phenylenediamine by using aniline. The method comprises the following steps of: reacting part aniline with NOx to obtain diazonium salt; reacting the diazonium salt with the non-reacted aniline to obtain 1,3-diphenyltriazene, wherein the reaction products contain less p-nitroaniline and o-nitroaniline; making the 1,3-diphenyltriazene in a rearrangement reactor generate the rearrangement reaction at 30 DEG C-120 DEG C to convert into p-aminoazobenzene, wherein 90% of the 1,3-diphenyltriazene is converted into the p-aminoazobenzene and the remaining 1,3-diphenyltriazene is converted into o-aminoazobenzene and few impurities after the rearrangement reaction; separating the low distillates from the rearranged materials; and carrying out the hydrogenation reaction: using Raney nickel as a catalyst, continuously inputting hydrogen gas and controlling the pressure and the temperature to be 0.2MPa-4MPa and 25 DEG C-150 DEG C respectively to synthesize the p-phenylenediamine. The method provided by the invention reduces the production cost of the p-phenylenediamine and also reduces environmental pollution. A DCS (distributed control system) computer control system is used so that the automation of the whole system is realized and meanwhile the production efficiency is high.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
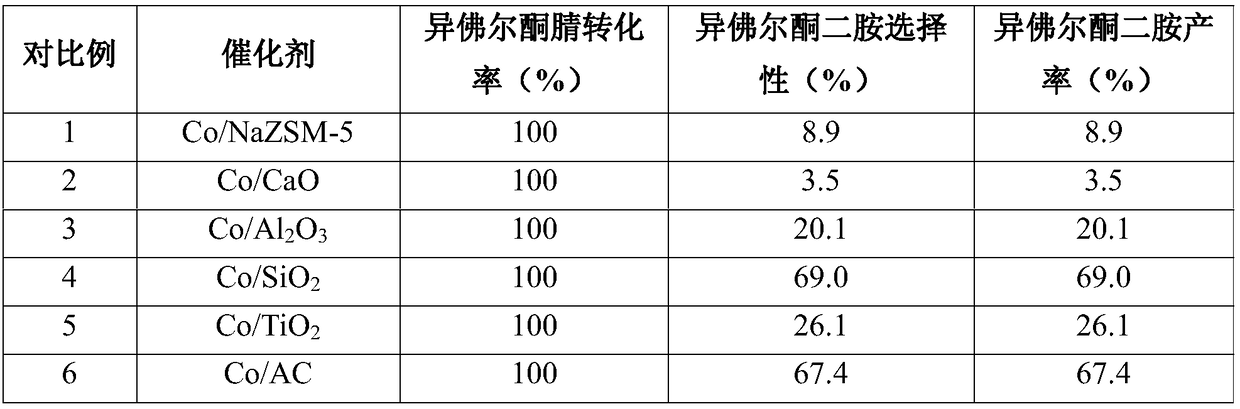
Catalyst for reductive amination of isophorone nitrile to synthesize isophorone diamine and preparation method and applications thereof
ActiveCN108686660AImprove stabilityHigh catalytic efficiencyPreparation by N-O/N-N bondsMetal/metal-oxides/metal-hydroxide catalystsActivated carbonIsophorone
