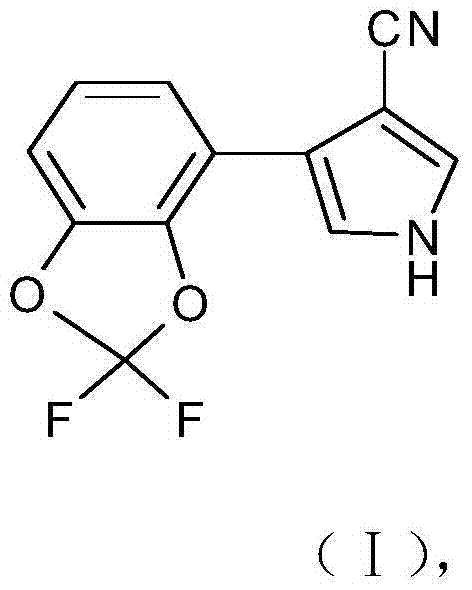
Synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile
A kind of technology of benzodioxol and dioxole, applied in 4-(2,2-difluoro-1,3-benzodioxol-4-yl)pyrrole -In the field of synthesis of 3-nitrile, it can solve the problems of difficult product quality control, large pollution and corrosion, easy hydrolysis and deterioration, etc., and achieve the effects of low production cost, good purity, and easy control of reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
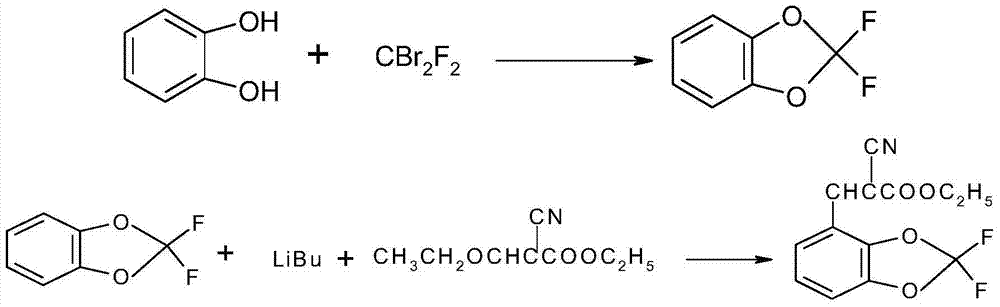
[0056] (1) In a 1000mL autoclave, mix catechol 55.0g (0.5mol), potassium carbonate 85.0g (0.62mol), NMP (N-methylpyrrolidone) 200ml, tetrabutylammonium iodide 0.5g and di Add bromodifluoromethane 157g (0.75mol) into the reaction kettle, heat up to 100°C, react for 8h, cool down to room temperature, pour the reaction solution into 500ml of crushed ice, stir until the ice is completely dissolved, let stand to separate the liquid, and remove the water phase, add 10g of anhydrous sodium sulfate to dry the organic phase to obtain the crude product 2,2-difluorobenzo-1,3-dioxole, and then perform rectification to obtain the refined product 2,2-difluorobenzo- 1,3 dioxole 63g (0.4mol);
[0057] 1 H NMR (CDCl 3 , 500MHz): 7.05 (s, 4H, ArH); IR (KBr, cm -1 ): 3076 (νAr-H), 1642, 1486 (νC=C), 1239 (νC-F), 1040 (νC-0-C), 905 (νO-C-O).
