Method for preparing aryl acetonitrile compound
A technology for aryl acetonitrile and compounds, applied in the field of compound synthesis, can solve the problems of tin reagent toxicity, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
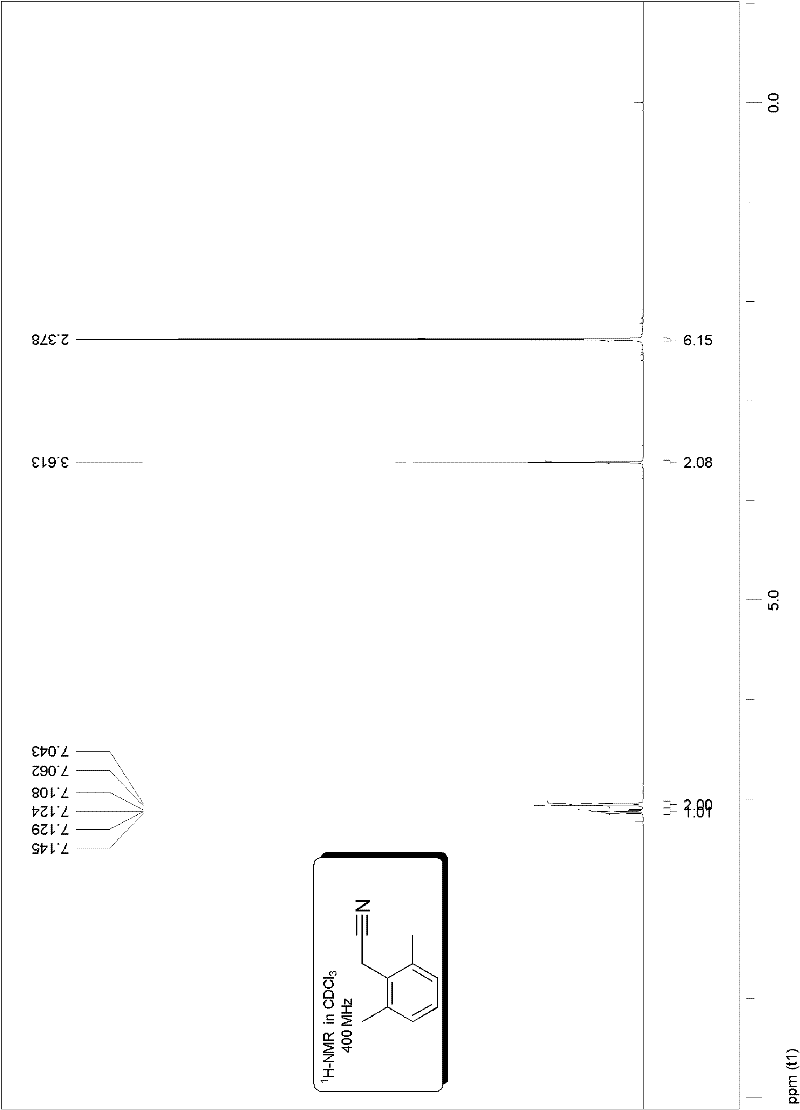
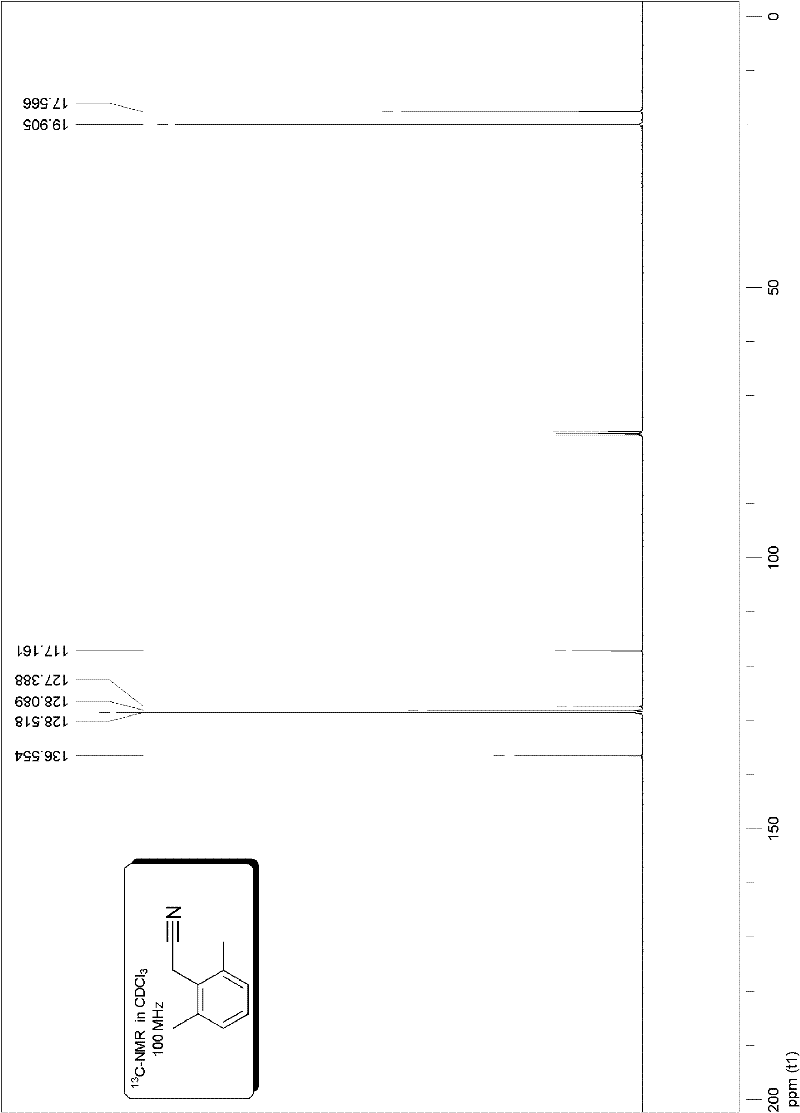
[0085] Embodiment 1, preparation 2,6-dimethylphenylacetonitrile:
[0086] Reaction formula:
[0087]
[0088] The specific method is as follows: add 0.75 mmoles of potassium cyanoacetate in the vacuum reactor, and the molar amount is allyl palladium chloride (Pd 2 (p-allyl) 2 Cl 2) Catalyst 0.01 millimole and electrophilic substrate molar dosage 0.6% S-Phos phosphine ligand 0.03 millimole, vacuumize, pass through high-purity argon, replace three times, add 0.5 millimole electrophilic substrate under the protection of argon flow 2, 6-dimethylbromobenzene