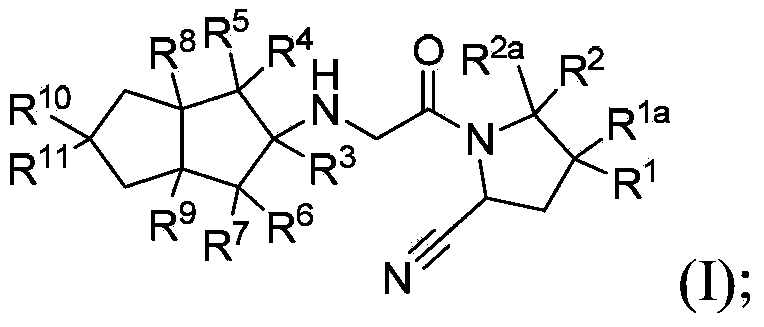
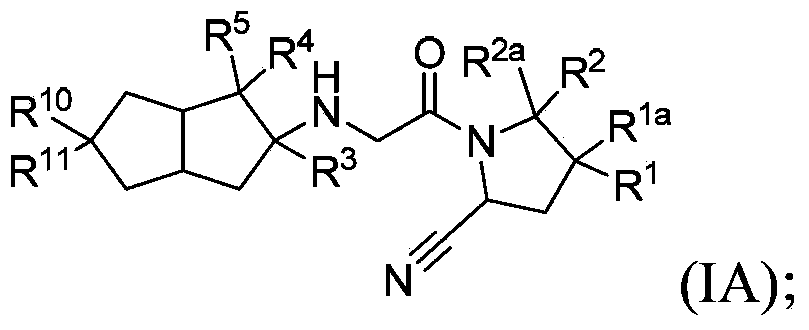
Hexahydropentaleno derivatives, preparation methods and applications in medicine thereof
一种化合物、杂环基的技术,应用在六氢并环戊二烯衍生物领域,能够解决失活等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
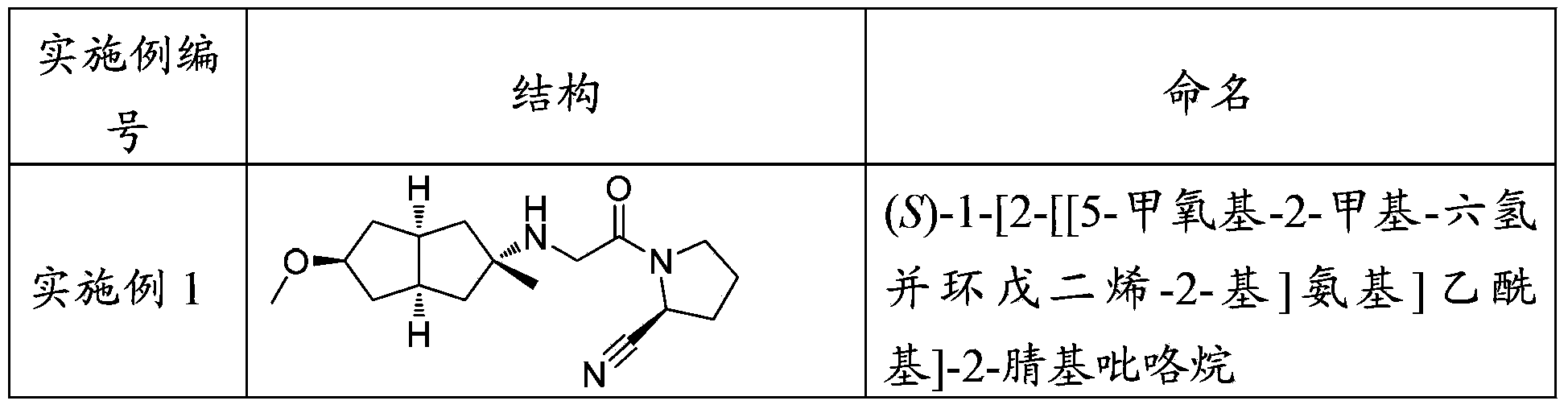
Embodiment 1
[0399] (S)-1-[2-[[5-Methoxy-2-methyl-hexahydropentalen-2-yl]amino]acetyl]-2-cyanopyrrolidine
[0400]
[0401]
[0402] Step 1) 5',5'-Dimethylspiro[hexahydropentadiene-5,2'-1,3-dioxane]-2-one
[0403] Hexahydropentacyclopentadiene-2,5-dione 1a (2.00g, 14.47mmol, Chengdu Aiertai Biotechnology Co., Ltd.), neopentyl glycol (1.51g, 14.49mmol, Aladdin), p-toluene A mixture of sulfonic acid (25 mg, 0.13 mmol, Guangzhou Huada Chemical Reagent Co., Ltd.), toluene (150 mL) was heated under reflux for 5 hours, cooled to room temperature, and then concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether:ethyl acetate (V / V)=4:1) to obtain the title compound 1b (1.96 g, 60.3%) as a yellow solid.
