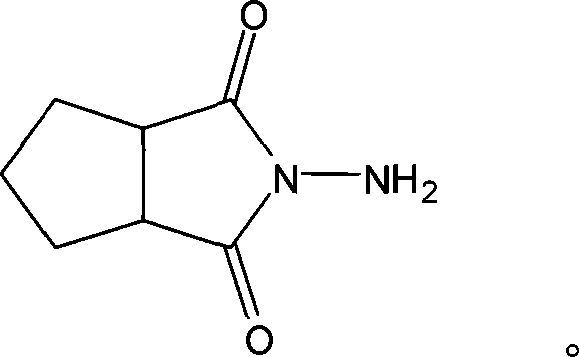
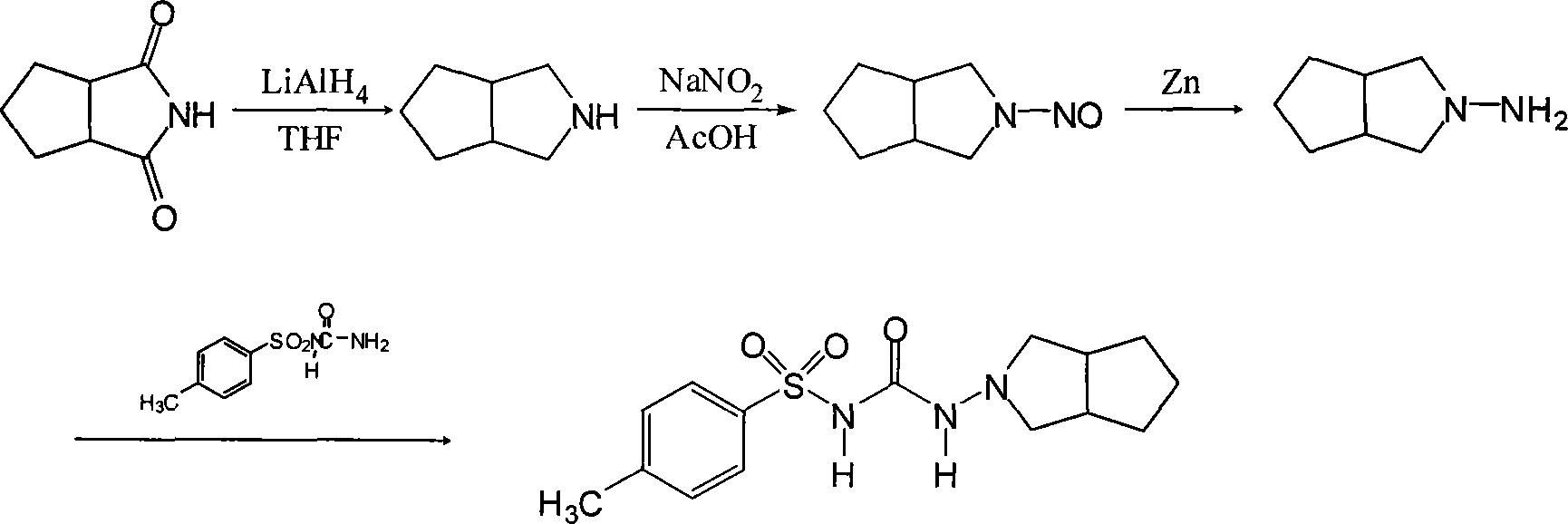
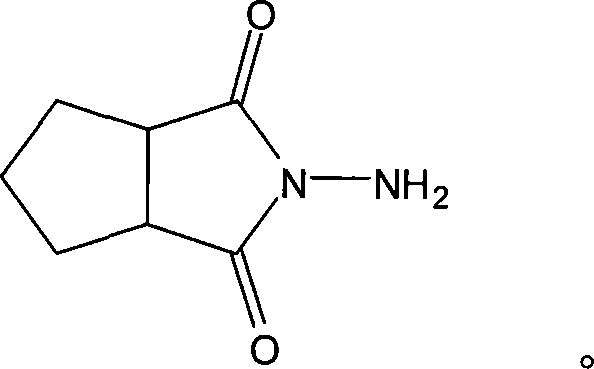
N-amino-1,2-cyclopentanediformylimine and preparation method thereof
A technology of cyclopentane dicarboximide and cyclopentane o, which is applied in the field of organic N-amino-1, can solve the problems of high reaction cost and cumbersome steps, and achieve the effect of shortening the reaction steps and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: A kind of preparation method of N-amino-1,2-cyclopentane dicarboximide, carries out following steps successively:
[0020] In a 250mL three-neck flask equipped with a reflux condenser and mechanical stirring, add 14g of cyclopentane phthalic anhydride and 50mL of methanol, after heating to reflux, slowly add 7mL of 85% hydrazine hydrate aqueous solution dropwise, and continue to reflux after the dropwise addition React for 6 hours.
[0021] After the reaction is over, stop heating, put the reaction solution to a rotary evaporator to distill off the solvent and water under reduced pressure to obtain a viscous liquid, and after cooling to obtain the product N-amino-1,2-cyclopentanedicarboximide, vacuum Dry at 30° C. for 12 hours to obtain 13.5 g of the product with a yield of 87.7%.
Embodiment 2
[0022] Embodiment 2: A kind of preparation method of N-amino-1,2-cyclopentane dicarboximide, carries out following steps successively:
[0023] In a 250mL three-necked flask equipped with a reflux condenser and mechanical stirring, add 14g of cyclopentane phthalic anhydride, 50mL of methanol, and add 7mL of 85% hydrazine hydrate dropwise at room temperature. After the dropwise addition, heat to reflux. The reaction time is 7 Hour.
[0024] After the reaction is over, stop heating, put the reaction solution to a rotary evaporator to distill off the solvent and water under reduced pressure to obtain a viscous liquid, and after cooling to obtain the product N-amino-1,2-cyclopentanedicarboximide, vacuum Dry at 30° C. for 12 hours to obtain 13.2 g of the product with a yield of 85.7%.
Embodiment 3~11
[0025] Embodiments 3-11: By changing the solvent, the concentration of hydrazine hydrate, the dosage of hydrazine hydrate, and the reaction time in the above-mentioned embodiment 1 or 2, corresponding embodiments 3-11 can be obtained. The specific content is shown in Table 1, and the product yield of each embodiment gained is shown in Table 1.
[0026] The concrete data of table 1, embodiment 3~11
[0027] Reality
[0028] 5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com