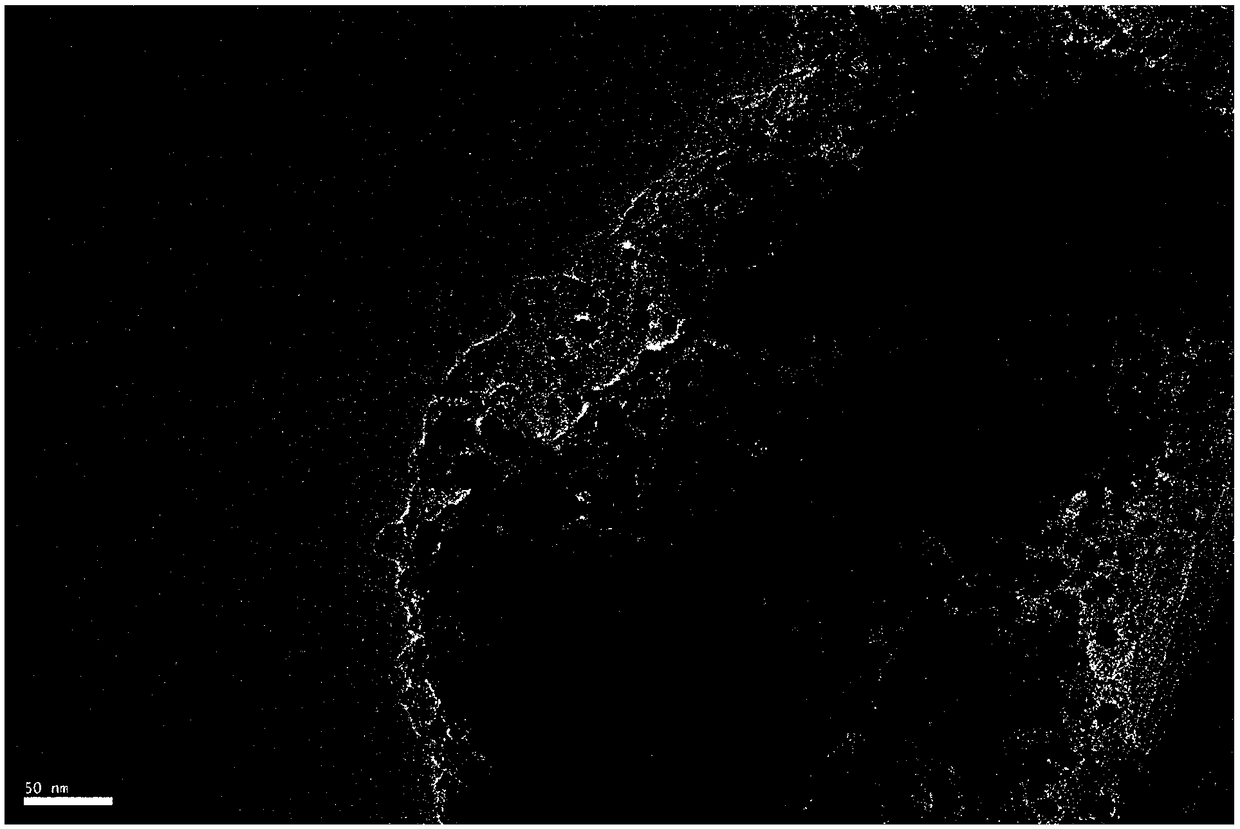
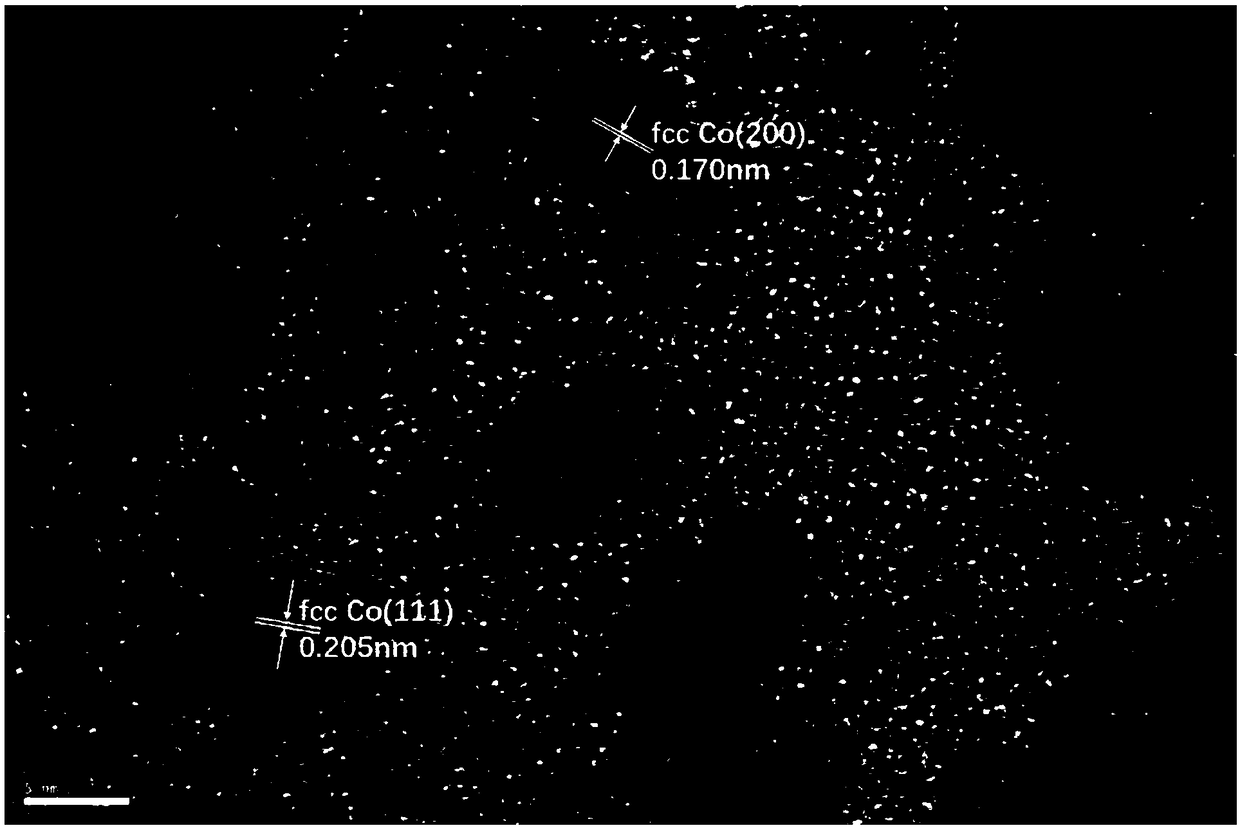
The invention discloses a catalyst for reductive amination of isophorone nitrile to synthesize isophorone diamine. The catalyst comprises an active component and a carrier. The active component is cobalt, the carrier is active carbon, and cobalt has a fcc crystal form when being loaded on active carbon. The disclosed novel catalyst has the advantages of little using amount, high catalytic activity, good stability, and resistance to inactivation and is capable of efficiently catalyzing the reductive amination of isophorone nitrile to synthesize isophorone diamine without a hydrogen reduction technology.
Owner:ZHEJIANG UNIV
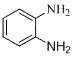
Method for preparing o-phenylenediamine and derivative of o-phenylenediamine
ActiveCN104262166AAvoid separation stepsAvoid pollutionPreparation by N-O/N-N bondsOrganic compoundSpent acid
The invention relates to a method for synthesizing organic compounds, and provides a method for preparing o-phenylenediamine and a derivative of o-phenylenediamine, for solving the problems that the process route is complex, a great amount of byproducts can be generated and are hard to purify, the reaction condition is rigorous, the environment pollution is severe and the like in a conventional method for synthesizing o-phenylenediamine derivatives. The method disclosed by the invention comprises the following steps: by taking azobenzene and a derivative of the azobenzene