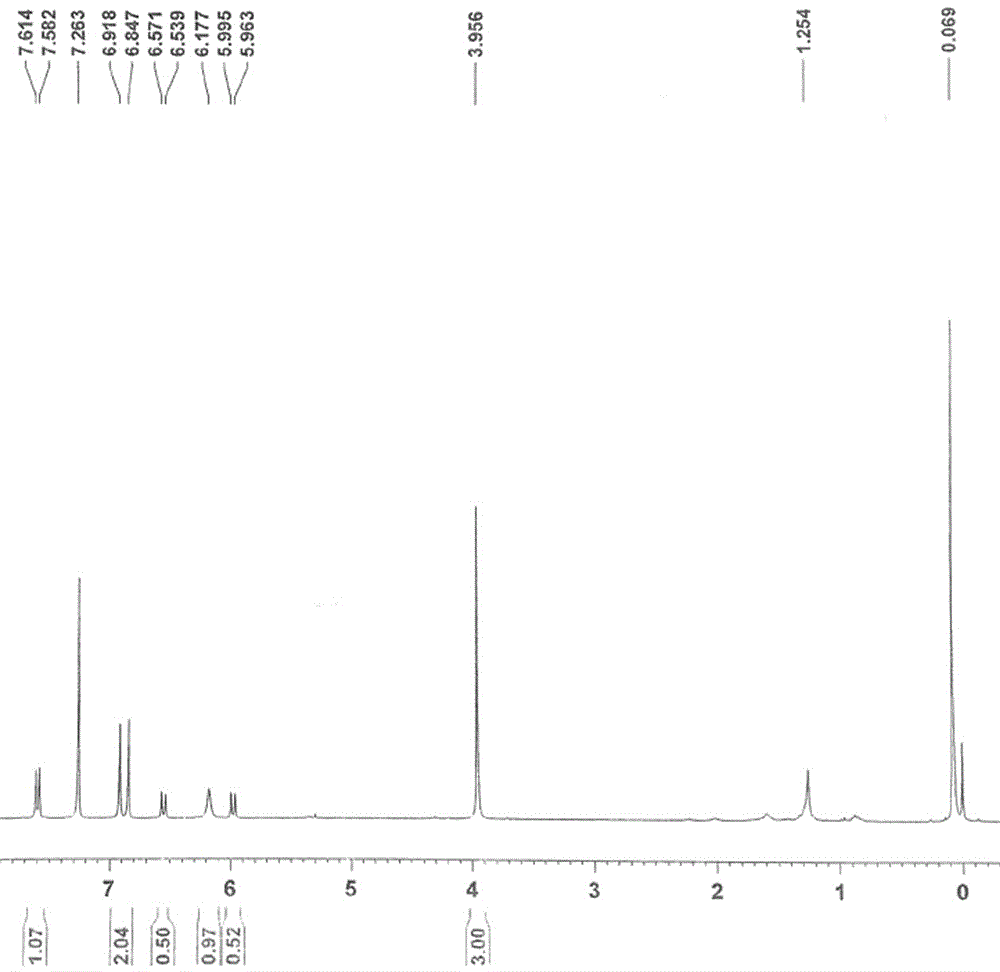
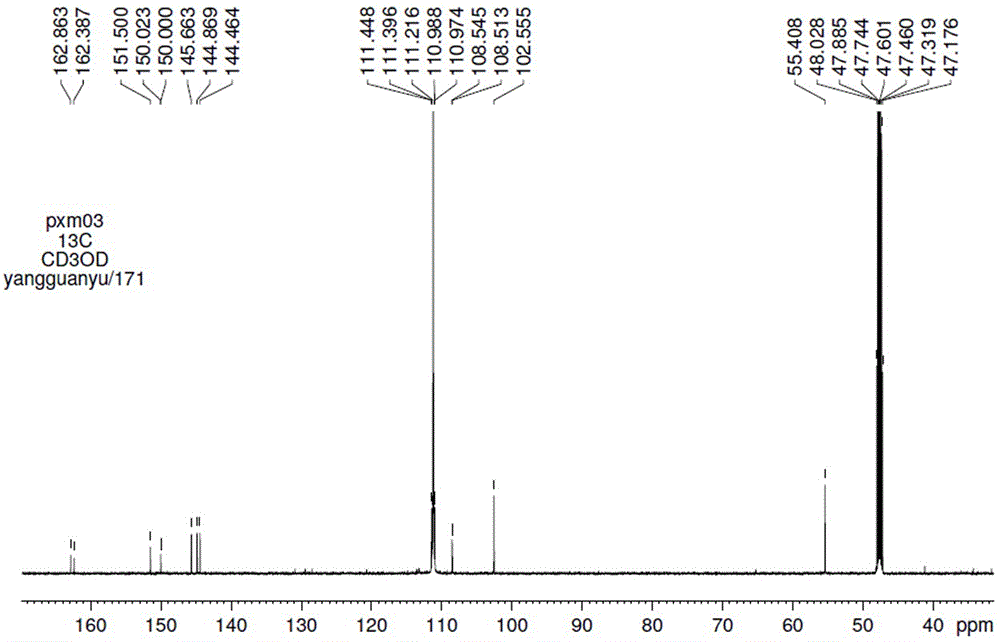
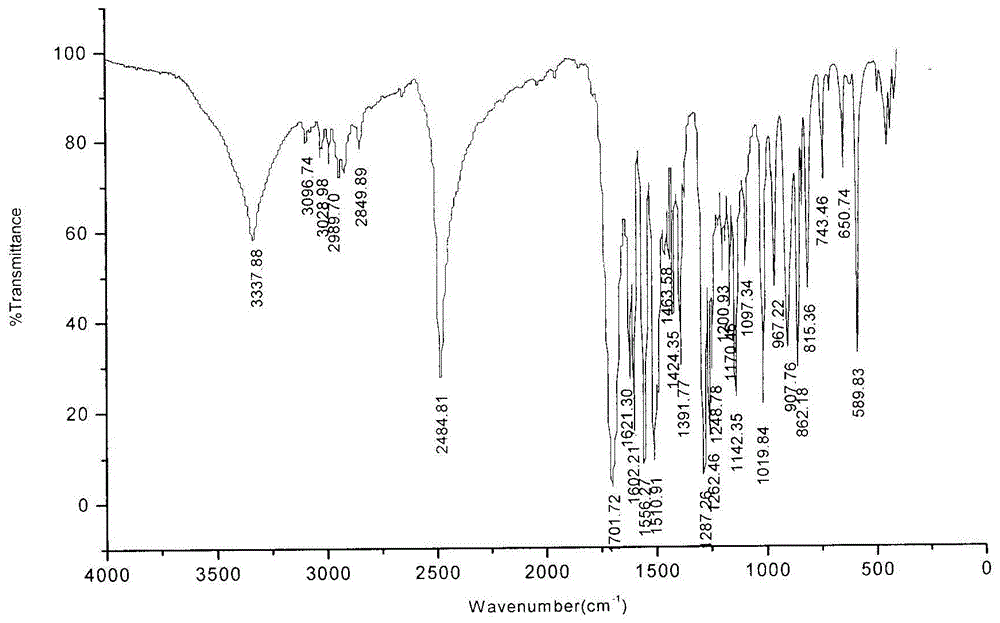
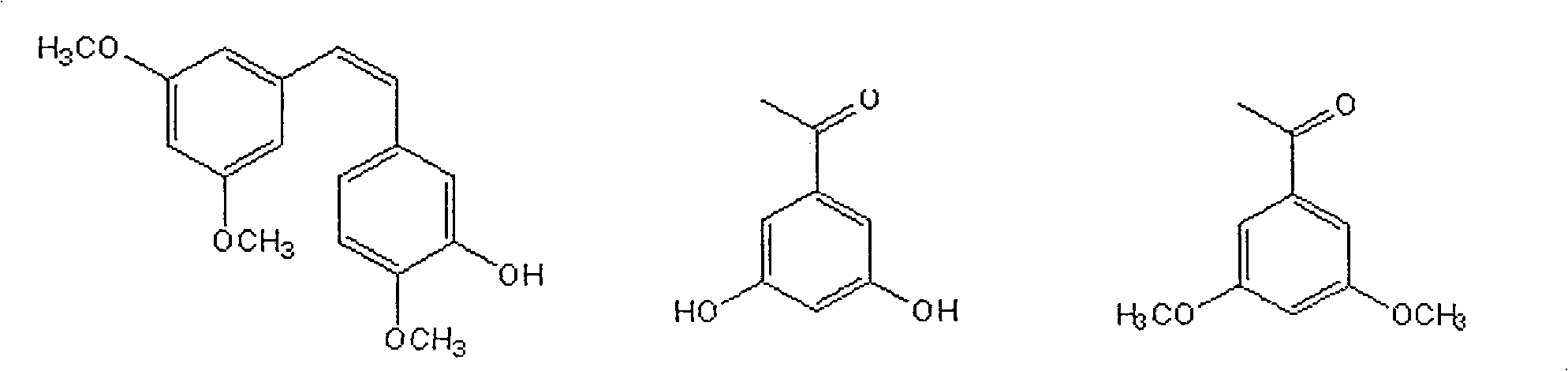
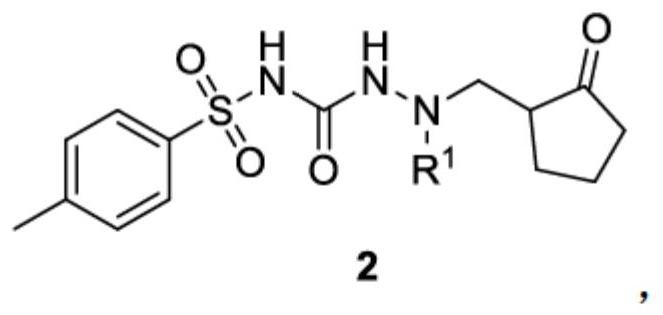
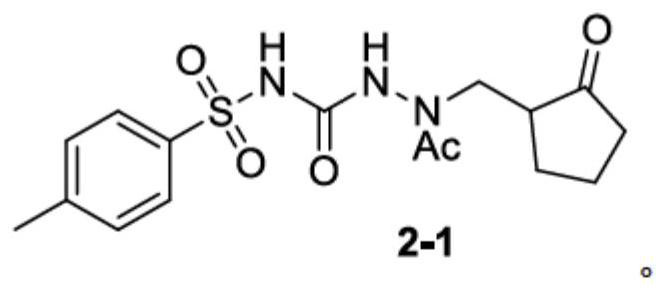
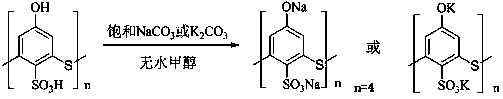Patents
Literature
37 results about "Perkin reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
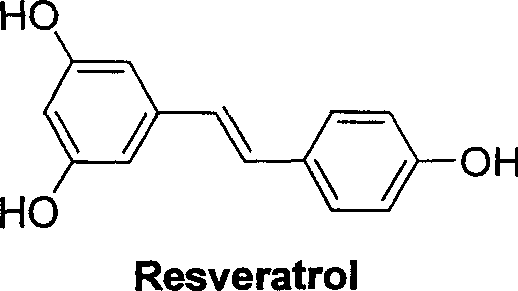
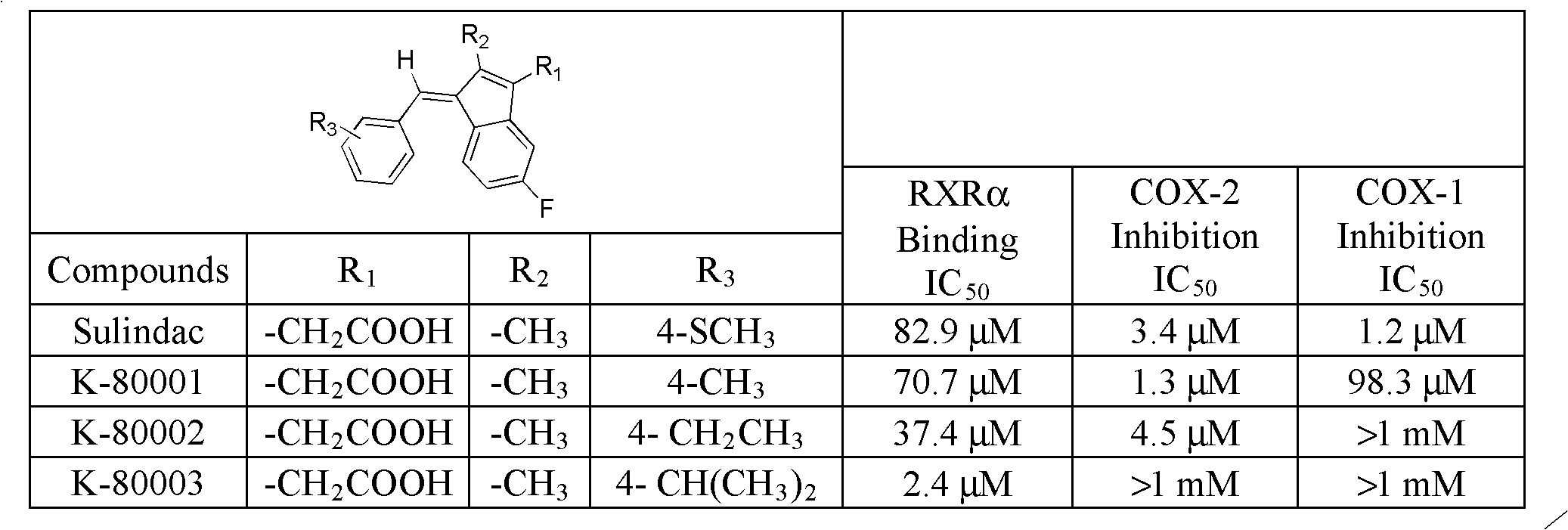
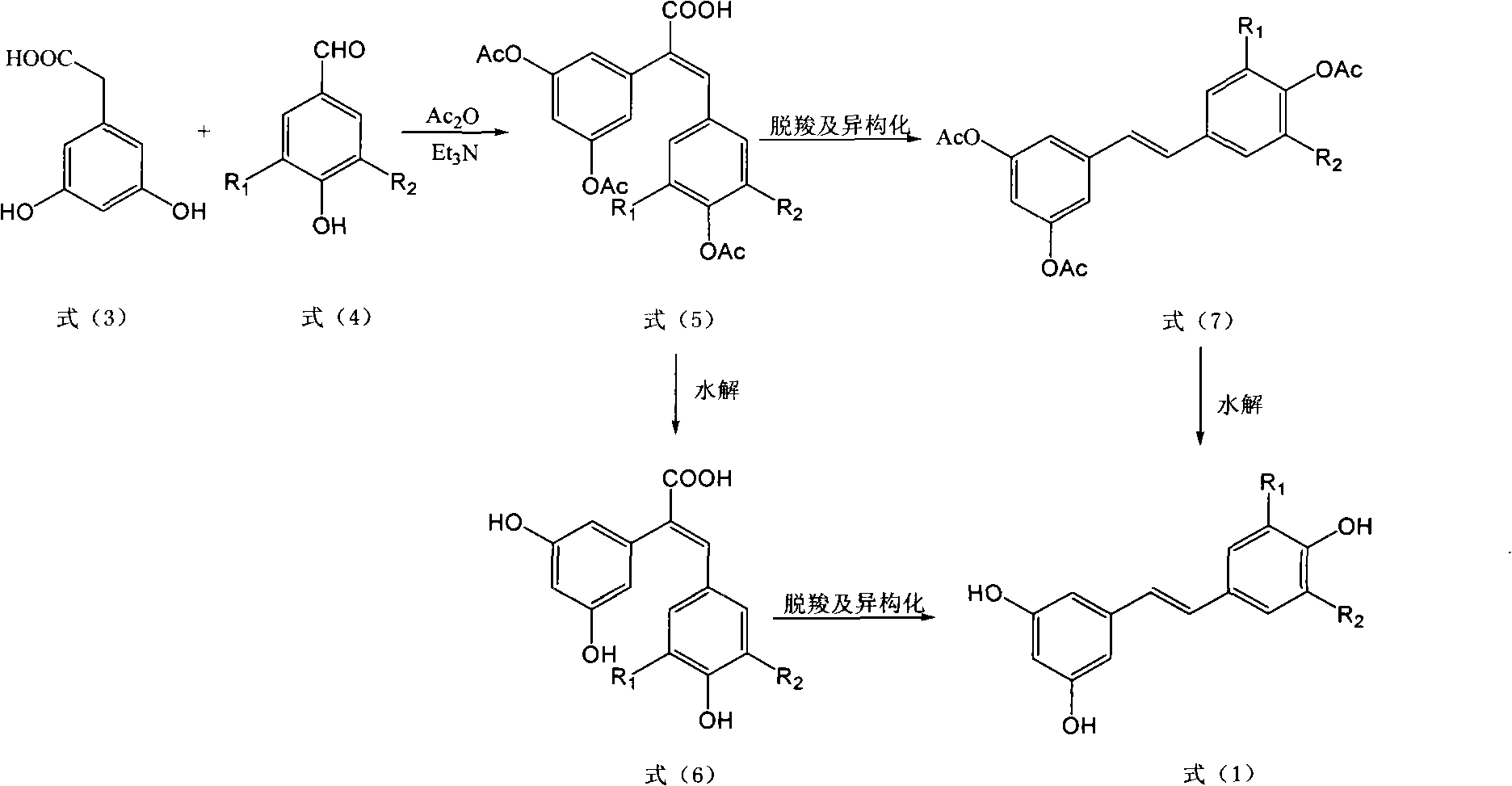
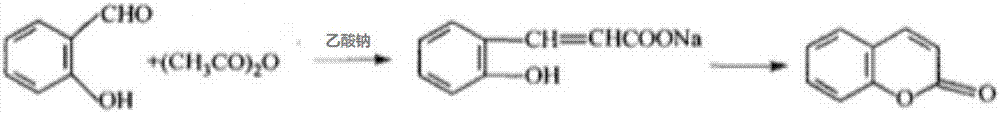
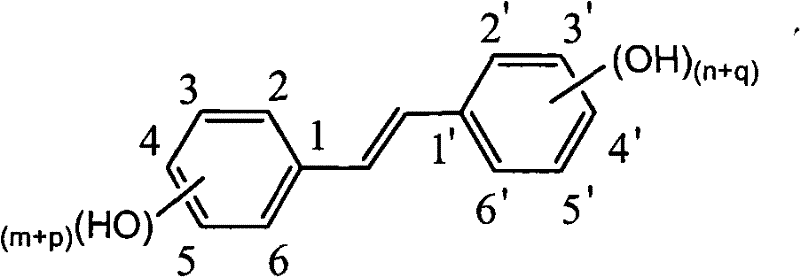
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.
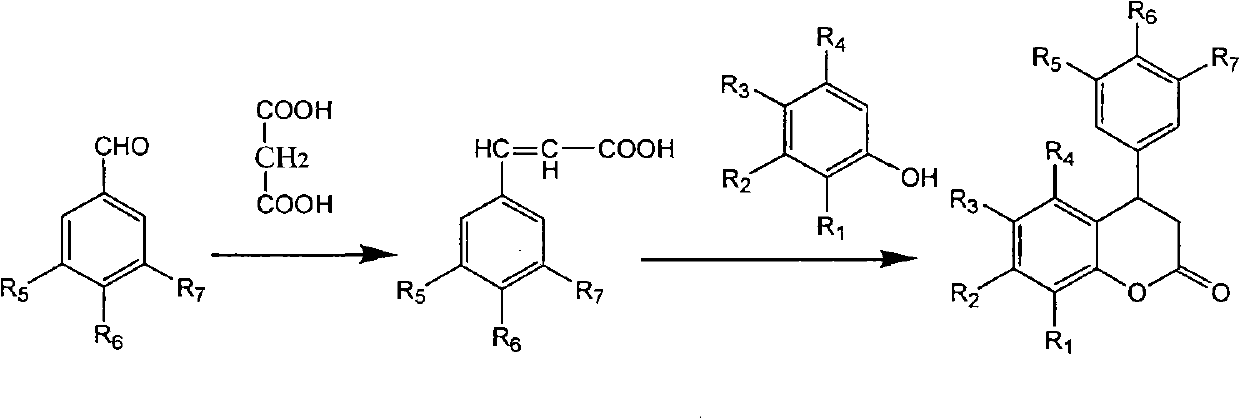
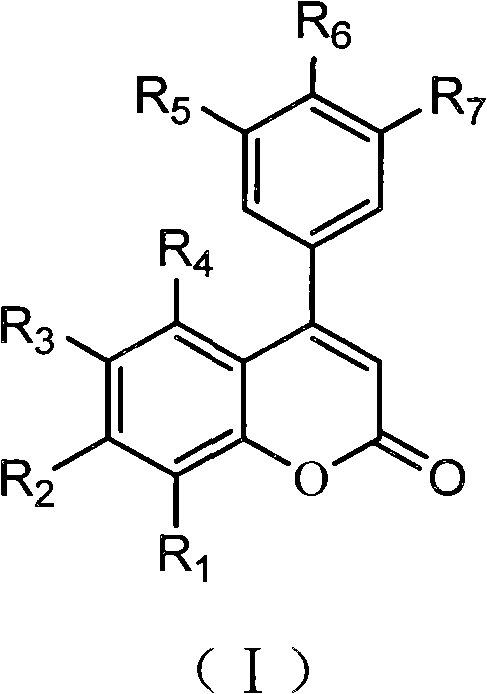
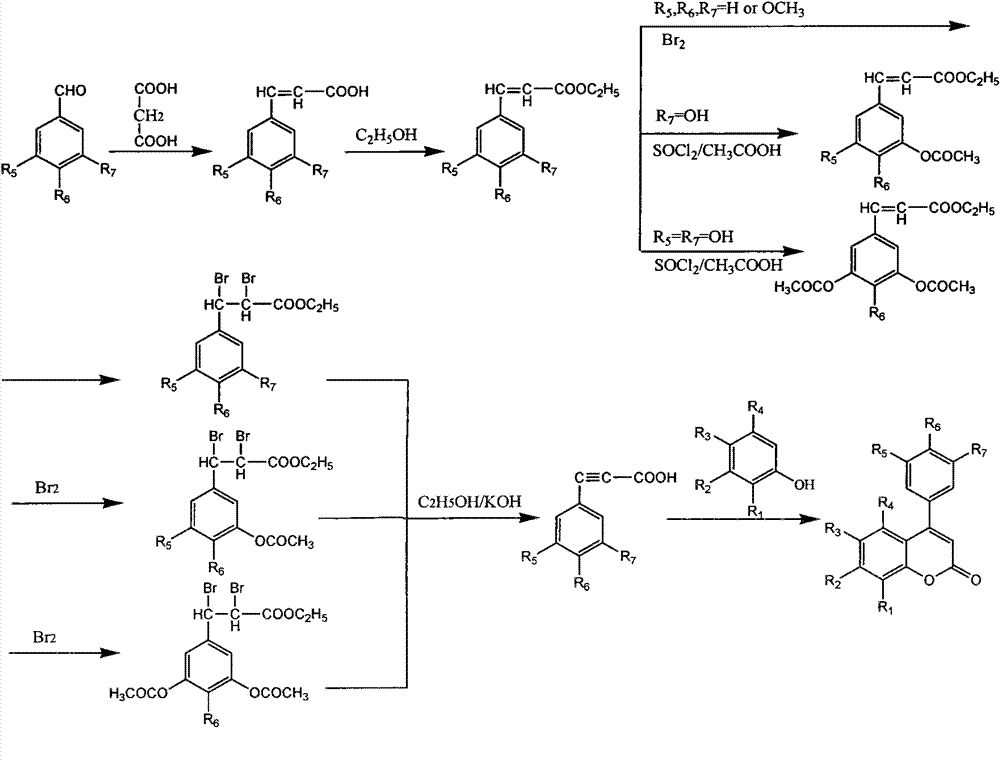
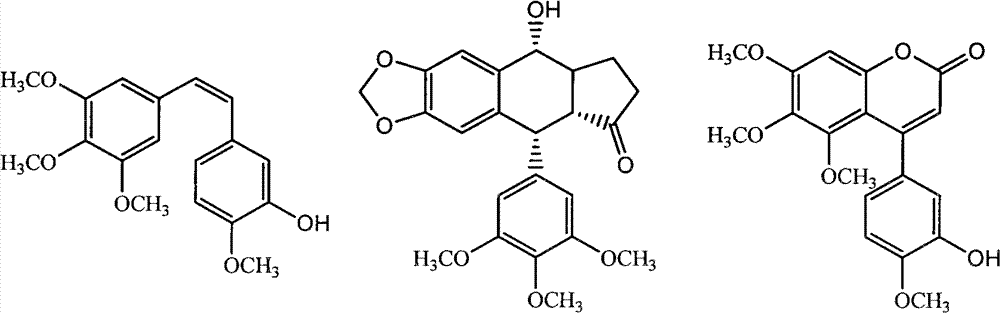
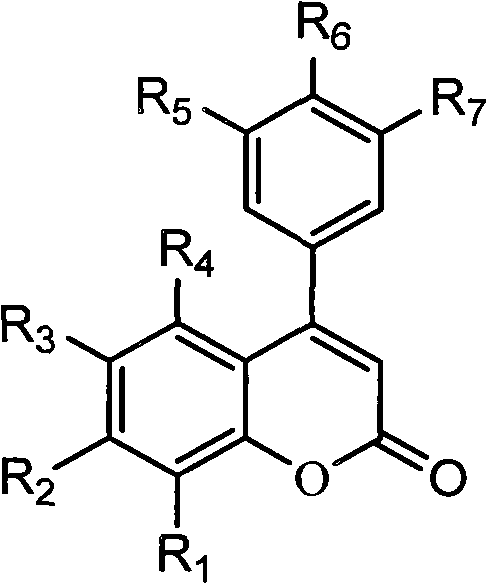
3,4-dihydro-4-aryl coumarin compounds as well as preparation method and application thereof
InactiveCN101906090AHas a chemical structureBiologically activeAntibacterial agentsOrganic chemistryVascular proliferationBenzaldehyde
The invention discloses 3,4-dihydro-4-aryl coumarin derivatives as well as a preparation method and application thereof. The preparation method comprises the following steps of: with substituted benzaldehyde and malonic acid as raw materials, heating in the presence of pyridine and piperidine and