Synthesis method of p-phenylenediamine
A technology of p-phenylenediamine and synthesis method, applied in the direction of preparation through nitrogen-oxygen/nitrogen-nitrogen bonds, organic chemistry, etc., can solve the problems of cumbersome routes and pollution of p-phenylenediamine, and achieve good industrial implementation prospects, reaction Simple steps and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
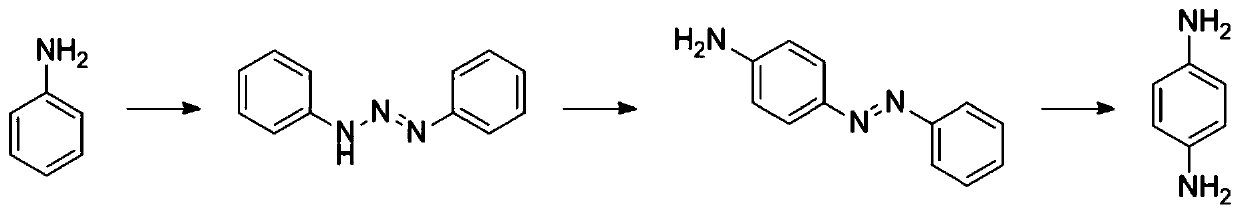
[0019] The synthetic method of p-phenylenediamine that the present invention proposes, its synthetic route is as figure 1 shown, including the following steps:
[0020] (1) Synthesis of 1,3-diphenyltriazene:
[0021] Add aniline, molar concentration in water and be the concentrated hydrochloric acid of 12mol / L, the volume ratio of aniline, concentrated hydrochloric acid and water is: aniline: concentrated hydrochloric acid: water=1:(1~2):(5~10), obtain the first solution; under ice-bath conditions, sodium nitrite solution is added dropwise to the first solution, so that the mol ratio of aniline and sodium nitrite is (1.5~2): 1, an alkaline solution is added in the reactant, and the pH value is adjusted to 4~6, react for 0.5~2 hours, get 1,3-diphenyltriazene precipitate, filter to get 1,3-diphenyltriazene;
[0022] (2) Synthesis of 4-aminoazobenzene:
[0023] Add the 1,3-diphenyltriazene and the concentrated acid of step (1) to the solvent, and the addition ratio is: 1,3-dip...
Embodiment 1
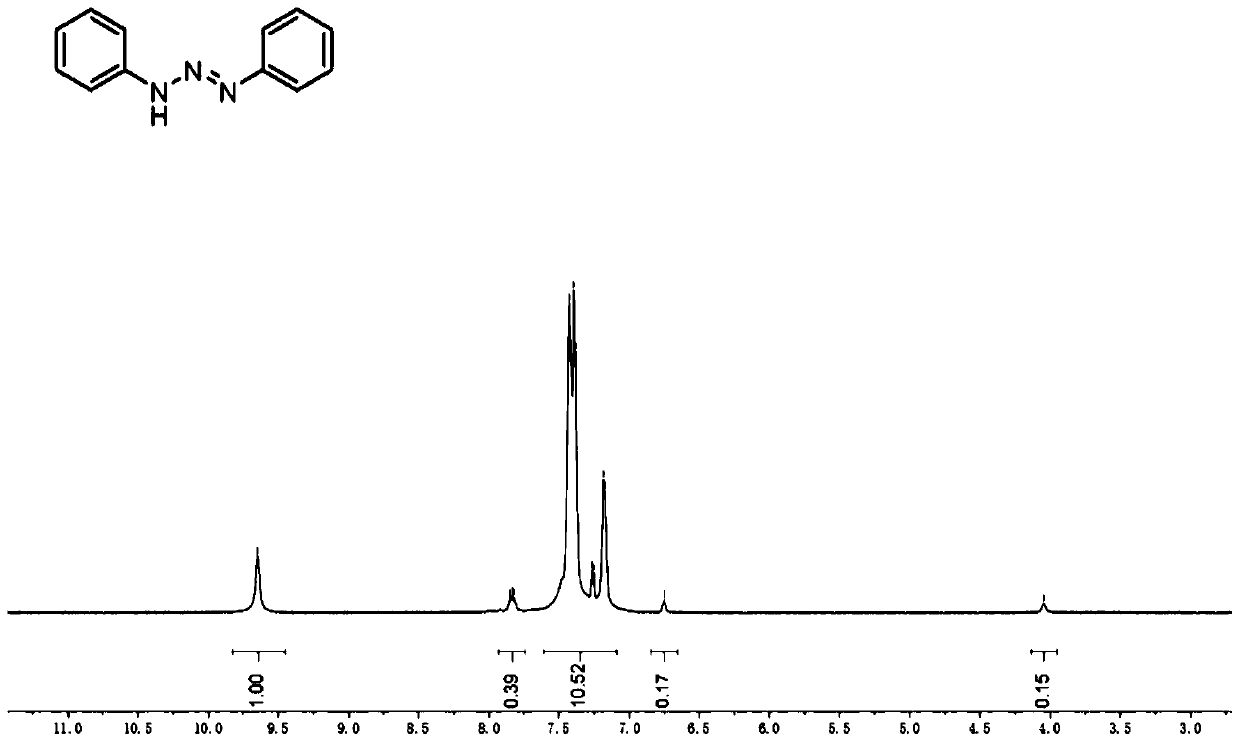
[0033] (1) Synthesis of 1,3-diphenyltriazene: Add aniline (6.5 mL), 12 mol / L concentrated hydrochloric acid (6.5 mL) and water (32.5 mL) into a flask. Stir for a period of time to fully dissolve the aniline in the system, then place the reaction bottle in an ice bath, and add aqueous sodium nitrite solution (molar ratio of sodium nitrite to aniline = 1:1.5) dropwise to the above system. After that, sodium carbonate solution (molar concentration 1mol / L) was added dropwise to adjust the pH to 4, a large amount of light yellow precipitates could be observed, and the reaction was stirred for 0.5 hours. Filtrate with suction and wash several times with ultrapure water to obtain 1,3-diphenyltriazene. figure 2 For the 1,3-diphenyltriazene prepared in embodiment 1 1 HNMR spectrum.
