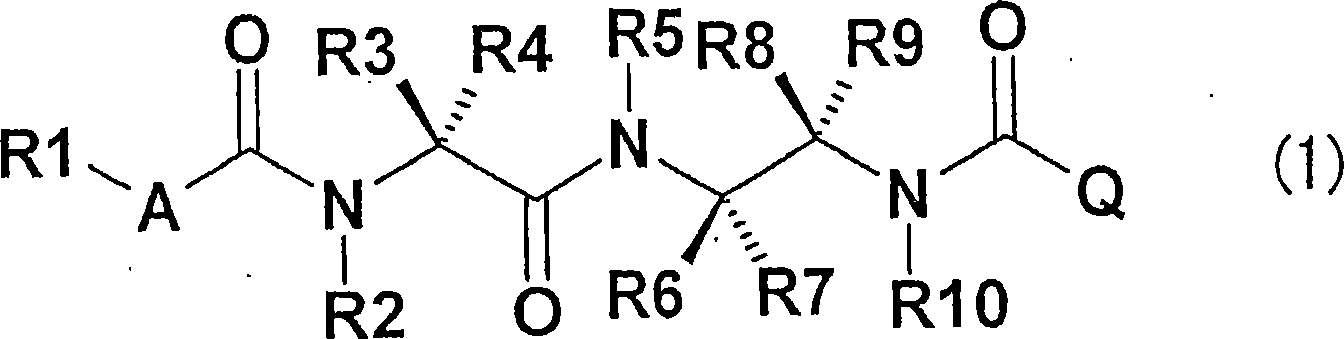
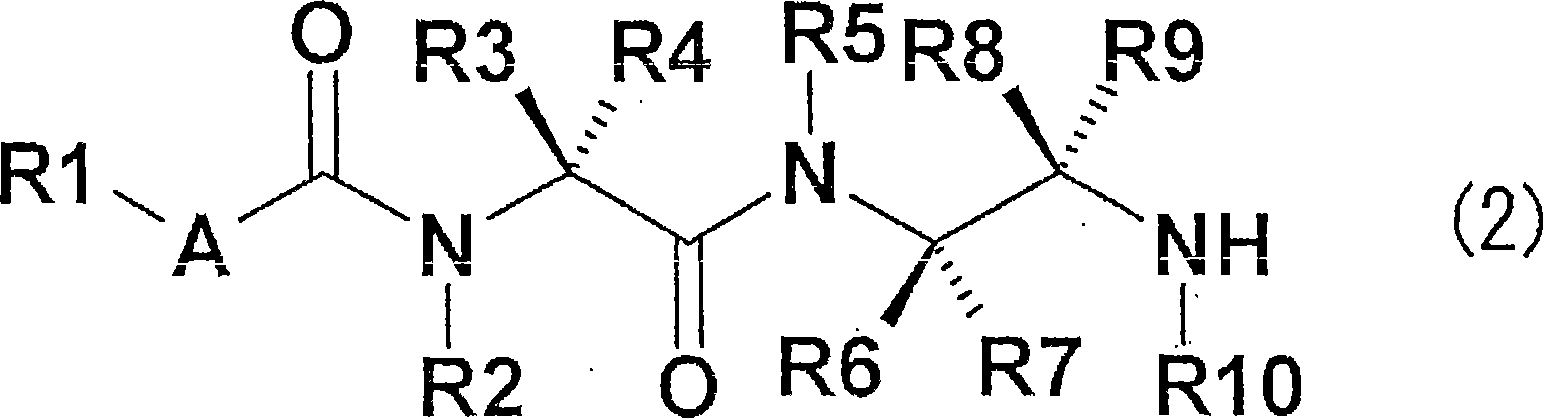
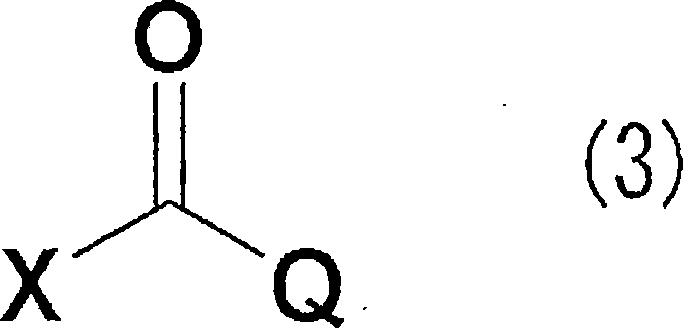
Diamine derivative, process for producing the same and fungicide containing the derivative as active ingredient
A technology of diamine derivatives and compounds, which is applied in the preparation of carbamic acid derivatives, botany equipment and methods, and the preparation of organic compounds, etc., which can solve the problem that the harmfulness of useful crops with bactericidal activity is not very ideal, drug-resistant bacteria, Does not show activity and other problems, and achieves high control effect, broad spectrum of sterilization, and excellent rain resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] N-[1,1-Dimethyl-2-[(4-methylbenzoyl)amino]ethyl][(1-methylethoxycarbonyl)amino]acetamide (Compound No. 14)
[0168] Add 1,1′-carbonyldi-1H-imidazole (0.25 g, 1.54mmol), stirred for 1 hour. N-(2-amino-2-methylpropyl)4-methylbenzamide (0.25 g, 1.21 mmol) was added to the reaction liquid at room temperature, and stirred at room temperature for 12 hours. The reaction solution was washed successively with 5% aqueous citric acid solution, saturated saline, saturated aqueous sodium bicarbonate, and saturated saline, and dried over anhydrous magnesium sulfate. After the inorganic salt was filtered, it was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (n-hexane:ethyl acetate=1:1) to obtain 0.29 g of the target product as a white solid (yield 69%).
Embodiment 2
[0170] N-[2-Methyl-1-(S)-[[(4-methylbenzoyl)amino]methyl]propyl]2-(S)-[(1,1-Dimethylethoxy Cylcarbonyl)amino]pentanamide (Compound No. 40)