generating a Perkin reaction and a decarboxylic reaction to obtain substituted phenylacrylic acid derivatives; and then subjecting the substituted phenylacrylic acid derivatives and phenol compounds to a reaction in the presence of the catalysis of boron trifluoride diethyl ether and phosphorus oxychloride to obtain the 3,4-dihydro-4-aryl coumarin compounds. The compounds can be used for preparing medicaments for resisting tumors, abnormal vascular proliferation, bacterial and oxidation.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
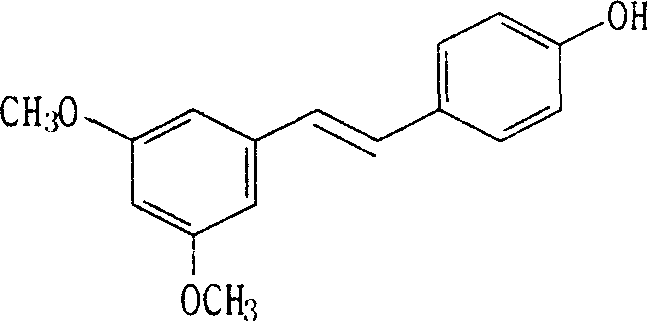
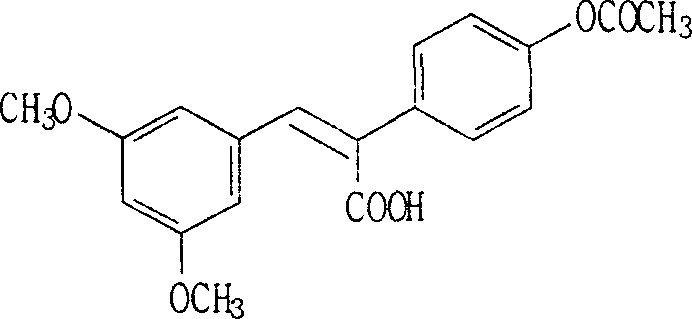
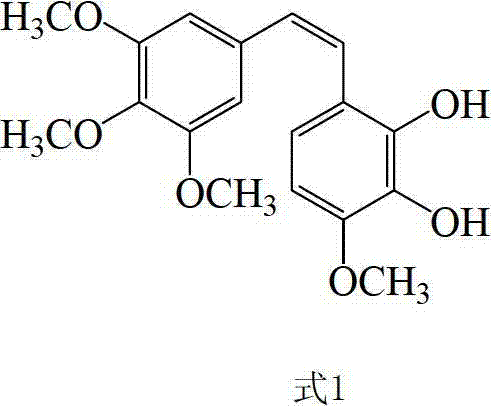
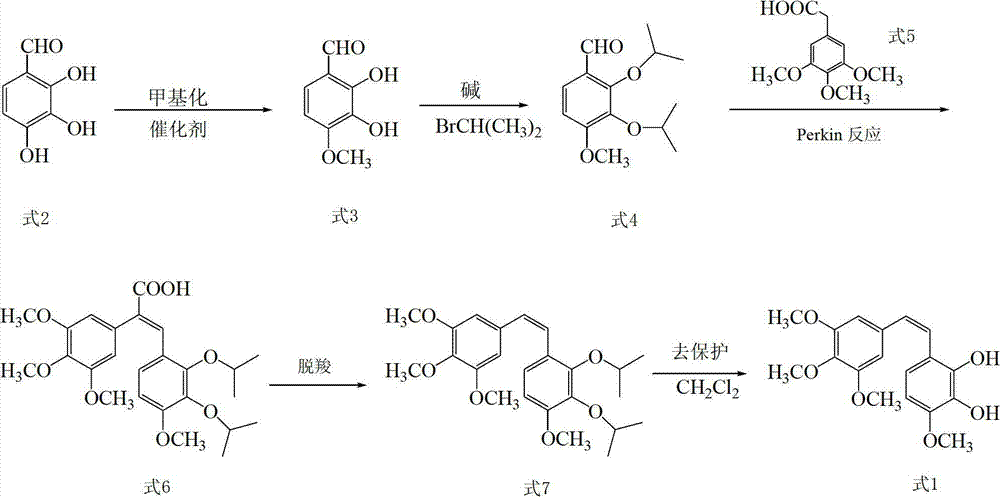
(E)-3,5-dimethox-4'-hydroxy diphenyl ethylene synthesis method
InactiveCN1876613AImprove economySimple reaction stepsOrganic chemistryOrganic compound preparationAcetic acidDecarboxylation
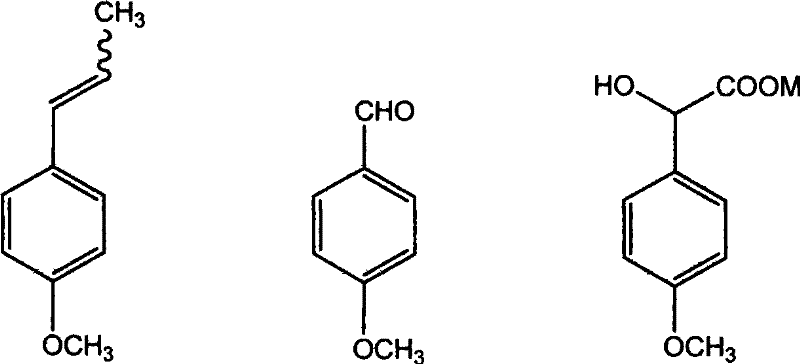
The invention discloses the synthesis of pterostilbene. The method comprises the following steps: using p-hydroxyphenyl acetic acid and veratraldehyde as raw material, carryingout Perkin reaction, getting (Z)-2(4-acetoxylation phenyl)-3-(3, 5- dimethoxy benzoic) acroleic acid, deacetylation, decarboxylation, and getting pterostilbene. The invention has the advantages of cheap raw material, simple technology, easy operation and high productivity. The invention has the advantages of low cost, good economy benefit and environment protection.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
