Improved process for preparing 2,3-dihydro-1H-indenes-1-amine and derivative thereof
A dihydrogen, hydroxylamine technology, applied in the preparation of amino-substituted functional groups, preparation through nitrogen-oxygen/nitrogen-nitrogen bonds, organic chemistry, etc., can solve the limitations of industrialization, high cost, and the difficulty of resolving agents. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
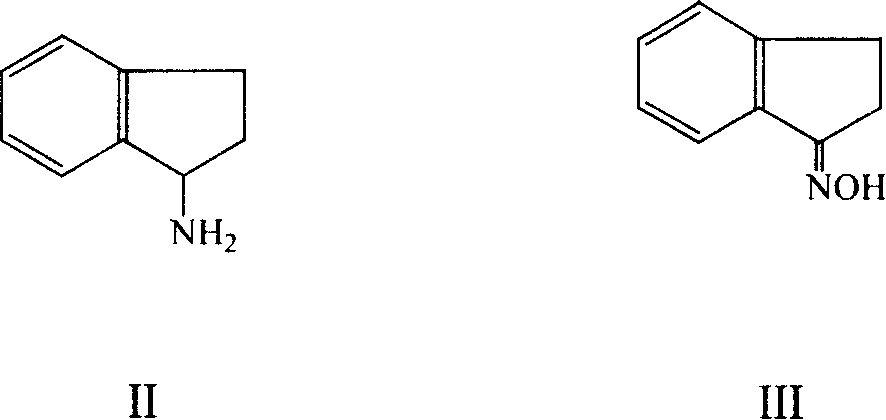
[0085] Preparation of 2,3-dihydro-1H-inden-1-amine (II)
[0086] In a 150ml three-necked flask, add 4.0g (27.2mmol) of 2,3-dihydro-1H-inden-1-one oxime (III), 40ml of ethanol, and 40ml of 20% sodium hydroxide solution, and control the temperature at 50-55°C , add aluminum-nickel alloy (nickel mass content 40-50%, 60-80 order) 6.0g in batches in 2~3 hours, react about 9 hours (TLC monitors reaction process, developer: normal hexane: ethyl acetate= 4:1). Filter, evaporate most of the ethanol in the filtrate under reduced pressure, extract with dichloromethane 30ml×3, combine the dichloromethane layers, wash with water and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and concentrate to obtain the title compound (II) 2.9 g, oil.
[0087] IR (KBr): 3356, 3285, 3068, 3021, 2946, 2852, 1588, 1476, 1456, 1374, 1297, 1156, 1022, 951, 809, 758, 601, 459cm -1
[0088] EI-MS: 133 (M + ), 132, 116, 117, 115, 133, 104, 65, 91, 77, 51.
Embodiment 2
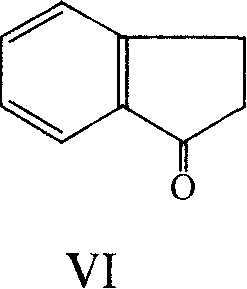
[0090] "One-pot" preparation of 2,3-dihydro-1H-inden-1-amine (II)
[0091]In a 10L reaction flask, add 890ml of water, 368.0g (5.296mol) of hydroxylamine hydrochloride, 890ml of ethanol and 350.0g (2.648mol) of 2,3-dihydro-1H-inden-1-one (VI), stir, and then add 1100ml of 20% sodium hydroxide solution; after the dropwise addition, heat and reflux for 30 minutes (TLC monitors the reaction process, developer: n-hexane: ethyl acetate = 4: 1); cool to 50°C, then add 1800ml of ethanol, 45 % sodium hydroxide solution 1100ml; control the internal temperature of the reaction system at 50-55°C, add 500g of aluminum-nickel alloy (nickel mass content 40-50%, 60-80 mesh) in batches within 2-3 hours; after adding, The reaction was continued at 50-55° C. for about 8 hours (TLC monitored the progress of the reaction, developing solvent: n-hexane: ethyl acetate = 4:1). Filter, extract the filtrate with dichloromethane 2L×3, combine the dichloromethane layers, wash with water until neutral; c...
Embodiment 3
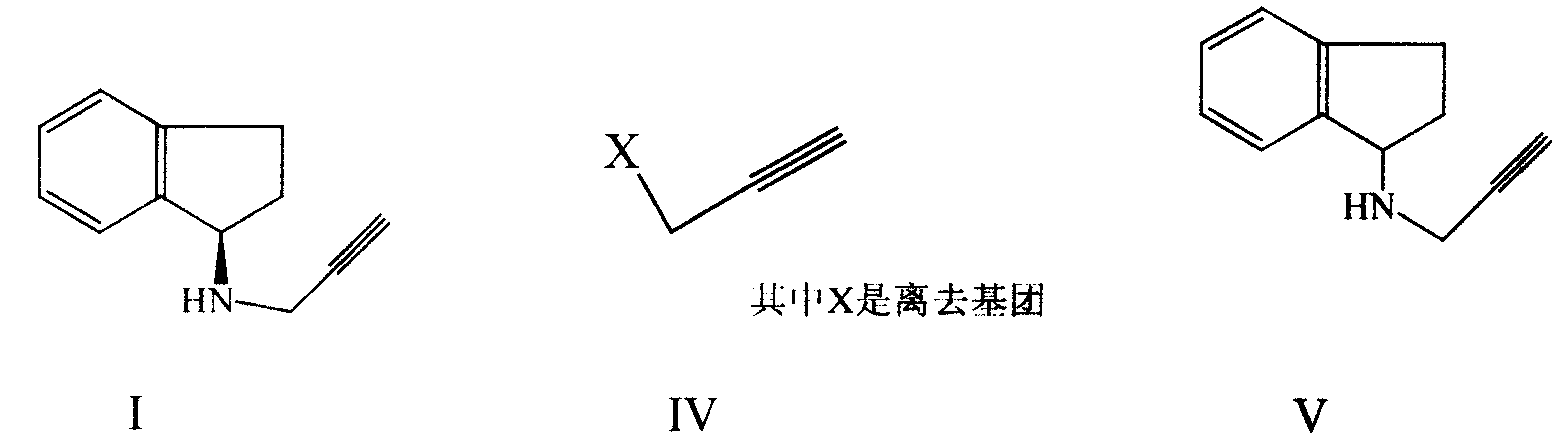
[0093] Preparation of 2,3-dihydro-N-2-propynyl-1H-inden-1-amine (V)
[0094] In a 5L three-necked flask, add 2,3-dihydro-1H-inden-1-amine (II) hydrochloride 340.0g (2.004mol), water 600ml, 20% sodium hydroxide solution 700ml and toluene 800ml; Add 393.0 g (2.003 mol) of propargyl benzenesulfonate dropwise at ~25°C for about 70 minutes; Process, developer: n-hexane:ethyl acetate=4:1). After the reaction, cool to room temperature, separate the toluene layer, wash with 10% sodium hydroxide solution; add 550ml of water to the toluene layer, adjust the pH of the water layer to about 3 with 30% sulfuric acid solution; separate the water layer after extracting the toluene layer , adjust the pH with 10% sodium hydroxide solution to be about 8, extract the aqueous layer with 400ml×3 toluene; combine the toluene layers, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the title compound (V) 271.3g, brown oil , HPLC: 93.08%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com