Hyper-branched chitosan or hyper-branched glycol chitosan and preparation method thereof
A technology for ethylene glycol chitosan and chitosan transformation is applied in the field of preparing chitosan, which can solve the problems of no reactivity, and chitosan has not yet been found, and achieves few reaction steps, mild reaction conditions and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1. The preparation of hyperbranched ethylene glycol chitosan
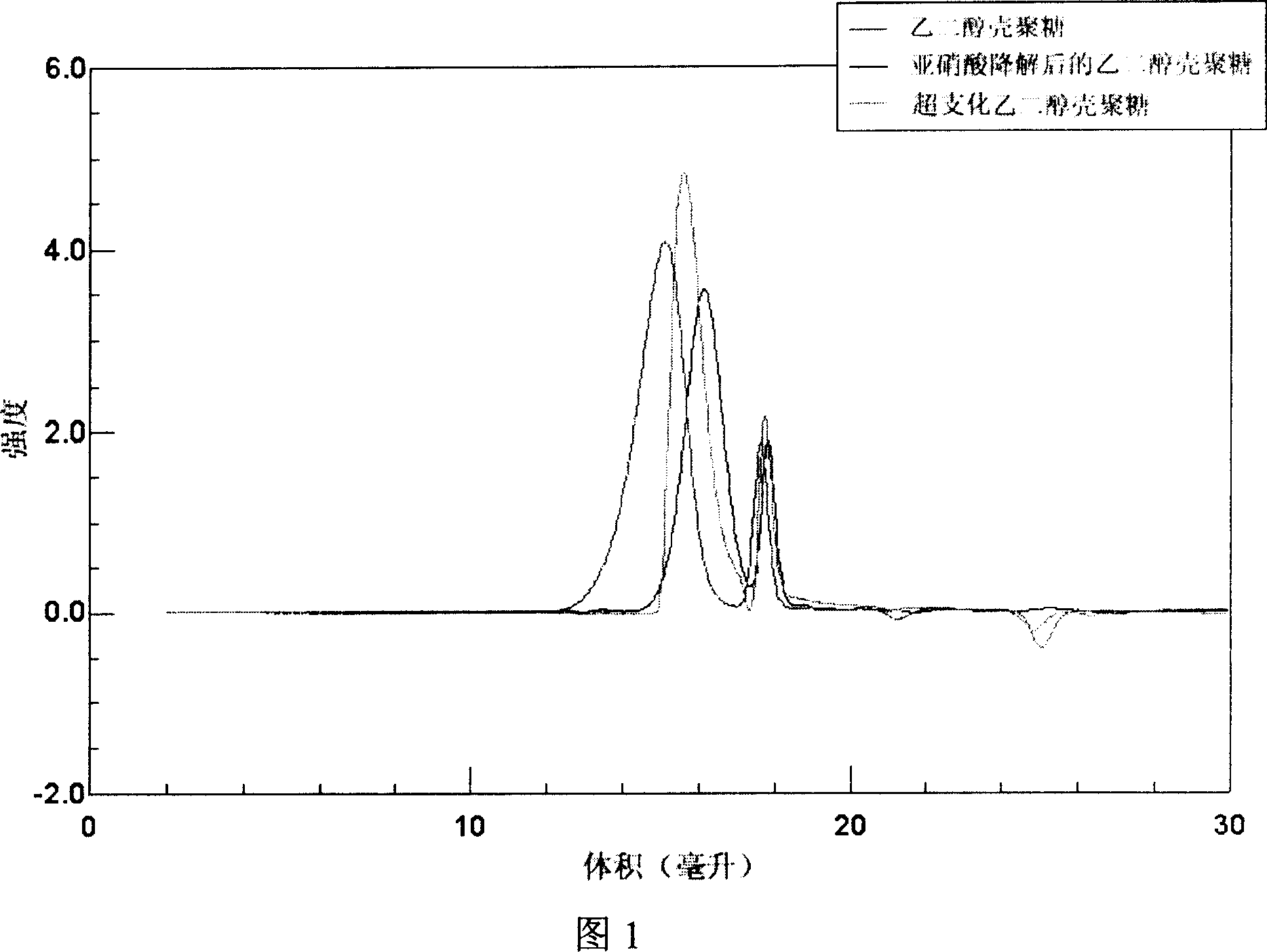
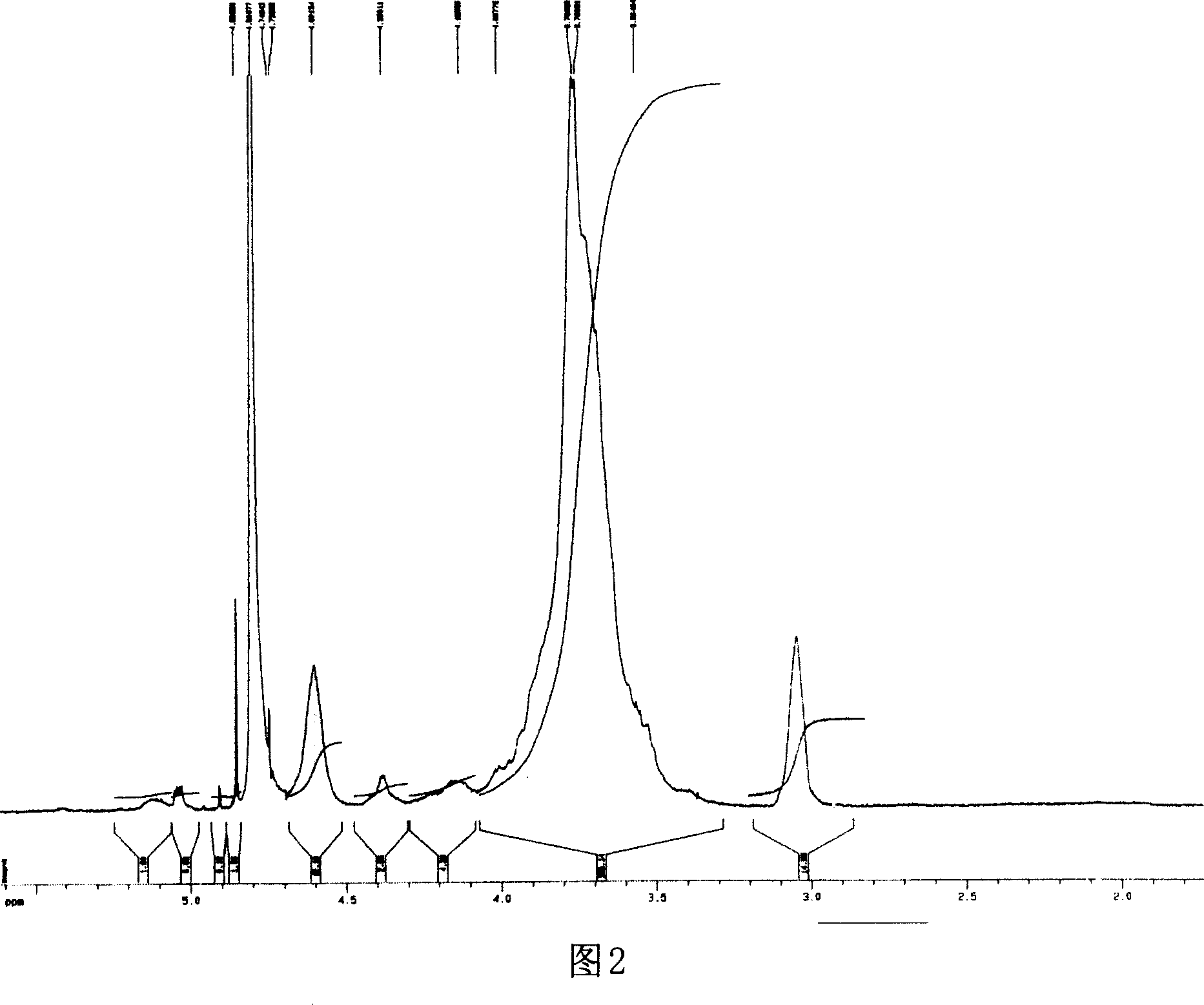
[0033] Ethylene glycol chitosan (Mw = 250 kDa, GPC / light scattering method) was dissolved in 0.2M acetic acid. Add NaNO equivalent to 5% of amino moles under high-speed stirring at 0~4°C 2 Aqueous solution, continue to react at 0 ~ 4 ℃ for 15 hours, then use 3 times the volume of acetone to precipitate. The precipitate was washed several times with acetone and methanol and then dried in vacuum. Obtain the ethylene glycol chitosan that low molecular weight has aldehyde end group after drying, its weight-average molecular weight is 5500 (GPC / light scattering method), and its nuclear magnetic resonance spectrum is shown in Fig. 2, wherein the peak at chemical shift 5.05 place is Aldehyde H. The low molecular weight ethylene glycol chitosan (Mw=5500) degraded by the above nitrous acid was dissolved in 0.1M acetic acid aqueous solution (5mg / mL), stirred at 25°C for 1 hour, then added dropwise with 0...
