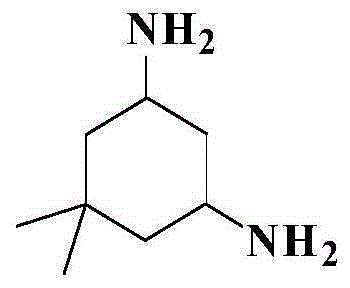
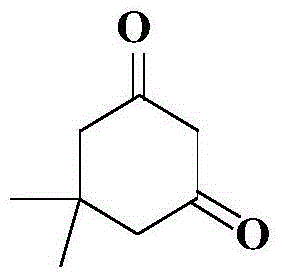
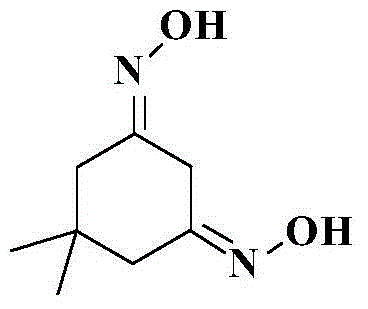
Preparation method of 5,5-dimethyl-1,3-cyclohexamethylenediamine
A technology of cyclohexanediamine and dimethyl, which is applied in the field of chemical synthesis, can solve the problems of being unsuitable for industrial production scale-up, low product yield and purity, and cumbersome post-treatment, and achieves benefits for scale-up of industrial production, low cost, and method novel effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1, the preparation of 5,5-dimethyl-1,3-cyclohexanedione
[0028] Add 64.4g (0.937mol) sodium ethylate, 138.4g (0.856mol) diethyl malonate, 80.0g (0.815mol) 4-methyl-3-penten-2-one, 257.8g absolute ethanol to In a 2L three-neck flask, stir and mix well, heat to reflux, and stir at reflux for 1 to 2 hours. TLC detects that 4-methyl-3-penten-2-one has reacted completely and there is no residue. Add a mixed solution of 123.0g (1.972mol) KOH and 566g tap water, and stir under reflux for 6-10h. Ethanol in the system was evaporated, and 300g4mol / L HCl aq was added dropwise to the remaining aqueous solution system to adjust the pH value of the system to 1-2, a yellow solid gradually precipitated in the system, cooled to room temperature, and suction filtered, the filter cake was a yellow solid, The filter cake was rinsed with tap water (100 g x 5). After the filter cake was dried, 90.0 g of light yellow granular solid was obtained, the yield was 78.8% (calculated as 4-me...
Embodiment 2
[0034] Step 1, the preparation of 5,5-dimethyl-1,3-cyclohexanedione
[0035] Add 50.6g (0.937mol) sodium methoxide, 138.4g (0.856mol) diethyl malonate, 80.0g (0.815mol) 4-methyl-3-penten-2-one, and 203g methanol to a 2L three-necked flask , stir and mix, heat to reflux, and stir at reflux for 1 to 2 hours. TLC detects that 4-methyl-3-penten-2-one has reacted completely and there is no residue. Add a mixed solution made of 78.9g (1.972mol) NaOH and 363g tap water, and stir under reflux for 6-10h. The methanol in the system was evaporated, and 300g4mol / L HCl aq was added dropwise to the remaining aqueous solution to adjust the pH value of the system to 1-2. A yellow solid gradually precipitated in the system, cooled to room temperature, and suction filtered. The filter cake was a yellow solid. Tap water (100g×5) rinses the filter cake. After the filter cake was dried, 88.5 g of light yellow granular solid was obtained, with a yield of 77.5% (calculated as 4-methyl-3-penten-2-o...
Embodiment 3
[0041] Step 1, the preparation of 5,5-dimethyl-1,3-cyclohexanedione
[0042] Add 76.9 g (0.937 mol) sodium isopropoxide, 138.4 g (0.856 mol) diethyl malonate, 80.0 g (0.815 mol) 4-methyl-3-penten-2-one, 269.8 g tetrahydrofuran to 2L In the three-neck flask, stir and mix well, heat to reflux, and stir at reflux for 1-2 hours. TLC detects that 4-methyl-3-penten-2-one has reacted completely and there is no residue. Add a mixed solution of 123.0g (1.972mol) KOH and 566g tap water, and stir under reflux for 6-10h. The tetrahydrofuran in the system was evaporated, and 300g4mol / L HCl aq was added dropwise to the remaining aqueous solution system to adjust the pH value of the system to 1-2. A yellow solid gradually precipitated in the system, cooled to room temperature, and suction filtered. The filter cake was a yellow solid. The filter cake was rinsed with tap water (100 g x 5). After the filter cake was dried, 87.2 g of a yellowish granular solid was obtained, with a yield of 76....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com