as raw materials, synthesizing the o-nitro azobenzene and the derivative of the o-nitro azobenzene under the coactions of a catalyst, an oxidant and a nitrating agent, and further reducing by using a reducing agent, thereby obtaining the o-phenylenediamine and the derivative of the o-phenylenediamine. The method provided by the invention has the advantages that the raw materials are cheap and easy to obtain, the operation is simple, convenient and safe, the synthesis steps are short, the purification is simple, no great waste acid pollution is caused, and the like.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
Preparation method of 2-aminoindan
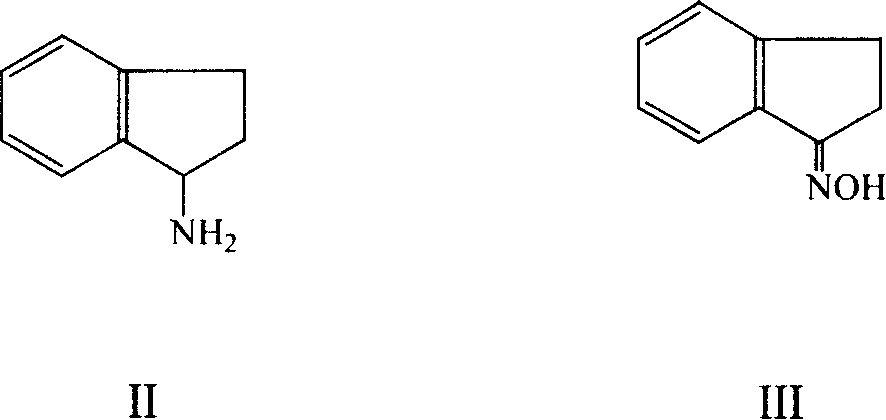
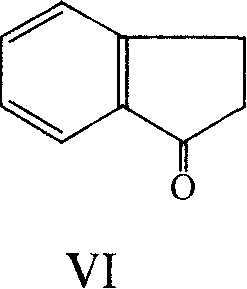
InactiveCN104628575AFew reaction stepsSimple and fast operationPreparation by N-O/N-N bondsAluminium chloridePotassium borohydride
The invention provides a preparation method of 2-aminoindan. Under the catalytic effect of lewis acid such as lithium chloride, aluminium chloride and magnesium chloride, 2-indene oxime is reduced by using potassium borohydride to prepare 2-aminoindan. The synthesis method disclosed by the invention can be completed in one step and has the advantages of less reaction steps, convenient operation, simple separation and purification method and operation, high yield and low cost; the product has good appearance, good color and luster and high purity (99.5%); in addition, the reducing agent used in the method is low in price and easily available; therefore, the preparation method is suitable for industrial production.
