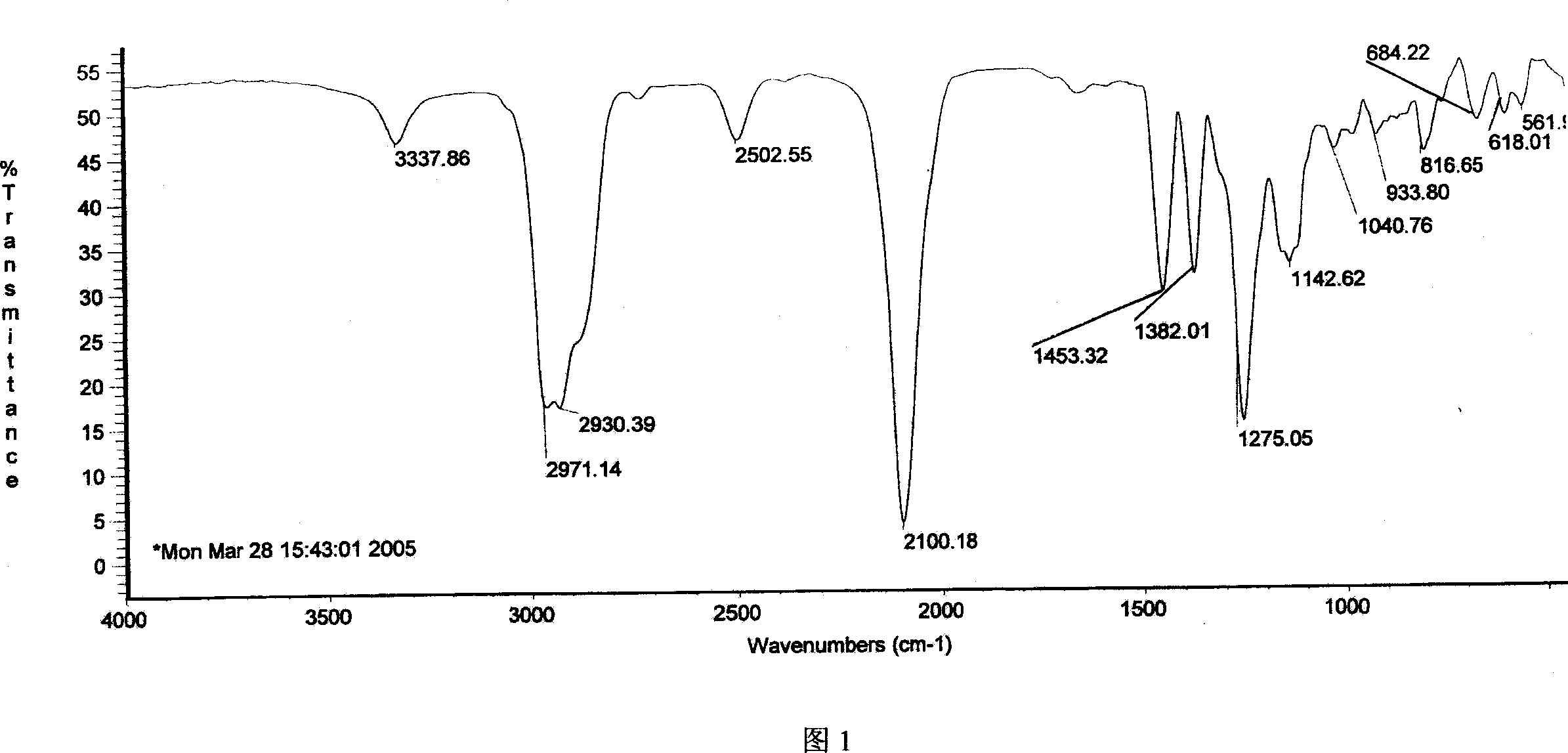
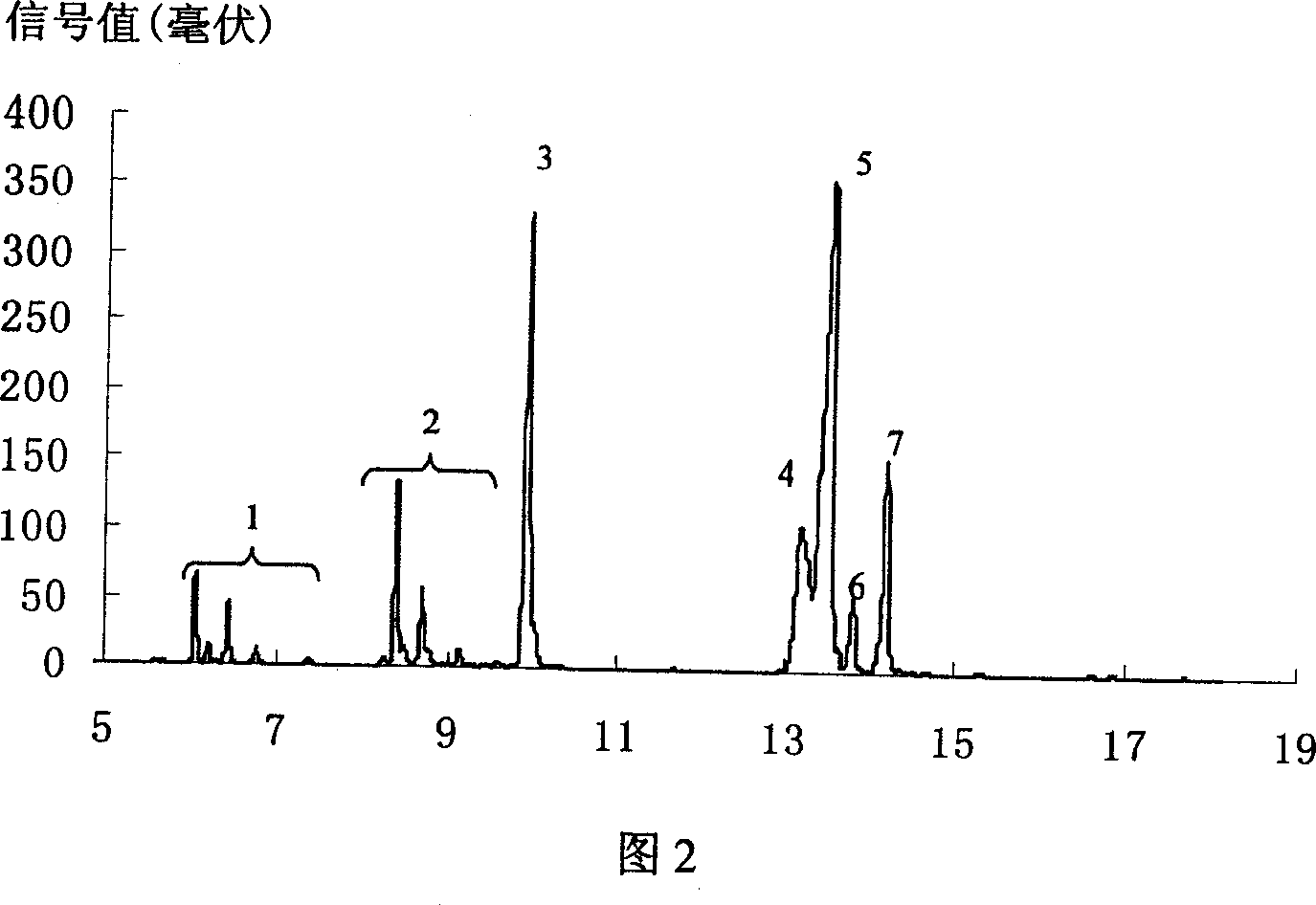
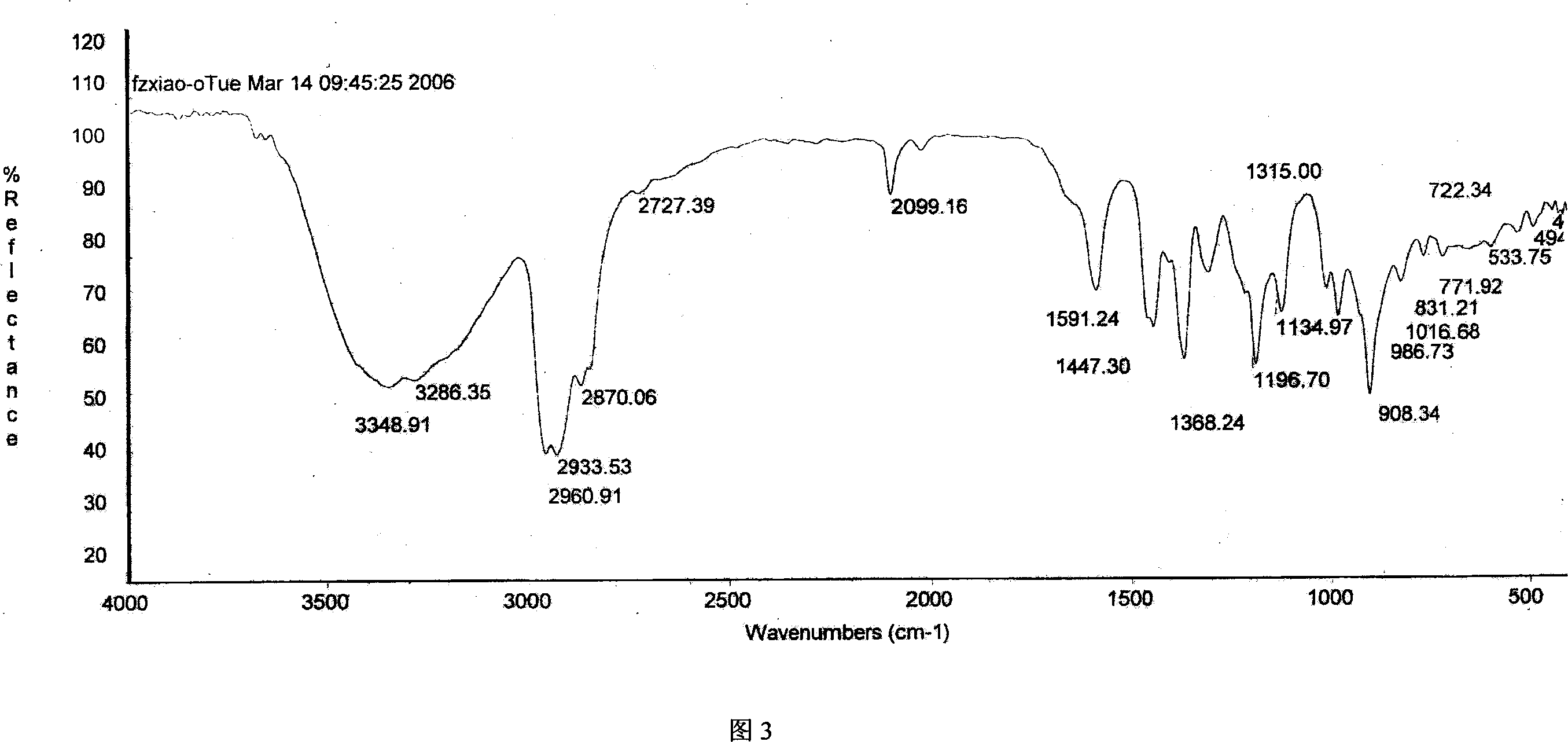
Method for preparing 4-amino-alpha, alpha,4-trimethyl-cyclohexanemethanamine from 1,8- terpinum
A technology for terpene diols and diazidoalkanes, which is applied in the field of preparing alkanediamines, can solve the problems of difficult industrialization, little practical significance, reduced yield and the like, and achieves simple and easy operation, reduced risk, and reduced toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 74.3g of water into a 250mL container that can be completely closed, add 90.8g of concentrated sulfuric acid dropwise under the condition of stirring and ice-water bath to prepare a 55% (wt) sulfuric