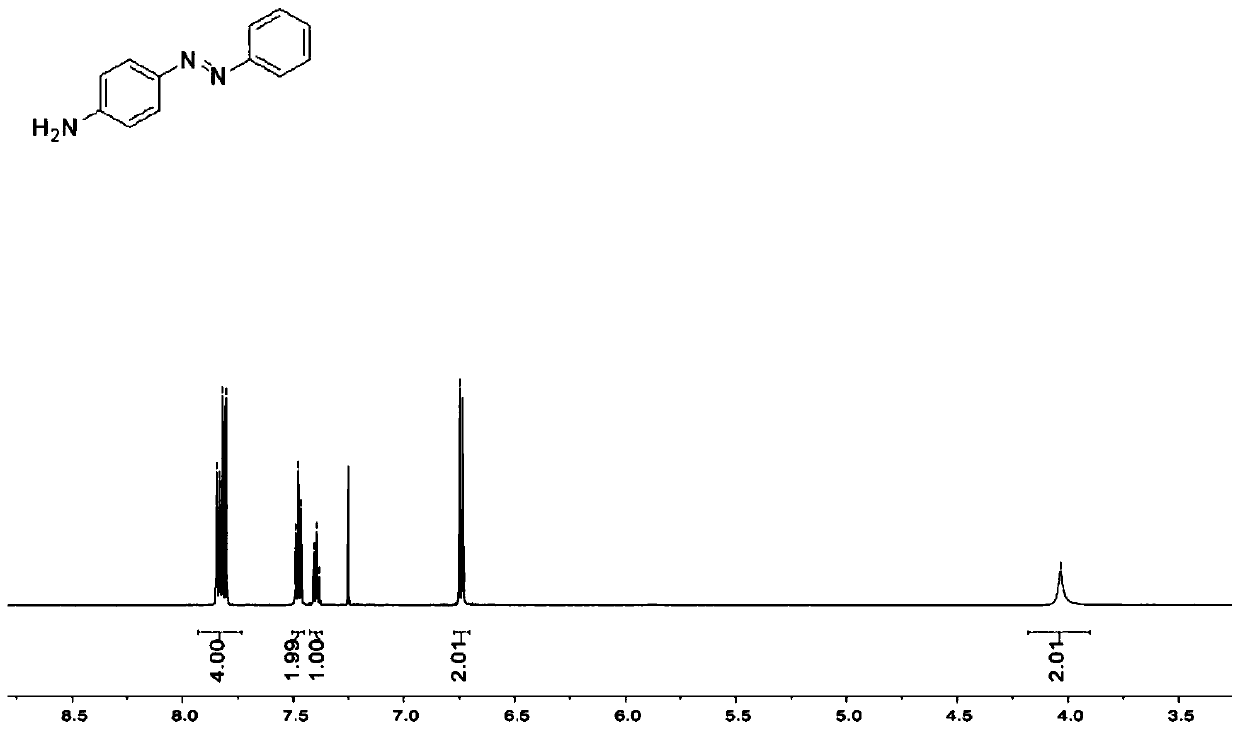
[0034] (2) Synthesis of 4-aminoazobenzene: Methanol (2 mL) and 1,3-diphenyltriazene (1 g) were added to a flask. Then add 12mol / L concentrated hydrochloric acid (0.5mL) to the system under stirring con...
Embodiment 2
[0037](1) Synthesis of 1,3-diphenyltriazene: Add aniline (6.5 mL), 12 mol / L concentrated hydrochloric acid (7.8 mL) and water (39 mL) into a flask. Stir for a period of time to fully dissolve the aniline in the system, then place the reaction bottle in an ice bath, and add aqueous sodium nitrite solution (molar ratio of sodium nitrite to aniline = 1:1.6) dropwise to the above system. Afterwards, sodium acetate solution (molar concentration: 1.2 mol / L) was added dropwise to adjust the pH to 4.4, a large amount of light yellow precipitates could be observed, and the reaction was stirred for 0.8 hours. Filtrate with suction and wash several times with ultrapure water to obtain 1,3-diphenyltriazene.
[0038] (2) Synthesis of 4-aminoazobenzene: Add ethanol (2.6 mL) and 1,3-diphenyltriazene (1 g) into a flask. Then add concentrated nitric acid (0.8mL) to the system under stirring condition, stir well to dissolve the solid, raise the temperature to 48°C, and react for 1.7 hours to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com