Owner:XUCHANG HAOFENG CHEM TECH
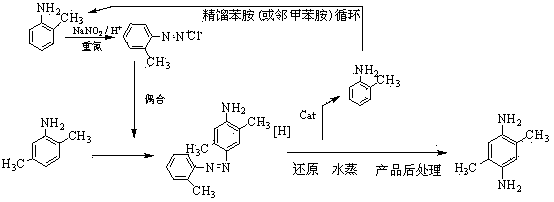
2,5-dimethyl-1,4-phenylenediamine preparation method
The invention relates to a preparation method of 2,5-dimethyl-1,4-phenylenediamine. The method is characterized in that aniline substances are used as a raw material and are made into 2,5-dimethyl-1,4-phenylenediamine through a diazotization reaction, a coupled reaction, a hydrogenolysis reaction and purification treatment. According to the invention, the main and the auxiliary raw materials are cheap and easy to obtain, the reaction steps are relatively few, the yield is high, the operation is simple and convenient, and the method is used for further replacing the old technology to realize and develop the novel industrialized production of the product; the aniline substances are reclaimed to be still used as auxiliary raw materials for preparing aniline diazonium salt, so that cost and pollution are reduced, circular economy is produced, and cleaner production is realized; the quality of the obtained product is improved greatly, and the content is far more than the old product, especially an exported product of the content of 75%; the prepared product can not only be used for dye and paints, but also can expand using functions of a high-performance material monomer.
Owner:ZHEJIANG DINGLONG TECH
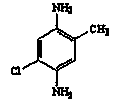
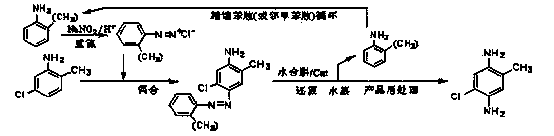
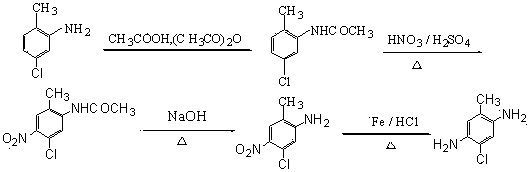
Preparation method for 5-chloro-2-methyl-1,4-phenylenediamine
ActiveCN103508904AMeet economic requirementsEasy to implementPreparation by N-O/N-N bondsMethanol waterMethyl benzene
The invention relates to a preparation method for 5-chloro-2-methyl-1,4-phenylenediamine. 5-chloro-2-methyl aniline is used as a raw material and subjected to coupled reaction with aniline diazonium salt prepared through diazotization of an aniline substance to prepare an azo-compound; the azo-compound is subjected to hydrazine hydrate normal-pressure reduction and hydrogenolysis with the existence of a catalyst; in the later period of reduction, after the aniline substance taken off by reaction is subjected to water steaming out while reduction is performed; a methanol lysate is added to remove the catalyst so as to obtain a methanol water solution of 5-chloro-2-methyl-1,4-phenylenediamine; the obtained methanol water solution is subjected to adsorption, decoloring and edulcoration, and then after-treatment technologies of cooling crystallization, filtering, water washing, drying and the like to prepare high-purity 5-chloro-2-methyl-1,4-phenylenediamine; a mixture of the aniline substance subjected to steam distillation and recovery and water is separated and rectified, and then recycled in preparation of aniline diazonium salt. The preparation method has the characteristics of novel route, easily accessible raw material, high atom utilization ratio, conventional equipment, simple and convenient operation, little pollution, capability of realizing circular economy and the like.