acid solution, and add NaN below 15°C 3 11.8g, stirred for 5min to dissolve completely, then added 15.7g of 1,8-terpene diol, heated to 45°C and kept at a constant temperature for 6h, separated and dried to obtain diazide 11.6g of the mixture of alkane, after analyzing the diazide group in the mixture The content of alkane is 58.59% (wt).
Embodiment 2
[0034] Add 90.8g of water into a 250mL container that can be completely closed, add 74.3g of concentrated sulfuric acid dropwise under the condition of stirring and ice-water bath to prepare a 45% (wt) sulfuric acid solution, and add NaN below 15°C 3 11.8g, stirred for 5min to dissolve completely, then added 15.7g of 1,8-terpene diol, heated to 50°C and kept at a constant temperature for 8h, separated and dried to obtain diazide 13.2g of the mixture of alkane, after analyzing the diazide group in the mixture The content of alkane is 89.24% (wt).
Embodiment 3
[0036] Add 90.8g of water into a 250mL container that can be completely closed, add 74.3g of concentrated sulfuric acid dropwise under the condition of stirring and ice-water bath to prepare a 45% (wt) sulfuric acid solution, and add NaN below 15°C 3 11.8g, stirred for 5min to dissolve completely, then added 15.7g of 1,8-terpene diol, heated to 35°C and kept at a constant temperature for 40h, separated and dried to obtain diazide 12.4g of the mixture of alkane, after analyzing the diazide group in the mixture The content of alkanes was 50.3% (wt).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com