2,5-dimethyl-1,4-phenylenediamine preparation method
A dimethylaniline and dimethyl technology, which is applied in the field of preparation of 2,5-dimethyl-1,4-phenylenediamine, can solve the problems of low product yield, high cost of reducing agent hydrosulfite, and reaction time. long-term issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
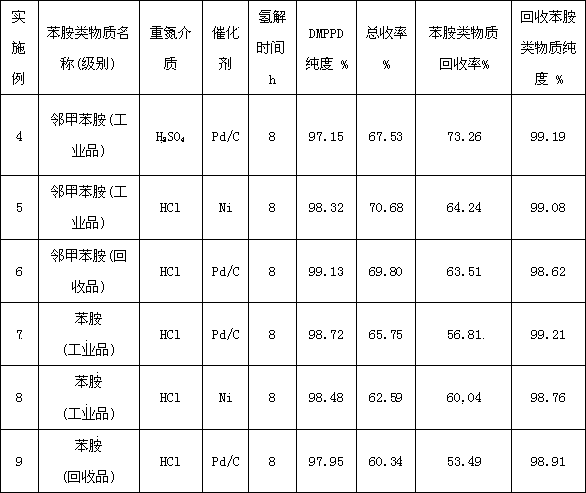
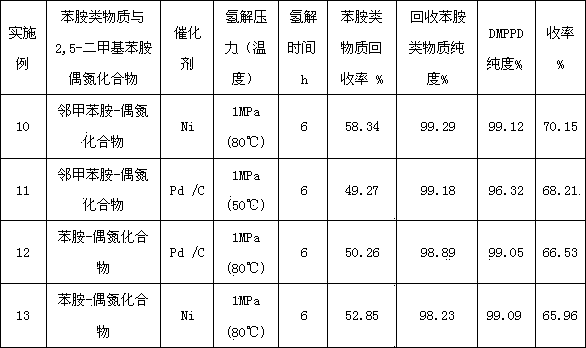
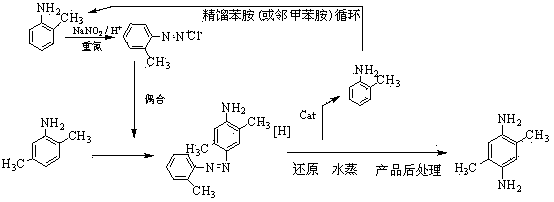
Image
Examples
Embodiment 1
[0049] Diazo coupling reduction hydrogenolysis direct method (2,5-dimethylaniline-o-toluidine method)
[0050] A preparation method of 2,5-dimethyl-1,4-phenylenediamine, comprising the steps of:
[0051] (1) Diazotization reaction: Add 0.4mol of o-toluidine to 150g of water and 100g of 30% hydrochloric acid in an aqueous solution of hydrochloric acid with a mass concentration of 12%, control the temperature at 0-3°C, and add 0.408mol of nitrous acid dropwise within 15 minutes Sodium, stirred and reacted for 15min to obtain o-toluidine diazonium salt solution;
[0052] (2) Coupling reaction: Add o-toluidine diazonium salt solution dropwise at 8-10°C to a solution containing 48.5g 2,5-dimethylaniline (0.40mol), 22.1g NaCO 3 and 460g of water in a stirred 1 L four-necked reaction flask, the pH is controlled at 8~8.5, after the dropwise addition is completed within 3 hours, continue to stir and react at 8~10°C for 1.5 hours to obtain the azo compound 4-amino-2 , the reaction so...
Embodiment 2
[0058] Diazo coupling reduction hydrogenolysis step-by-step method (2,5-dimethylaniline-aniline method)
[0059] A preparation method of 2,5-dimethyl-1,4-phenylenediamine, comprising the steps of:
[0060] (1) Diazotization reaction: Add 0.4 mol of aniline to 250 g of aqueous hydrochloric acid solution with a mass concentration of 12%, control the temperature at 0-5°C, and add 0.408 mol of sodium nitrite dropwise within 15 minutes, stir and react for 15 minutes to obtain aniline Diazonium salt solution;
[0061] (2) Coupling reaction: Aniline diazonium salt solution was added dropwise at 6-8°C with 48.5g 2,5-dimethylaniline, 22.1g NaCO 3 and 460g of water in a stirred 1 L four-necked reaction flask, the pH is controlled at 8.0-8.5, after the dropwise addition is completed within 3.5 hours, continue to stir and react at 8-10°C for 2.5 hours to obtain 4-amino-2,5- Dimethyl azobenzene solution;
[0062] (3) Hydrogenolysis reaction: filter the reaction suspension of the prepar...
Embodiment 3
[0067] Diazo coupling reduction hydrogenolysis step-by-step method (2,5-dimethylaniline-o-toluidine method)
[0068] Carry out the operation of diazo coupling reaction according to the same feeding as in Example 1, first filter the reaction suspension of the prepared azo compound 4-amino-2,5-dimethyl-2'-methylazobenzene, and use 360ml of water was beaten, washed and filtered to obtain 109.3g of azo compound wet product (82.28g after drying, 0.365mol, purity 96.86%, yield 91.3%); then 109.3g of 4-amino-2,5-dimethyl - The wet product of 2'-methylazobenzene was put into the autoclave, followed by adding 620mL of water and 2.0g of 5%Pd / C catalyst with a solid content of 50% to adjust the pH to 8-8.5, then sealed back into the autoclave, and replaced with nitrogen After replacing the nitrogen with hydrogen in the air, stir and heat up to 55°C and start to introduce hydrogen under the liquid, control the pressure at 1.0MPa, rotate at a speed of 600r / min, and carry out hydrogenation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com