Owner:ZHEJIANG DINGLONG TECH +1
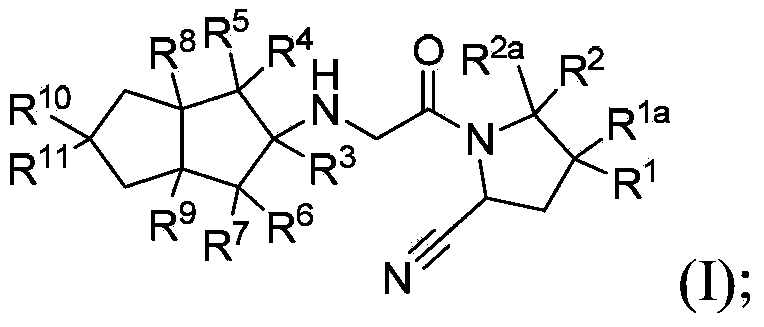
Hexahydropentaleno derivatives, preparation methods and applications in medicine thereof
The invention relates to hexahydropentaleno derivatives, the preparation method and use in medicine thereof, and in particular to hexahydropentaleno derivatives or stereo-isomers or pharmaceutically acceptable salts thereof as shown in general formula (I), and to the preparation method therefor and pharmaceutical compositions comprising the derivatives, and to the use thereof as a therapeutical agent, especially as a DPP-IV inhibitor. The definition of each substituent in formula (I) is the same as the definition in the description.
Owner:SUNSHINE LAKE PHARM CO LTD
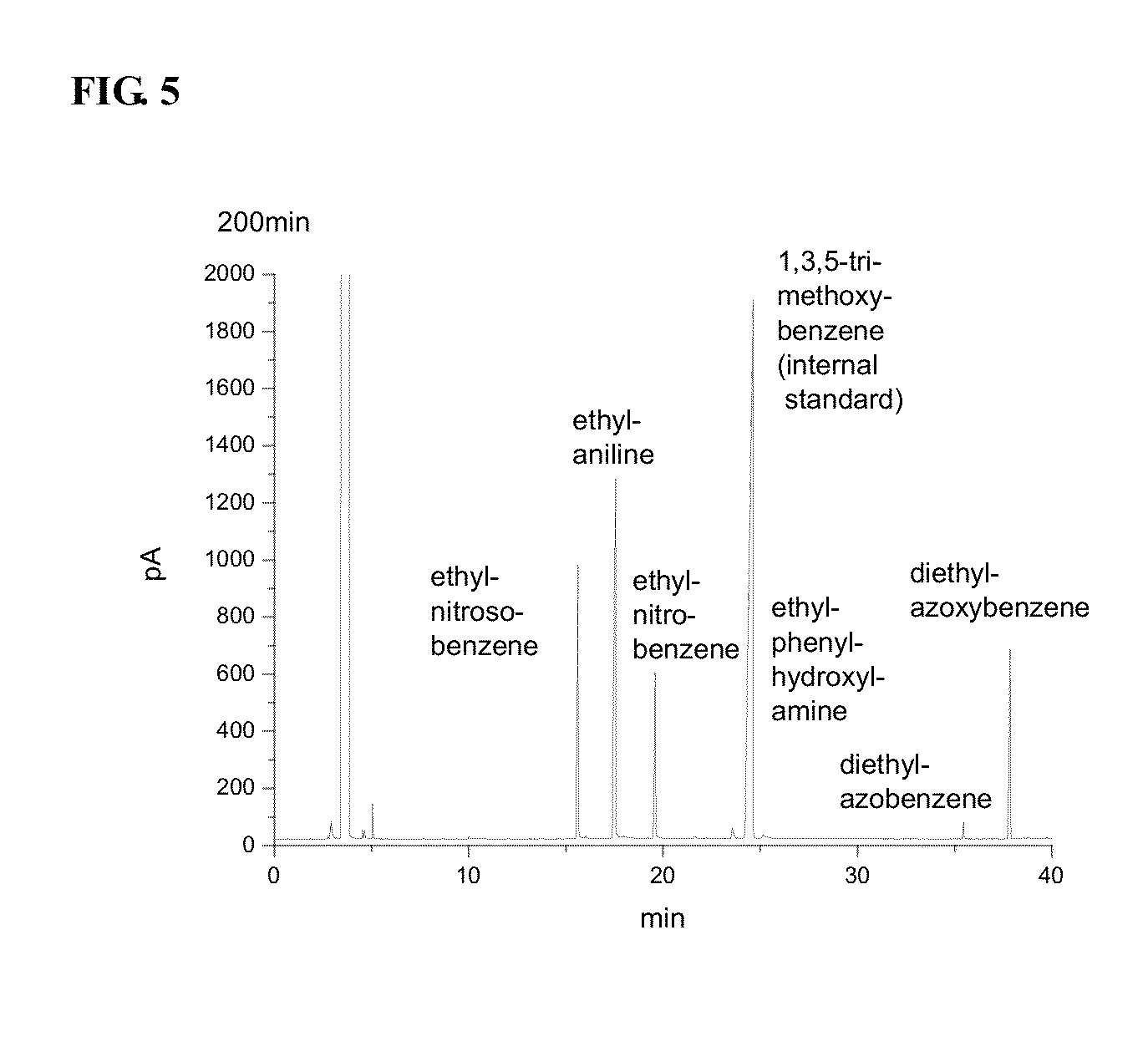
Polymer supported reagents and methods or reducing aromatic nitro compounds by using the same
ActiveUS20130253083A1High yieldLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitro compoundCrosslinked polymers
The present invention relates to a polymer supported reagent comprising a novel crosslinked mesoporous polymer, enabling a simple and easy production of an azoxy compound or an azo compound from an aromatic nitro compound, and a method of selectively reducing an aromatic nitro compound by using the same. The polymer supported reagent comprises a certain acrylamide mesoporous crosslinked polymer.
Owner:LG CHEM LTD +1
Synthesis method of p-phenylenediamine
ActiveCN110818572AHigh purityThe preparation process is safe and environmentally friendlyPreparation by N-O/N-N bondsPtru catalystDistillation