[0404] MS m / z(ESI):225.0(M+1);
[0405] 1 H NMR (400MHz, CDCl 3 )δ:3.50(s,2H),3.45(s,2H),2.80(m,2H),2.44(m,2H),2.27(m,2H),2.15(m,2H),1.80(m,2H ),0.96(s,6H).
[0406] Step 2) 5,5-Dimethylspiro[1,3-dioxane-2,5′-hexahydropentadiene]-2′-ol
[0...
Embodiment 2
[0441] (S)-1-[2-[5-methoxy-2-methyl-hexahydropentalen-2-yl]amino]acetyl]-4-fluoro-2-cyanopyrrolidine
[0442]
[0443] N,N-dimethylformamide of the product 5-amino-5-methyl-hexahydropentalen-2-ol 2a (1.50g, 8.87mmol) prepared in step 7) of Example 1 Potassium iodide (1.62g, 9.76mmol), potassium carbonate (1.35g, 9.76mmol), (S)-1-(2-chloroacetyl )-4-fluoro-2-cyano-pyrrolidine 2b (1.86g, 9.76mmol, Chengdu Aiertai Biotechnology Co., Ltd.). The reaction was reacted at room temperature for 14 hours, then concentrated in vacuo. The residue was purified by silica gel column chromatography (dichloromethane:methanol (V / V)=20:1) to obtain the title compound 2 (0.87 g, 30.3%) as a yellow solid.
[0444] MS m / z(ESI):324.2(M+1);
[0445] 1 H NMR (400MHz, CDCl 3 )δ:5.22-5.49(m,1H),4.94(d,1H),3.87(m,2H),3.72(m,1H),3.33(s,2H),3.29(s,3H),2.71(m ,2H),2.63(m,1H),2.24(m,1H),1.90(m,4H),1.73(m,2H),1.59(m,2H),1.18(s,3H).
Embodiment 3
[0447] (S)-1-[2-[[5-Hydroxy-2-methyl-hexahydropentalen-2-yl]amino]acetyl]-4-fluoro-2-cyanopyrrolidine
[0448]
[0449]
[0450] Step 1) 5-Hydroxy-hexahydropentalen-2-one
[0451] To the stirred hexahydropentadiene-2,5-dione 3a (100g, 0.725mol) ethyl acetate solution (88mL) was added lithium tri-tert-butoxy aluminum hydride (184g, 0.725mol, Beijing Coupling Technology Co., Ltd. company). The resulting mixture was stirred at room temperature for 15 hours. Water (100 mL) was added dropwise, and the mixture was filtered and washed with ethyl acetate (200 mL×3). The organic layer was separated, dried over anhydrous sodium sulfate, concentrated in vacuo, and the resulting residue was purified by column chromatography (petroleum ether: ethyl acetate (V / V) = 6:1) to obtain the title compound 3b as a pale yellow oil (42g, 41.3%).
[0452] GC-MS m / z(EI):140.1(M);
[0453] 1 H NMR (400MHz, CDCl 3 )δ: 4.38(m,1H), 2.80(m,2H), 2.52(m,2H), 2.28(m,2H), 2.15(m,2H), 1.59(m,2H).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com