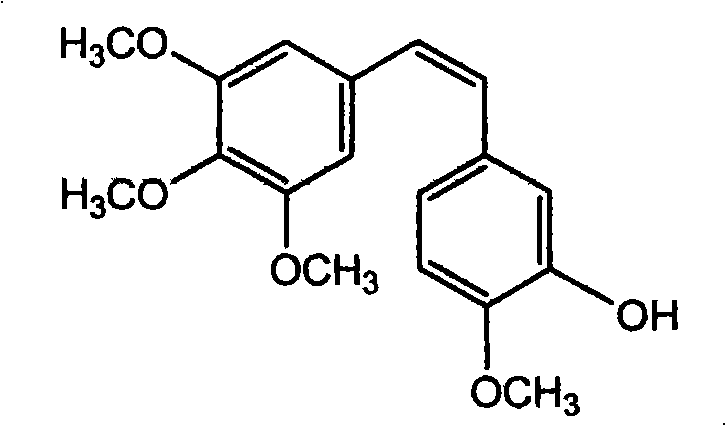
Method of preparing (Z)-3'-hydroxyl-3,4,4',5-tetramethoxy toluylene
InactiveCN101402555AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
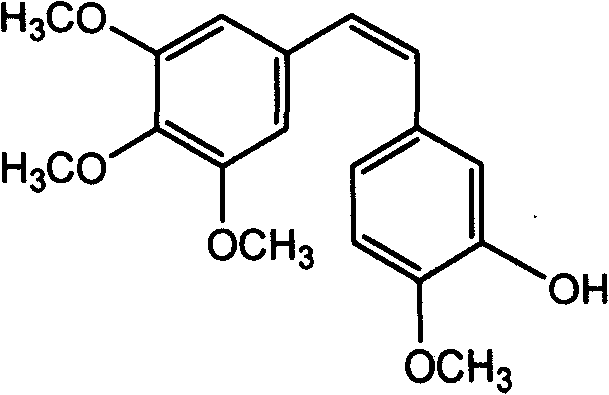
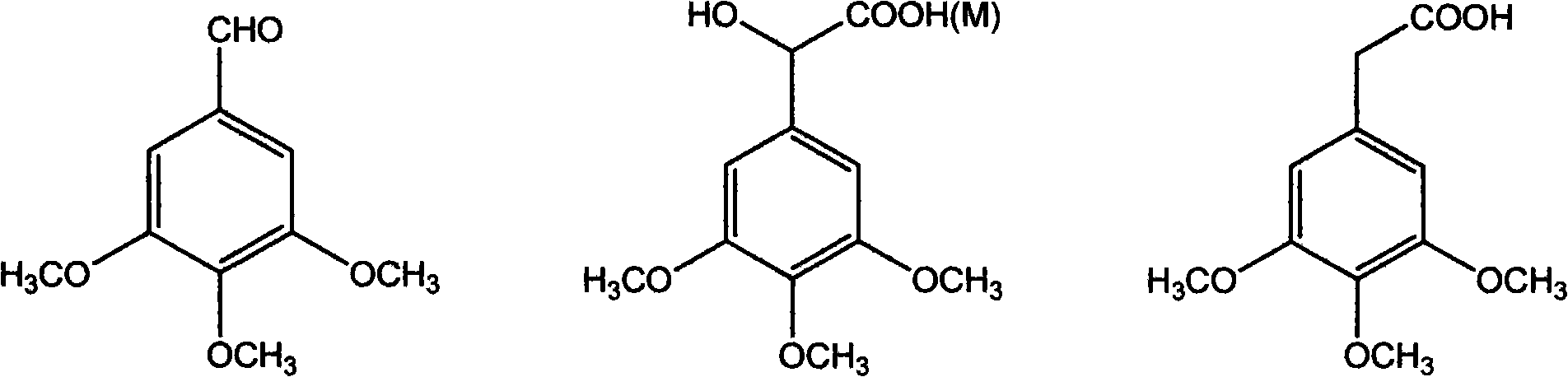
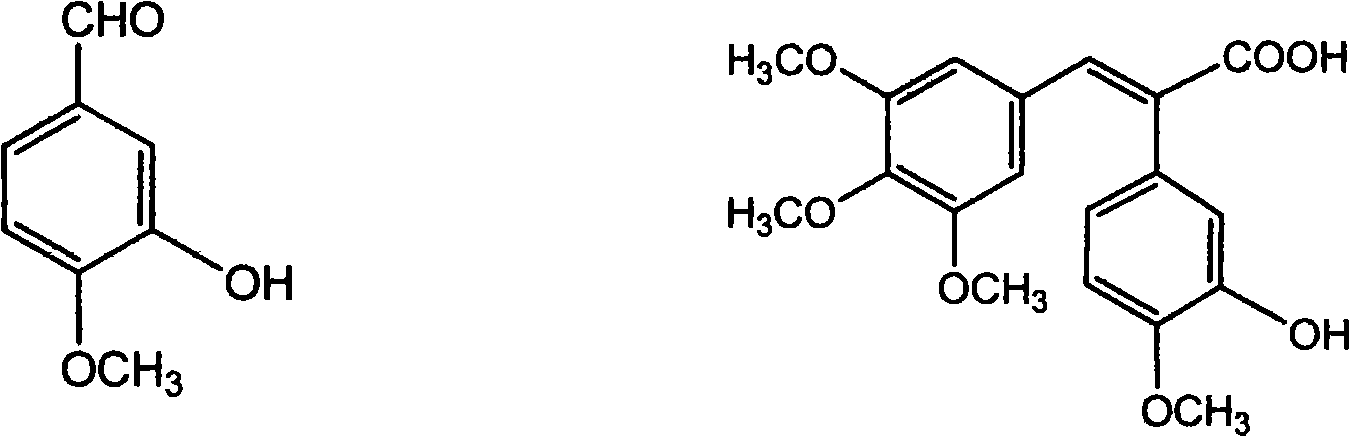
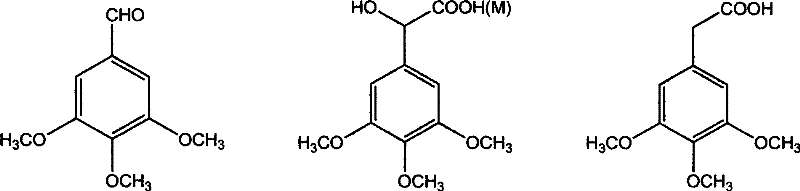
The invention discloses a method for preparing (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene by the material of renewable natural plant resource. Naturally sourced 3,4,5-tri-methoxybenzaldehyde (a derivative extracted from Chinese gall) is taken as the raw material; and 3,4,5-tri-methoyl mandelic acid is obtained through a dichlorocarbene insertion reaction and is reduced to obtain 3,4,5-tri-methoyl phenylacetic acid. The compound can have a Perkin reaction with naturally sourced isovanillin to construct a cis-form diphenyl ethylene backbone, and the (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene is obtained after a decarboxylic reaction. The method adopts the renewable resource-isovanillin and the 3,4,5-tri-methoxybenzaldehyde rich in China to replace increasingly exhausted petrochemical materials, thereby having a good sustainable development capability and remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