The invention relates to a synthesis method of p-phenylenediamine, and belongs to the technical field of organic material preparation. Aniline adopted as a raw material undergoes a diazotization reaction and a heating rearrangement reaction to generate 4-aminoazobenzene, a proper amount of an acid is added, and the 4-aminoazobenzene is separated out; the 4-aminoazobenzene is dissolved in a propersolvent, a catalyst is added, the p-phenylenediamine product is obtained through a hydrogenation reduction reaction, filtration and reduced pressure distillation, and aniline is recovered as a byproduct and can be recycled as the raw material. Compared with an existing p-phenylenediamine production method, the method of the invention has the advantages of safe and environmentally-friendly preparation process, little emission of three wastes, easily available reaction raw materials, simple reaction steps, and high purity of the finally prepared product, so the method has good industrial implementation prospects.
Owner:TSINGHUA UNIV
Method for preparing 3,3'-dichlorobenzidine hydrochloride by hydrochloric acid translocation
The invention discloses a method for preparing 3,3'-dichlorobenzidine hydrochloride by hydrochloric acid translocation. The method comprises the following steps: catalytically hydrogenating ortho-nitrochlorobenzene by hydrogen in the presence of catalysts, such as organic solvents, rare metal and the like to prepare 2,2'-dichlorohydrazobenzene; performing a translocation and rearrangement reactionin hydrochloric acid to finally prepare the 3,3'-dichlorobenzidine hydrochloride with yield reaching over 90 percent. The method has reasonable process route; and the finished product prepared by adopting the method has higher yield and purity, and has larger industrial prospect.