and solvent trimethylbenzene (the amount of solvent is added 2 milliliters of solvent per millimole of electrophilic substrate), placed at 140 ° C, heated and stirred for 10 hours, decarboxylated according to the following method The reaction system after the coupling reaction is purified to obtain the target product 2,6-dimethylphenylacetonitrile: the reaction system mixture after the reaction is purified by fast silica ...
Embodiment 2
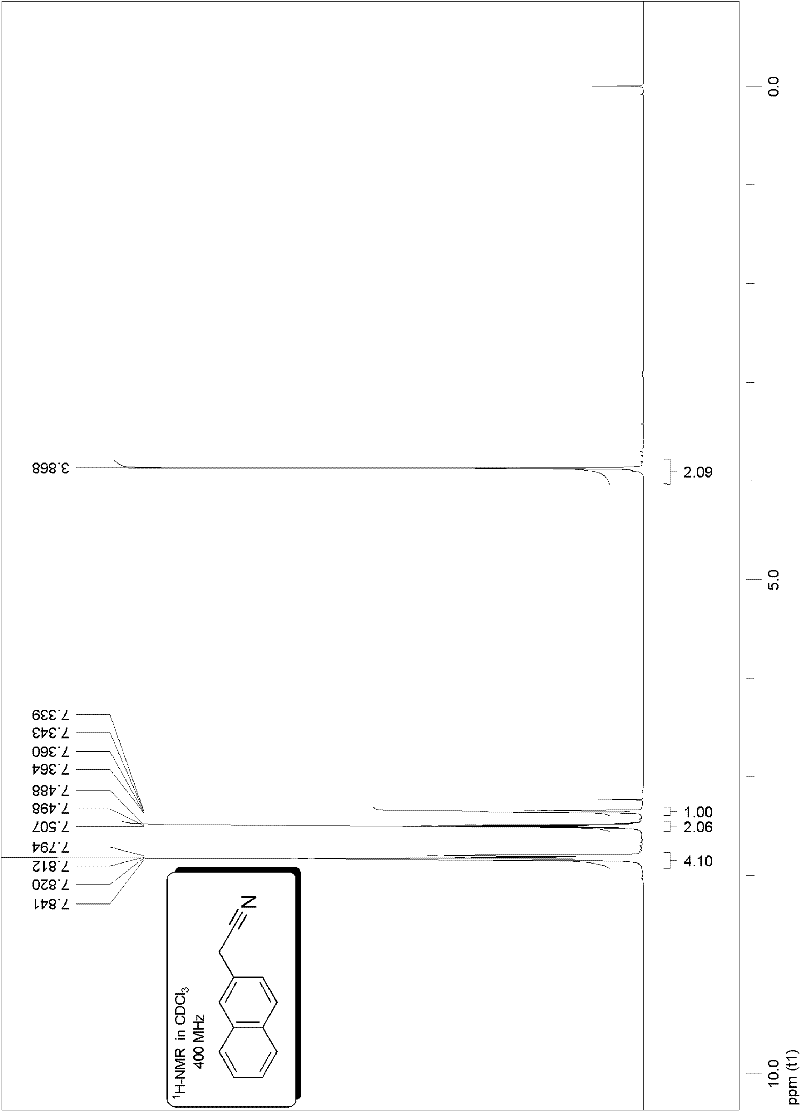
[0090] Embodiment 2, preparation benzyl nitrile:
[0091] Reaction formula:
[0092]
[0093] The method is the same as in Example 1, and the productive rate is shown in Table 1.
Embodiment 3
[0094] Embodiment 3, preparation benzyl nitrile:
[0095] Reaction formula:
[0096]
[0097] The method is the same as in Example 1, and the productive rate is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com