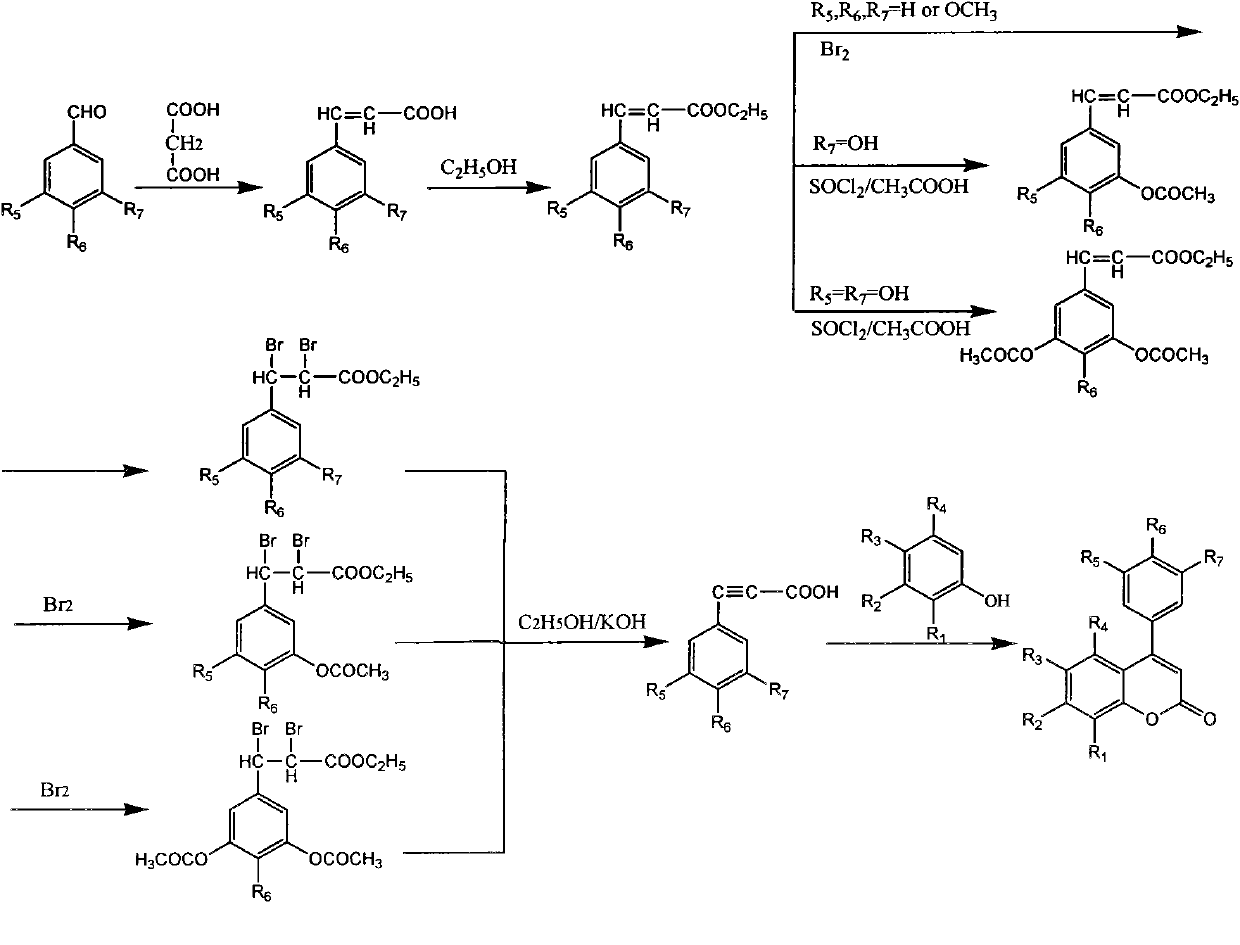
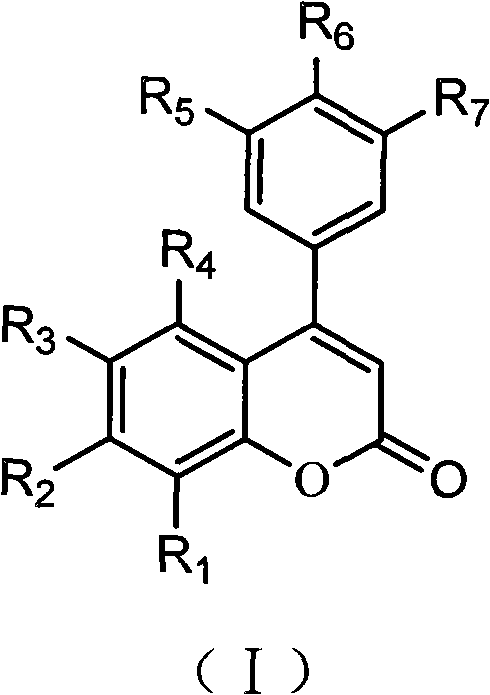
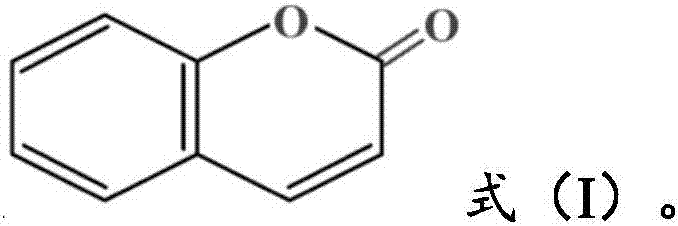
4-aryl coumarin compound and preparation method and application thereof
InactiveCN101967135AHas a chemical structureBiologically activeAntibacterial agentsOrganic chemistryBenzaldehydeElimination reaction
The invention discloses a 4-aryl coumarin compound and a preparation method and application thereof. The 4-aryl coumarin compound has a structure shown in a formula (I). The preparation method comprises the following steps: taking substituted benzaldehyde and propandioic acid as raw materials, heating under condition of existing pyridine and piperidine, and generating Perkin reaction and decarboxylic reaction to obtain a series of substituted phenylacrylic acid compounds; carrying out bromination and elimination reaction on the substituted phenylacrylic acid compounds or acetylation to protect a phenolic hydroxyl group, and then carrying out bromination and elimination reaction to obtain substituted phenylpropiolic acid compounds; and enabling the substituted phenyl propargylic acid compounds and phenolic compounds to react under the catalysis of boron trifluoride etherate and phosphorus oxychloride or trifluoroacetic acid to obtain a series of 4-aryl coumarin compounds. The compounds can be used for preparing antineoplastic medicine, anti-abnormal-angiogenesis medicine, antimicrobial medicine, antioxidation medicine and antimalarial medicine.