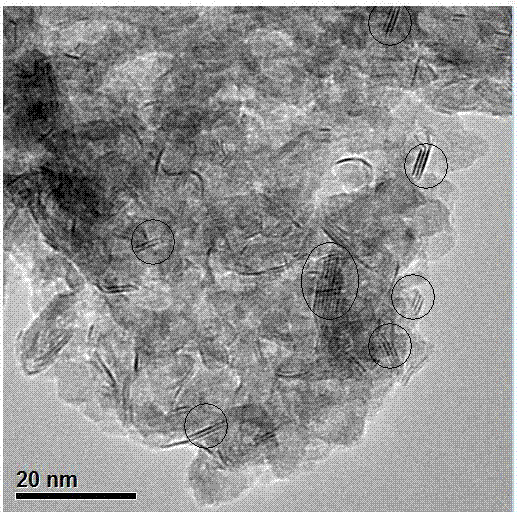
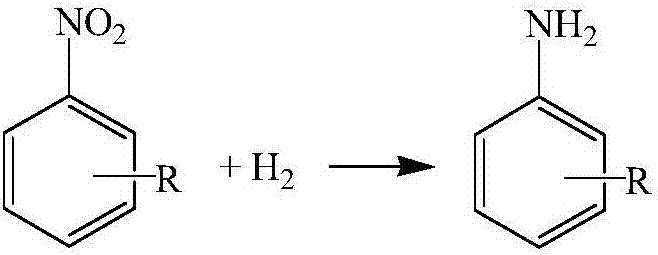
Catalyst for hydrogenation production of aniline or derivatives thereof by nitrobenzene or derivatives thereof, and preparation method and application thereof
A technology of derivatives and catalysts, which is applied in the field of catalysts, preparation methods and applications for the hydrogenation of nitrobenzene or its derivatives to produce aniline or its derivatives, can solve the problems of complicated separation process and poor selectivity of target products, and achieve Low equipment investment, good application prospects and practical value, high activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Take 0.086g of nickel nitrate, 0.522g of ammonium heptamolybdate, add 5g of ammonia water, heat the mixed solution to 80°C, add ionic water to make the solution volume 16.271ml, and obtain a mixed solution with a total solution concentration of 0.2mol / L, and use 0.5mol / L nitric acid to adjust the pH to 8.0. Add 9.5g of alumina for impregnation for 2 hours, and stir continuously at a rate of 30 rpm during the impregnation process to make the impregnation uniform. After 2 hours, the sample was filtered out and dried. During drying, the temperature was slowly raised from room temperature to 100°C at a rate of 10°C / min, and dried at this temperature for 24h. The resulting dried samples were calcined at 400 °C for 12 h. The obtained roasted sample was put into a fixed-bed reactor for vulcanization, the hydrogen pressure was 0.5 MPa, the vulcanizing agent was dimethyl disulfide, the dosage was 0.293 g, the carrier oil was cyclohexane, and the sulfur content was 1000 ppm. Ra...
Embodiment 2
[0040] Get 6.522g ferric nitrate nonahydrate, 2.850g ammonium heptamolybdate, add 20g ammoniacal liquor, the mixed solution is heated to 50 ℃, add ionized water to make the solution volume be 9.224ml, obtain the total solution concentration and be 3.5mol / L mixed solution, use 0.5mol / L hydrochloric acid to adjust the pH value to 13.0. Add 6.0g of alumina for impregnation for 24 hours, and stir continuously at a rate of 300 rpm during the impregnation process to make the impregnation uniform. After 24 hours, the sample was filtered out and dried. During drying, the temperature was slowly raised from room temperature to 100°C at a rate of 10°C / min, and dried at this temperature for 6 hours. The obtained samples were calcined at 600°C for 3h. The obtained sample was put into a fixed bed reactor for vulcanization, the hydrogen pressure was 3.0MPa, the vulcanizing agent was dimethyl disulfide, the dosage was about 2.281g, the carrier oil was n-hexane, and the sulfur content was abo...
Embodiment 3
[0043] Take 1.354g of copper nitrate, 2.550g of ammonium heptamolybdate, add 9g of ammonia water, heat the mixed solution to 60°C, add ionic water to make the solution volume 12.036ml, and obtain a mixed solution with a total solution concentration of 1.8mmol / L, and use 0.5mol / L ethylenediamine to adjust the pH to 12.0. Add 7.0g of alumina for impregnation for 16 hours, and stir continuously at a rate of 150 rpm during the impregnation process to make the impregnation uniform. After 18 hours, the sample was filtered out and dried. During drying, the temperature was slowly raised from room temperature to 120°C at a rate of 15°C / min, and dried at this temperature for 10h. The obtained samples were calcined at 500°C for 8h. The obtained sample was put into a fixed-bed reactor for vulcanization, the hydrogen pressure was 2.0 MPa, the vulcanizing agent was dimethyl disulfide, the dosage was about 1.700 g, the carrier oil was cyclohexane, and the sulfur content was about 3000 ppm....
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com