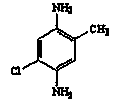
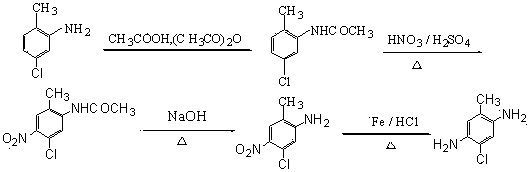
Preparation method for 5-chloro-2-methyl-1,4-phenylenediamine
A technology of methylaniline and phenylenediamine, which is applied in the field of preparation of chemical intermediates, can solve the problems of complex process, large gap, and many steps, and achieve the effects of high yield, low production cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
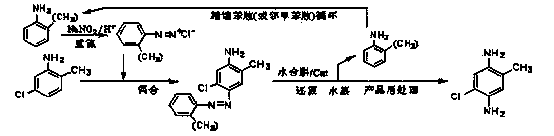
[0047] Diazo coupling reduction direct method (5-chloro-2-methylaniline-o-toluidine method)
[0048] Add 42.9g of o-toluidine (OT, 0.40mol) to the hydrochloric acid aqueous solution (mass concentration: 12%) mixed with 150g of water and 100g of 30% hydrochloric acid, control the temperature at 0-5°C, and drop 112.6g of mass within 15min 25% sodium nitrite (0.408mol) aqueous solution was stirred and reacted for 15 minutes to obtain a diazotized solution of o-toluidine. This o-toluidine diazonium salt solution was added dropwise at 8-10°C to a solution containing 56.6g 5-chloro-2-methylaniline (0.40mol), 22.1g Na 2 CO 3 (0.208mol) and 460g of water in a stirred 1 L four-necked reaction flask, the dropwise addition was completed within 3 hours, the pH was controlled at 8~8.5, and the reaction was continued at 22~25°C for 1.5 hours to obtain the azo compound 4-amino - The reaction solution of 2-chloro-5-methyl-2'-methylazobenzene; the suspension after the reaction was directly t...
Embodiment 2
[0053] Diazo coupling reduction hydrogenolysis step-by-step method (5-chloro-2-methylaniline-o-toluidine method)
[0054] The reaction solution of the azo compound prepared by the same feeding and process as in Example 1 was filtered, and the wet product of the azo compound (yield 92.44%) was taken out, and was beaten and washed with 360ml of water and then filtered to obtain (4-amino-2-chloro -5-methyl-2'-methylazobenzene) wet product 127.8g (92.88g after drying, 0.358mol, purity 97.17%, yield 89.4%); add 620mL process water in proportion to the autoclave , 127.8g 4-amino-2-chloro-5-methyl-2'-methylazobenzene wet product (solid content 72.7%), 5g Raney-Ni catalyst and 80% hydrazine hydrate 50g (0.80mol) after sealing Return to the autoclave, after replacing the air with nitrogen, stir and heat up to 80°C, and carry out hydrazine hydrate reduction for 4 h at a speed of 600 to complete the hydrogenolysis reaction. With reference to the concentrated water vapor of Example 1, o-...
Embodiment 3
[0056] Diazo coupling reduction direct method (5-chloro-2-methylaniline-o-toluidine method)
[0057] Add 37.3g of aniline (0.40mol) to an aqueous solution of hydrochloric acid mixed with 150g of water and 100g of 35% hydrochloric acid, control the temperature at 0-5°C, and add 126.7g of sodium nitrite (0.45mol) with a mass concentration of 25% dropwise within 15 minutes ) aqueous solution, the diazotization solution of aniline was obtained after stirring for 1 h. Add this aniline diazotization solution dropwise at 8-10°C to a solution containing 56.6g 5-chloro-2-methylaniline (0.40mol), 28.3g Na 2 CO 3 (0.50mol) and 560g of water in a stirred 1 L four-necked reaction flask, the dropwise addition was completed within 4 hours, the pH was controlled at 8 to 8.5, and the stirring reaction was continued at 20°C for 3.5 hours to obtain the azo compound 4-amino-2- The reaction solution of chloro-5-methylazobenzene; the suspension after the reaction is directly transferred to a 2L a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com