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
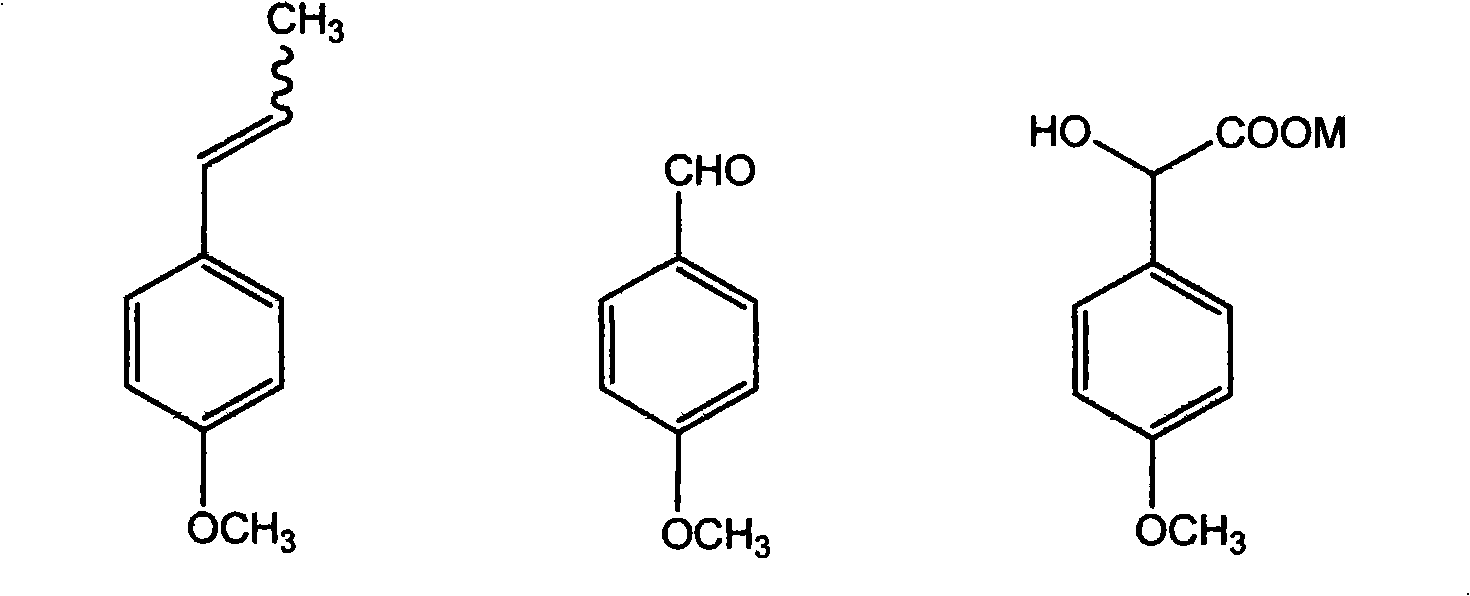
Method for preparing (Z)-3'-hydroxy-3,4,4',5-tetramethoxy diphenyl ethylene from regenerative natural plant resource
InactiveCN101353296AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
The invention discloses a preparation method for (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. A diphenyl ethylene framework structure is built by the Perkin reaction method, and natural aniseed fat-soluble components and propenyl anisole (anethole) are taken as raw materials, and oxidized to obtain anisaldehyde; dichlorocarbene insertion reaction is carried out on the anisaldehyde to obtain p-methoxyl-mandelic acid which is reduced to obtain methoxyl-phenylacetic acid, the methoxyl-phenylacetic acid is brominized to obtain 3-bromo-4-methoxyl-phenylacetic acid. The compound and the natural 3,4,5-trimethoxybenzaldehyde (nutgall extract derivative) carry out Perkin reaction to build a cis-form diphenyl ethylene framework which is further converted and decarboxylated by functional groups to obtain the (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. The raw materials of