Embodiment 2
[0034] Embodiment 2. The preparation of hyperbranched chitosan
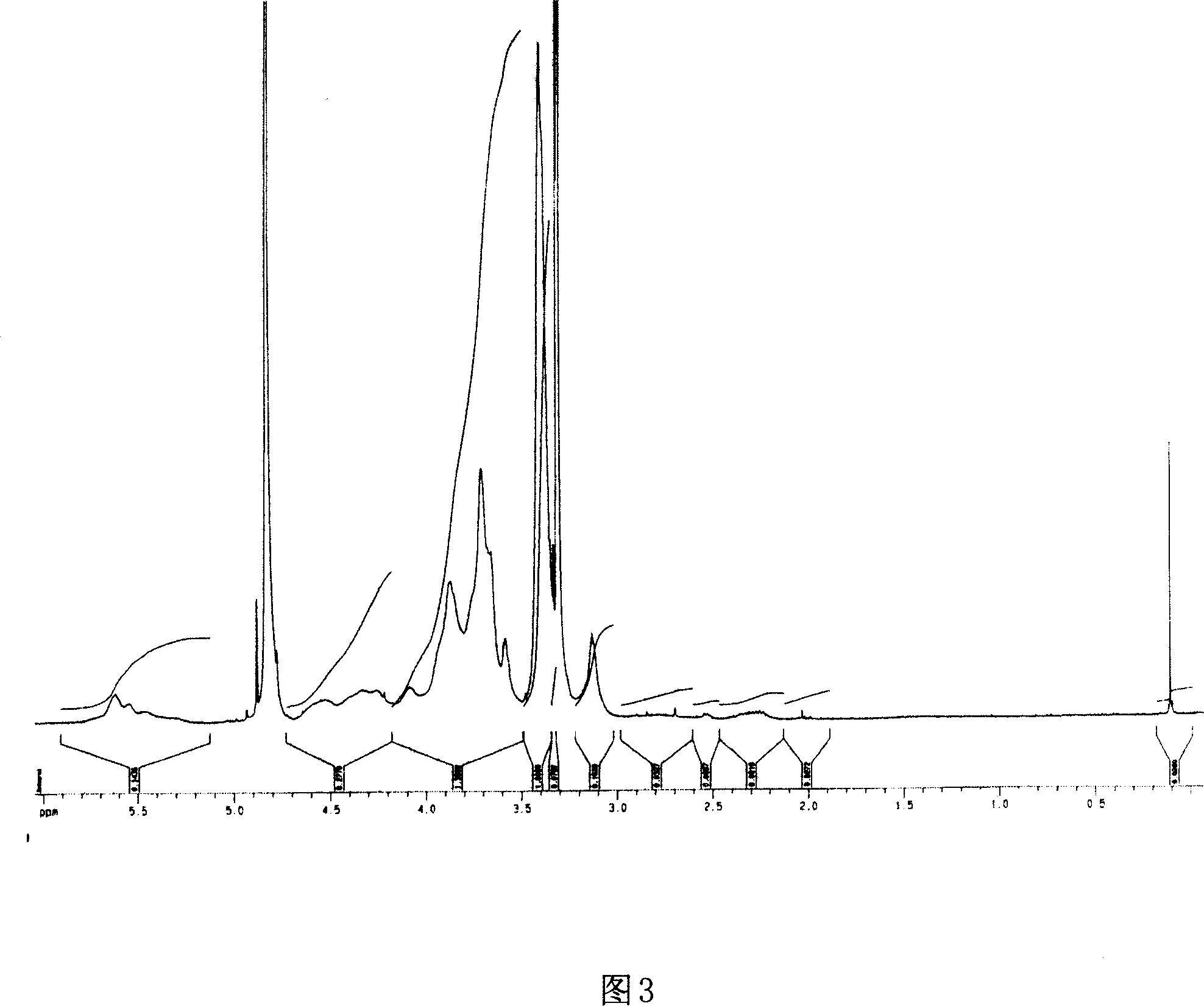
[0035] 50g of chitosan was dissolved in 1000mL of 5% acetic acid aqueous solution and cooled to 0-4°C. 5gNaNO 2 Dissolve in 50mL of water, add the above chitosan solution dropwise, react at 0-4°C for 9 hours, add concentrated NaOH aqueous solution until all the precipitates are precipitated, filter, wash with methanol and water for several times, and vacuum-dry the obtained product with aldehyde groups. based low molecular weight chitosan. The product (Mw=2300, Mw / Mn=1.5, GPC / light scattering method) of low molecular weight chitosan after methanol precipitation and fractionation was dissolved in 0.1M acetic acid (2mg / mL), stirred at 10°C for 1 hour, and then added dropwise with 0.1 M NaOH in water to pH 5.5. After continuing to stir for 2 hours, add NaCNBH equivalent to 1.0 times the mole number of aldehyde groups 3 , reacted at 10°C for 24 hours and then added the same amount of NaCNBH 3 , continue to react...
Embodiment 3
[0036] The preparation of the hyperbranched chitosan of embodiment 3.quaternary amination
[0037] 5g of chitosan was dissolved in 50mL of 0.2M acetic acid aqueous solution and cooled to 0-4°C. 0.15gNaNO 2 Dissolve in 1mL of water, add the above chitosan solution dropwise, react at 0-4°C for 15 hours, add concentrated NaOH until all the precipitates are precipitated, filter, wash with methanol and water several times, and vacuum-dry to obtain the product with aldehyde end groups Low molecular weight chitosan (Mw=3700, GPC / light scattering method). The obtained low-molecular-weight chitosan was dissolved in 0.1M acetic acid (2 mg / mL), stirred at 60°C for 1 hour, and then 0.1M NaOH aqueous solution was added dropwise until the pH was 5.0. After continuing to stir for 2 hours, add NaCNBH equivalent to 2.0 times the mole number of aldehyde groups 3 , reacted at 60°C for 72 hours. During the control pH value is not higher than 6.0. After the reaction was completed, concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com