Owner:夏恩将
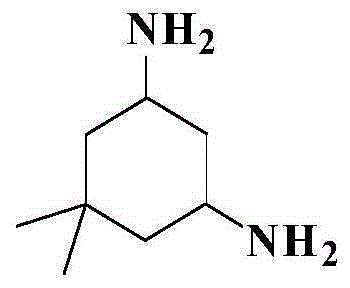
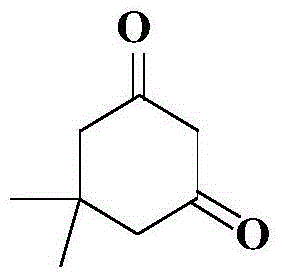
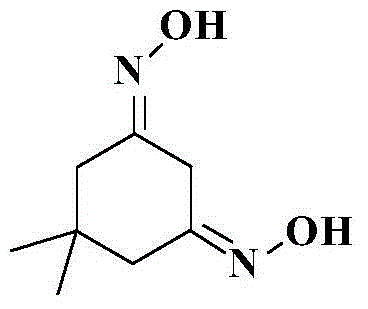
Preparation method of 5,5-dimethyl-1,3-cyclohexamethylenediamine
The invention relates to a preparation method of 5,5-dimethyl-1,3-cyclohexamethylenediamine, which comprises the following steps: 1. preparing 5,5-dimethyl-1,3-cyclohexyldione from 4-methyl-3-pentenyl-2-one and diethyl malonate; 2. adding the 5,5-dimethyl-1,3-cyclohexyldione into a polar organic solvent, carrying out condensation reaction with hydroxylamine hydrochloride under the action of an alkaline reagent to prepare 5,5-dimethyl-1,3-cyclohexyldiketodioxime; and 3. adding a polar organic solvent into the 5,5-dimethyl-1,3-cyclohexyldiketodioxime, and carrying out catalytic hydrogenation reduction reaction under the catalytic action of a metal catalyst to prepare the 5,5-dimethyl-1,3-cyclohexamethylenediamine. The total yield of the method is 50-55%, the gas-phase purity and content can reach 98.0% above, and the method has high safety and is simple to operate and beneficial to industrial production amplification.
Owner:VALIANT CO LTD
Synthetic method for cyclohexylamine
InactiveCN100528830CHigh purityMild reaction conditionsPreparation by N-O/N-N bondsHydrogenCyclohexanone oxime
The process of synthesizing cyclohexylamine includes the reduction reaction of butanone oxime and hydrogen inside solvent with ammonia at pressure of 1-5 MPa and temperature of 20-150 deg.c in the presence of Raney Ni catalyst; and the dewatering, drying and rectification of the reaction product. The Raney Ni catalyst amount is 0.3-10 wt% of butanone oxime, and the weight ratio between ammonia and butanone oxime is 0-1 to 1. The cyclohexylamine synthesizing process has low cost and high yield.
Owner:ZHEJIANG UNIV
Anthracene derivatives and light-emitting devices using the anthracene derivatives
ActiveUS8288012B2High luminous efficiencyLong lastingDischarge tube luminescnet screensOrganic compound preparationAnthraceneLight emitting device
The present invention provides novel anthracene derivatives. In particular, the present invention provides light-emitting elements with high luminous efficiency, and light-emitting elements with long lifetime. Further, the present invention provides light-emitting devices and electronic devices having long lifetime by using these light-emitting elements. An anthracene derivative represented by the general formula (1) is provided. In addition, since the anthracene derivative represented by the general formula (1) has high luminous efficiency, a light-emitting element using the anthracene derivative represented by the general formula (1) can also have high luminous efficiency. By using the anthracene derivative represented by the general formula (1), light-emitting elements with long lifetime can be provided.
Owner:SEMICON ENERGY LAB CO LTD
Polymer supported reagents and methods or reducing aromatic nitro compounds by using the same
ActiveUS9169200B2High yieldLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitro compoundCrosslinked polymers
The present invention relates to a polymer supported reagent comprising a novel crosslinked mesoporous polymer, enabling a simple and easy production of an azoxy compound or an azo compound from an aromatic nitro compound, and a method of selectively reducing an aromatic nitro compound by using the same. The polymer supported reagent comprises a certain acrylamide mesoporous crosslinked polymer.
Owner:LG CHEM LTD +1
Process for producing hexamethylenediamine and aminocapronitrile from adiponitrile, wherein the hexamethylenediamine contains less than 100 ppm tetrahydroazepine
InactiveUS7060819B2Amino compound purification/separationCarboxylic acid nitrile preparationHexamethyldiamineDistillation
Process for making both ACN and HMD from partial hydrogenation of ADN by using a combination of distillations resulting in the formation of a mixture of HMD and THA that can be hydrogenated to produce a mixture of HMD and HMI that can be separated easily by simple distillation.
Owner:INVISTA NORTH AMERICA R L
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com