the invention are reproducible natural resources-anethole which are rich in China and 3,4,5-trimethoxybenzaldehyde, and replace non-renewable petrochemical materials which are used by the prior art and become less and less so that the method has the advantages of good sustainable development capability as well as remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
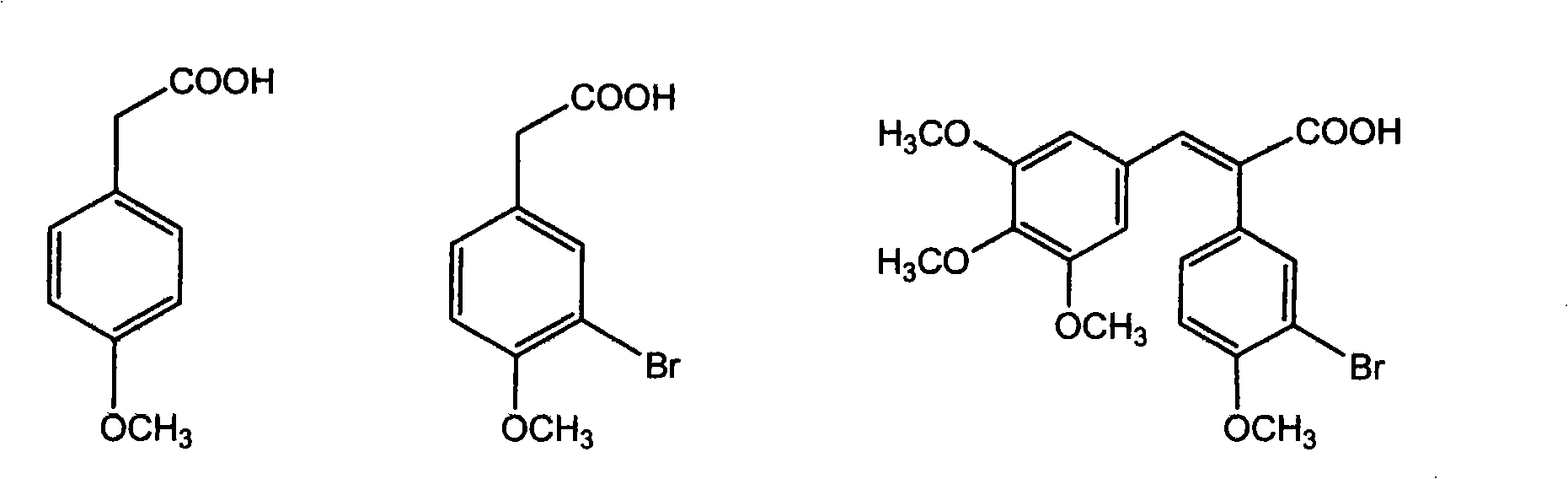
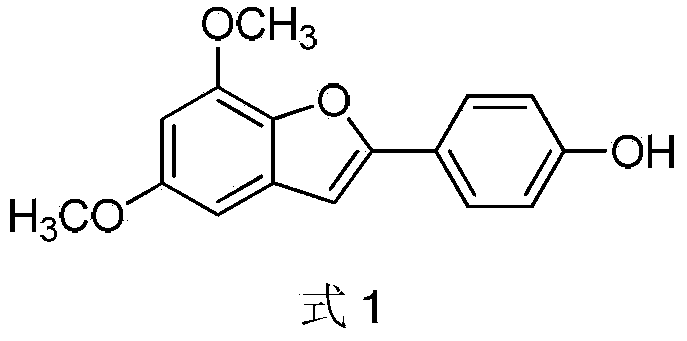
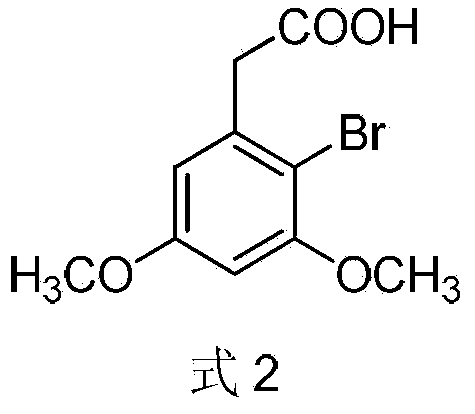
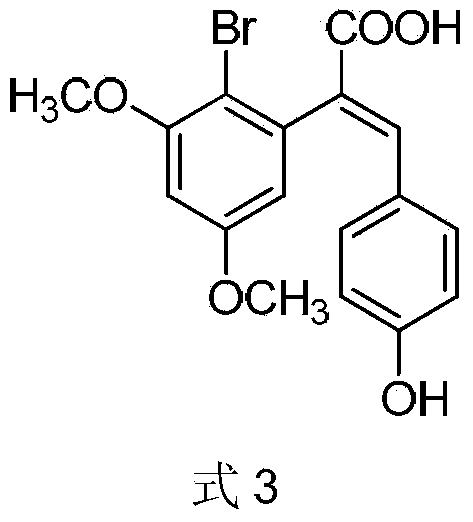
Preparation method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran
The invention discloses a synthetic method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. The synthetic method comprises the following steps of: firstly, performing bromination reaction and Perkin reaction on 3,5-dimethoxy phenylacetic acid and parahydroxyben-zaldehyde which are used as raw materials to obtain 2-(2-bromo-3,5-dimethoxy phenyl)-3-(4-hydroxyphenyl) acroleic acid; then performing series-wound hydroxylation / intramolecular cyclization / dehydrogenation reaction to obtain 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran-3-carboxylic acid; and finally, performing decarboxylic reaction to prepare the 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. According to the method, raw materials are low in price and easy to obtain, the synthetic route is simple, quick and efficient, a noble metal and a ligand are not needed for catalysis, and the method is simple and convenient to operate and good in atom economy.