Process of preparing trans-polyhydroxy diphenyl ethylene
A technology of polyhydroxy stilbene and stilbene, which is applied in the chemical industry, can solve the problems of complex steps and high cost, and achieve the effects of low reaction cost, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
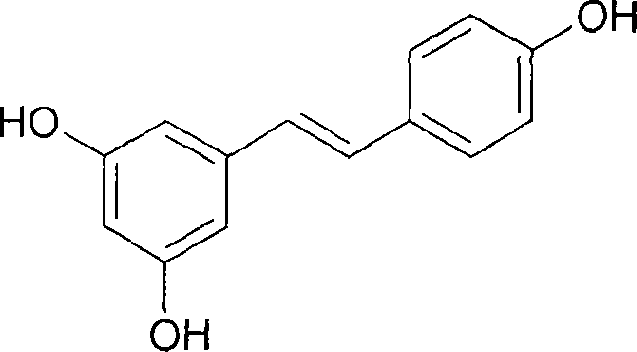
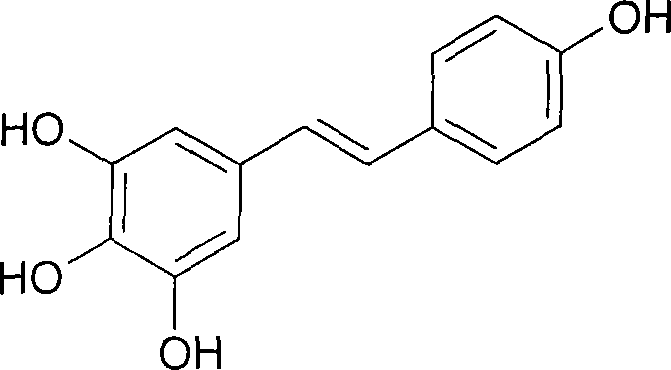
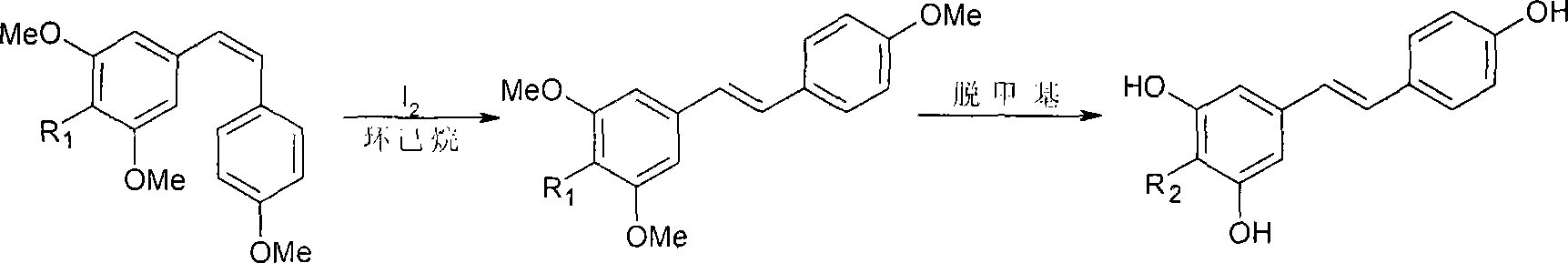
[0032] Add 15.22g (0.0373mol) of All 3 and 75ml of acetonitrile, then slowly dropwise added 2.70g (0.01mol) of (Z)-3,4',5-trimethoxystilbene dissolved in 5ml of acetonitrile, stirred and refluxed at 82°C for 3h, cooled, and the reaction The solution was concentrated to dryness, and a yellow-brown solid was produced, and an appropriate amount of water was added, and a light-yellow solid was obtained by suction filtration, and then recrystallized with ethanol / water to obtain 1.91 g of white needle-like crystals, namely (E)-3,4′,5- Trihydroxystilbene (resveratrol), the yield is 83.8%. The test data are as follows: Mp: 255-260°C. MS m / z (%): 228 (M + ). 1 H-NMR (CDCl 3 ): 6.27 (t, 1H, J = 2.2Hz), 6.54 (d, 2H, J = 2.4Hz), 6.96 (d, 2H, J = 16.4), 7.10 (d, 4H, J = 8.8Hz), 8.22 (s, 2H, 2 x OH), 8.50 (s, 1H, OH).
Embodiment 2
[0034] Add 15.22g (0.0373mol) of All 3 and 75ml of acetonitrile, then slowly dropwise added 2.70g (0.01mol) of (Z)-3,4',5-trimethoxystilbene dissolved in 5ml of acetonitrile, stirred at 65°C for 2h, cooled, and the reaction solution Concentrate to dryness, a yellow-brown solid is produced, add an appropriate amount of water, and filter with suction to obtain a light-yellow solid, and then recrystallize with ethanol / water to obtain 1.42 g of white needle-like crystals, namely (E)-3,4′,5-tri Hydroxystilbene (resveratrol), the yield is 62.3%.
Embodiment 3
[0036] Add 15.22g (0.0373mol) of All 3 and 75ml of acetonitrile, then slowly dropwise added 2.70g (0.01mol) of (Z)-3,4',5-trimethoxystilbene dissolved in 5ml of acetonitrile, stirred and refluxed at 90°C for 5h, cooled, and the reaction solution was Concentrate to dryness, a yellow-brown solid is produced, add an appropriate amount of water, and filter with suction to obtain a light-yellow solid, and then recrystallize with ethanol / water to obtain 1.71 g of white needle-like crystals, namely (E)-3,4′,5-tri Hydroxystilbene (resveratrol), the yield is 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com