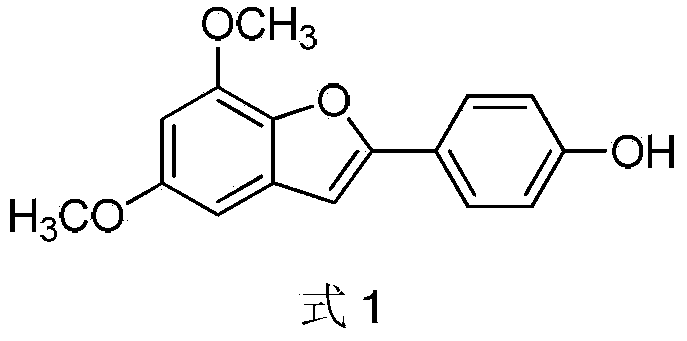
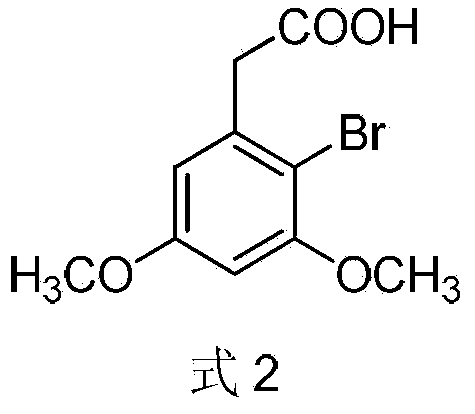
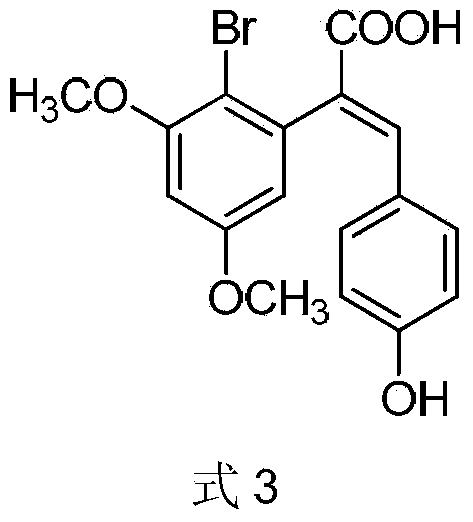
Preparation method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran
A technology of dimethoxybenzene and dimethoxyphenyl, which is applied in the field of preparation of 2--5,7-dimethoxybenzofuran, can solve the problems of poor substrate adaptability, complex catalytic system, Harsh reaction conditions and other issues, to achieve the effects of easy large-scale preparation, simple post-treatment process, and no need for noble metal catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of 2-bromo-3,5-dimethoxyphenylacetic acid (formula 2)
[0034] In a 150mL reaction flask, add 3,5-dimethoxyphenylacetic acid (9.80g, 50mmol) and 100mL of chloroform, respectively, stir and heat, and when the temperature rises to 40°C, slowly add dibromohydantoin (DBDMH , 7.15g, 25mmol), after the addition, continue to react for 15h, pour the reaction solution into ice water, stir for 1.5h, filter with suction, collect the filtrate and wash 3 times with saturated sodium bisulfite, 30mL / time, concentrate under reduced pressure, Recrystallized with distilled water to obtain 11.46 g of 2-bromo-3,5-dimethoxyphenylacetic acid, yield: 83.3%. m.p.122-124°C. 1 H NMR (400MHz, DMSO-d 6 ):δ12.44(s,1H),6.61(d,J=2.8Hz,1H),6.58(d,J=2.8Hz,1H),3.81(s,3H),3.90(s,3H),3.77 (s,2H); MS(EI):m / z=276[M+2] + ,274[M] + ,195[M-Br] + ,180[M-COOCH 3 ] + ,77[C 6h 5 ] + .
Embodiment 2
[0035] Example 2: Preparation of 2-(2-bromo-3,5-dimethoxyphenyl)-3-(4-hydroxyphenyl)acrylic acid (Formula 3)
[0036] In a 150ml reaction flask, add 2-bromo-3,5-dimethoxyphenylacetic acid (6.88g, 25mmol), p-hydroxybenzaldehyde (3.05g, 25mmol), triethylamine (7.0ml, 50mmol) 1. 15ml of acetic anhydride, stirred and heated to 110°C, and maintained at this temperature for 8h. After the reaction was completed, the reaction system was slowly poured into 50ml of ice-water solution, stirred, left overnight, suction filtered, washed with water, and the filter cake was purified by column chromatography (ethyl acetate:petroleum ether=2:1) to obtain 2-(2 -Bromo-3,5-dimethoxyphenyl)-3-(4-hydroxyphenyl)acrylic acid 8.47g, yield: 89.4%. m.p.110-112°C. 1 HNMR (400MHz, DMSO-d 6 ):δ10.36(s,1H),7.18(dd,J 1 =8.00,J 2 =1.74Hz,1H),6.89(d,J=1.74Hz,1H),6.75(d,J=8.00Hz,1H),5.30(s,1H),3.96(s,2H),3.74(s,3H ),3.65(s,3H); MS(EI):m / z=239[M] + ,208[M-OCH 3 ] + ,180[M-COOCH 3 ] + ,77[C 6 h 5 ]...
Embodiment 3
[0037] Example 3: Preparation of 2-(4-hydroxyphenyl)-5,7-dimethoxybenzofuran-3-carboxylic acid (formula 4)
[0038] In a 150ml normal-pressure stainless steel reaction flask, add 2-(2-bromo-3,5-dimethoxyphenyl)-3-(4-hydroxyphenyl)acrylic acid (3.79g, 10mmol), anhydrous Copper sulfate (4.8g, 30mmol), the mass fraction is 10% sodium hydroxide solution (NaOH solid 4.0g, 100mmol, add 36ml water to dissolve), warm up to 120°C, reflux for 72h, stop the reaction. Cool to 25°C, remove insoluble matter by suction filtration, slowly add dilute hydrochloric acid with a mass fraction of 5% to the filtrate and adjust the pH to 4-5, let it stand, and precipitate a large amount of black gray solid, and use ethyl acetate-petroleum ether (1: 2) The mixed solvent was recrystallized to obtain a light yellow solid, 2-(4-hydroxyphenyl)-5,7-dimethoxybenzofuran-3-carboxylic acid, weight: 1.48g, yield: 76.8%. m.p.212-214°C. 1 H NMR (400MHz, DMSO-d 6 ):δ12.75(s,COOH),10.05(s,OH),7.81(d,J=8.8Hz,2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com