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for catalytic synthesis of cinnamic acid by water-soluble calixarene phenolate
InactiveCN108164410ALow yieldLow costOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from carboxylic acid anhydridesAcetic anhydrideBenzaldehyde
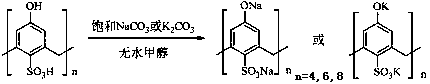
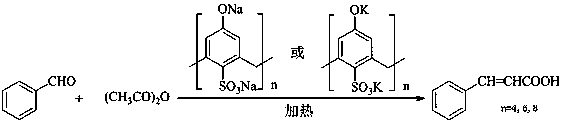
The invention is a method for catalytic synthesis of cinnamic acid by water-soluble calixarene phenolate, belonging to the technical field of preparation of organic compounds. The method comprises thefollowing steps: with the water-soluble calixarene phenolate as a catalyst, allowing the water-soluble calixarene phenolate to catalyze benzaldehyde and acetic anhydride to generate a perkin reaction, or catalyze benzaldehyde and propane diacid to generate a Knoevenagel reaction so as to prepare the cinnamic acid, wherein the calixarene phenolate can be repeatedly utilized for a plurality of times. According to the invention, the calixarene phenolate is calixarene phenolate sodium(possitium) salt which is formed through a reaction of calix[n]arene sulfonic acid (n is equal to 4, 6 and 8) andthiocalix[4]arene sulfonic acid