Synthetic method of 2,5-diaminotoluene and sulphate thereof
A technology of diaminotoluene and aminoazotoluene, which is applied in 2 fields, can solve the problems of poor economy, utilization of o-toluidine, and difficulty in industrialization implementation, and achieve the effect of reducing unit consumption and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
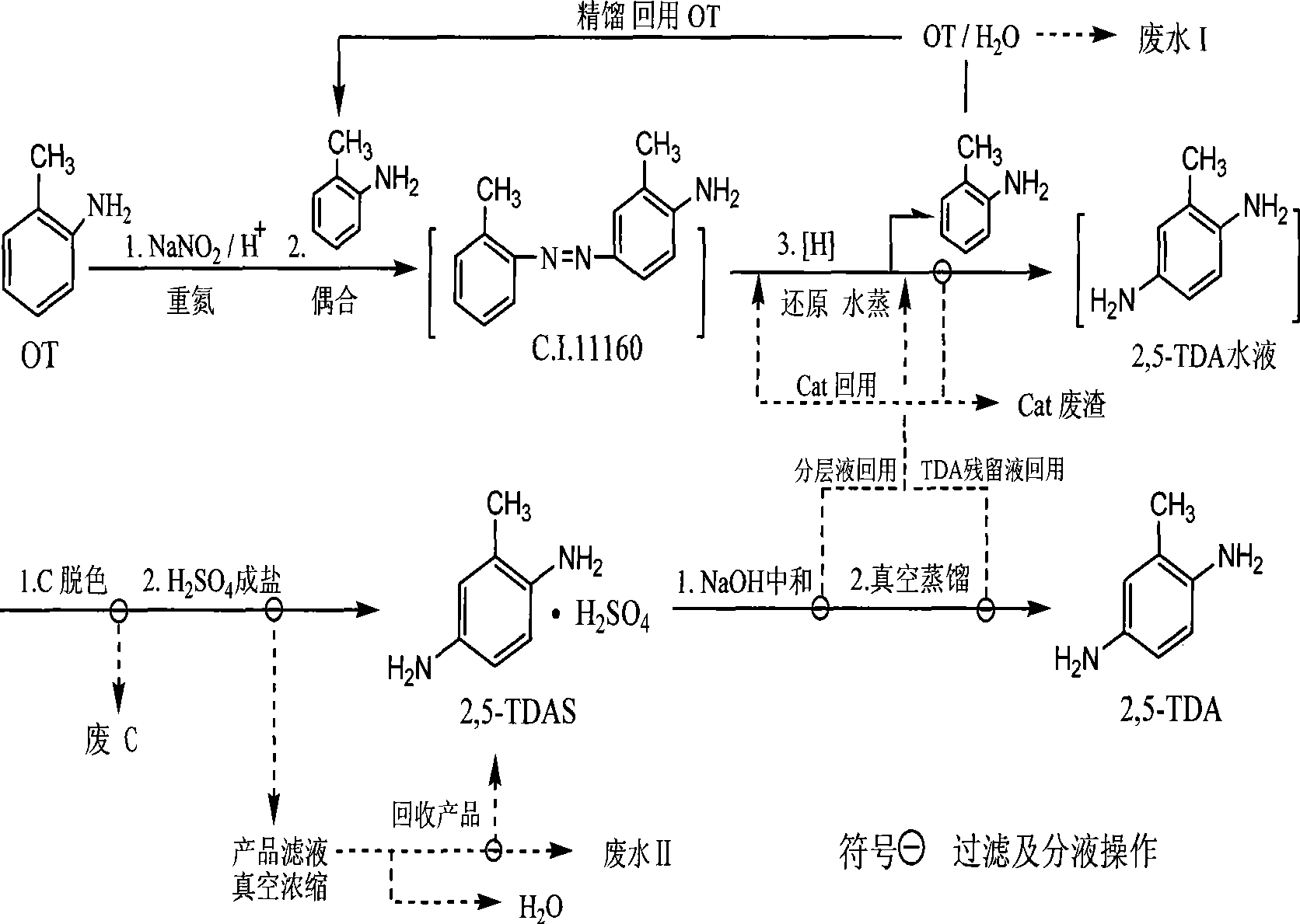
[0066] Example 1: OT one-pot in-situ generation of 2,5-TDA and recovery and recycling of OT
[0067] Under stirring, 85.6g of o-toluidine (OT, 0.8mol) was added into a four-necked flask equipped with 390g of sulfuric acid (0.88mol) with a mass concentration of 22%, and the temperature was controlled at 0 to 5°C. Sodium nitrite aqueous solution (0.804mol) is slowly added dropwise to the sulfuric acid aqueous solution of OT over 30 minutes, after the addition is completed and stirred for 30 minutes to form the diazotization solution of OT, then slowly added dropwise over 60 minutes while maintaining 0-5°C as the coupling component 85.6g of OT (0.8mol, composed of 60.0g of recovered OT and 25.6g of industrial product OT) in the diazotization solution, the feeding temperature was controlled below 20°C, and the temperature was raised to 25°C and stirred for 6 hours after the addition, and the coupling reaction was completed. Add 350mL of water and stir to raise the temperature to 8...
Embodiment 2
[0069] Embodiment 2: 2,5-diaminotoluene sulfate (2,5-TDAS) preparation
[0070] The crude 2,5-TDA aqueous solution obtained in Example 1 was first added with 3.0 g of activated carbon for alkaline adsorption at 70° C., stirred for 10 minutes and then filtered hot, and a small amount of sulfuric acid was added to the filtrate from which the waste carbon was removed to adjust the pH to 6.0. Then add 3.0g of activated carbon for weak acid adsorption decolorization operation, stir and decolorize for 10min, then heat filter, the filtrate is light brown transparent liquid; continue to carry out repeated cycle decolorization operations under this weak acidic condition, until the filtrate is light yellow and transparent , slowly add 45mL of sulfuric acid (98%) dropwise under stirring to carry out acid precipitation and salt formation, stir and cool to below 10°C, filter, filter cake with 300mL cold water beating and washing and then filter, and vacuum dry at 60°C for 8 hours to obtain ...
Embodiment 3~7
[0072] Embodiment 3~7: One-pot synthesis 2,5-TDA and preparation 2,5-TDAS protection range test
[0073] Adopt the basic operation of embodiment 1 and embodiment 2, select different diazo components and coupling components, metallic zinc to replace iron, and adopt the feeding mode of different methods, i.e. the step-by-step method in embodiment 1 and simultaneous method (See Example 8), according to the method of the present invention and the different scope of protection, OT is the raw material one-pot synthesis of 2,5-TDA crude product aqueous solution and its OT recovery, and further prepares the test of 2,5-TDAS, The results are shown in the table below:
[0074] Table 1: Examples and comparisons of different diazo-coupling operations, OT quality and recovery in one-pot in situ synthesis
[0075] Reality
[0076] * The OT of diazo component all uses 99.8% industrial product, and the OT of coupling component selects 30% industrial product (99.8%) for use, 70% r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com