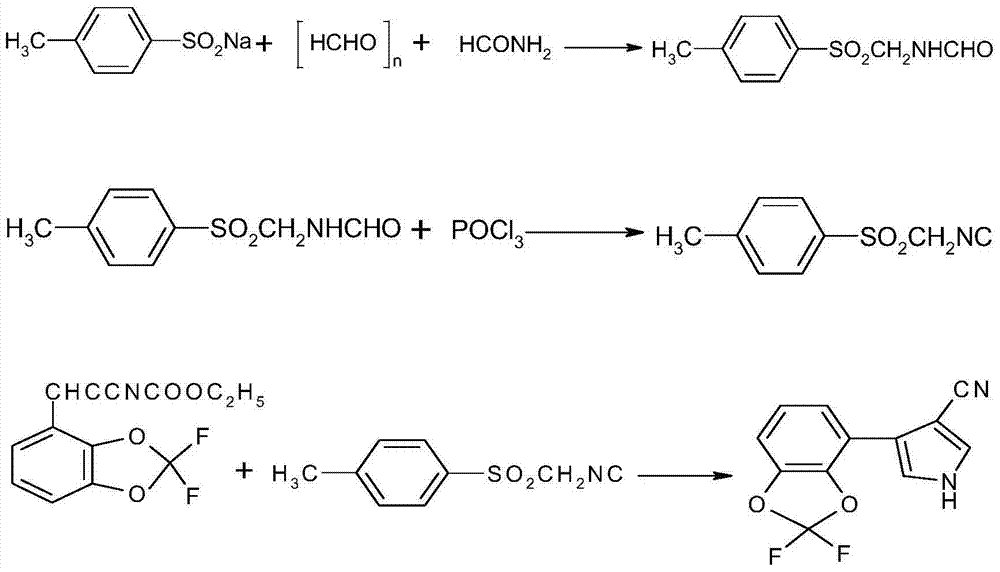
[0058] (2) Cool down to -50°C, under nitrogen protection, add 100ml of n-hexane and 90ml (0.2mol) tert-butyllithium solution into a 500ml four-neck fla...
Embodiment 2
[0067] (1) In a 1000mL autoclave, add 55.0g (0.5mol) of catechol, 85.0g (0.62mol) of potassium carbonate, 200ml of DMF, 0.5g of 18-crown ether and 157g (0.75mol) of dibromodifluoromethane In the reaction kettle, raise the temperature to 80°C, react for 8 hours, cool down to room temperature, pour the reaction solution into 500ml of crushed ice, stir until the ice is completely dissolved, let stand to separate the liquid, remove the water phase, add 10g of anhydrous sodium sulfate to dry the organic phase to obtain the crude product 2,2-difluorobenzo-1,3-dioxole, and then rectify to obtain the refined product 2,2-difluorobenzo-1,3-dioxole 67 g (0.43 mol).
[0068] (2) In a 500ml four-neck flask equipped with a stirring and condenser, cool down to -50°C, under the protection of nitrogen, add 100ml of n-hexane and 90ml (0.2mol) of n-butyllithium solution, and then dropwise add 2,2 -Difluorobenzo-1,3-dioxole 32g (0.2mol), heat preservation reaction for 4h, 47.6g (0.28mol) ethyl e...
Embodiment 3
[0074] (1) In a 1000mL autoclave, mix catechol 55.0g (0.5mol), potassium hydroxide 85.0g (0.62mol), DMSO 200ml, 18-crown ether 0.5g and dibromodifluoromethane 157g (0.75mol) Add it into the reaction kettle, raise the temperature to 80°C, react for 8 hours, cool down to room temperature, pour the reaction liquid into 500ml of crushed ice, stir until the ice is completely dissolved, let stand to separate the liquid, remove the water phase, add 10g of anhydrous sodium sulfate to dry Organic phase, to obtain the crude product 2,2-difluorobenzo-1,3-dioxole, and then carry out rectification to obtain the refined product 2,2-difluorobenzo-1,3-dioxole Alkene 66g (0.41mol).
[0075] (2) In a 500ml four-neck flask equipped with a stirring and condenser, cool down to -50°C, under nitrogen protection, add 100ml tetrahydrofuran and 90ml (0.2mol) tert-butyllithium solution, and then dropwise add 2,2- Difluorobenzo-1,3-dioxole 32g (0.2mol), keep warm for 4h, add 47.6g (0.28mol) of ethyl eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com