[0171] At room temperature, 1,1'- Carbonyldi-1H-imidazole (1.90 g, 11.72 mmol), stirred for 1 hour. Add N-[2-(S)-amino-3-methylbutyl]4-methylbenzamide hydrochloride (2.00g, 7.79mmol), triethylamine (0.87g, 8.60 mmol), stirred at room temperature for 12 hours. The reaction solution was washed successively with 5% aqueous citric acid solution, saturated saline, saturated aqueous sodium bicarbonate, and saturated saline, and dried over anhydrous magnesium sulfate. After the inorganic salt was filtered, it was concentrated under reduced pressure, and the obtained crude product was washed with diisopropyl ether to obtain 2.86 g of the target product as a white solid (yield 88%).
Embodiment 3
[0173] N-[2-methyl-1-(S)-[[(4-methylbenzoyl)amino]methyl]propyl]2-
[0174] (S)-[(1,1-Dimethylethoxycarbonyl)amino]-2-phenylacetamide (Compound No. 60)
[0175] At room temperature, add 1 , 1'-carbonyldi-1H-imidazole (1.90 g, 11.72 mmol), stirred for 1 hour. Add N-[2-(S)-amino-3-methylbutyl]4-methylbenzamide hydrochloride (2.00g, 7.79mmol), triethylamine (0.87g, 8.60 mmol), stirred at room temperature for 12 hours. The reaction solution was washed successively with 5% aqueous citric acid solution, saturated saline, saturated aqueous sodium bicarbonate, and saturated saline, and dried over anhydrous magnesium sulfate. After the inorganic salt was filtered, it was concentrated under reduced pressure, and the obtained crude product was washed with diisopropyl ether to obtain 3.06 g of the target product as a white solid (yield 87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com