with NaOH, KOH, Na2CO3 and K2CO3; and the usage amount of the calixarene phenolate accounts for 5 to 10% of the mass of the benzaldehyde. In the perkin reaction, the mass ratio of the benzaldehyde to the acetic anhydride is 1: 1 to 1: 4; the temperature of the reaction is 140 to 170 DEG C; and the time of the reaction is 1 to 2 h. In the Knoevenagel reaction, the mass ratio of the benzaldehyde to the propane diacid is 1: 1 to 1: 4; the temperature of the reaction is 120 to 150 DEG C; and the time of the reaction is 1 to 2 h. The process of the reaction does notgenerate three industrial wastes like waste gas, waste water and waste residue and is a green chemical process, so the pollution to the environment is reduced; and after completion of the reaction, the product is easily to be separated from the catalyst. The method provided by the invention has the advantages of simple process, high yield, environmental friendliness and low cost, and is a method applicable to industrial production.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
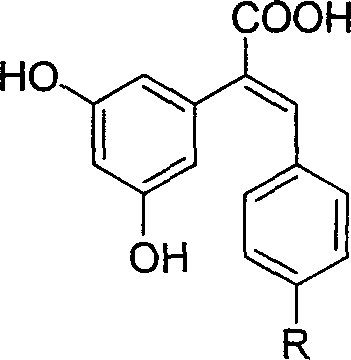
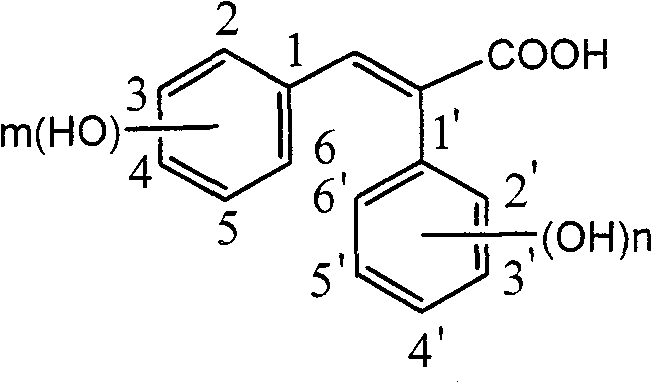
Carboxyl substituted resveratrol analog compound and its preparation method
InactiveCN1807391AEnhance anti-tumorImprove protectionPreparation from nitrilesAntineoplastic agentsHydrogenBenzaldehyde
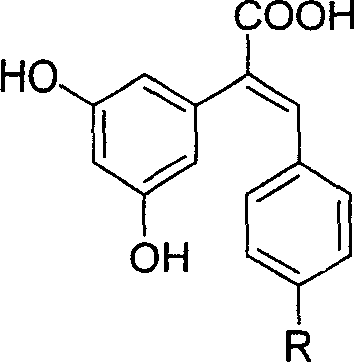
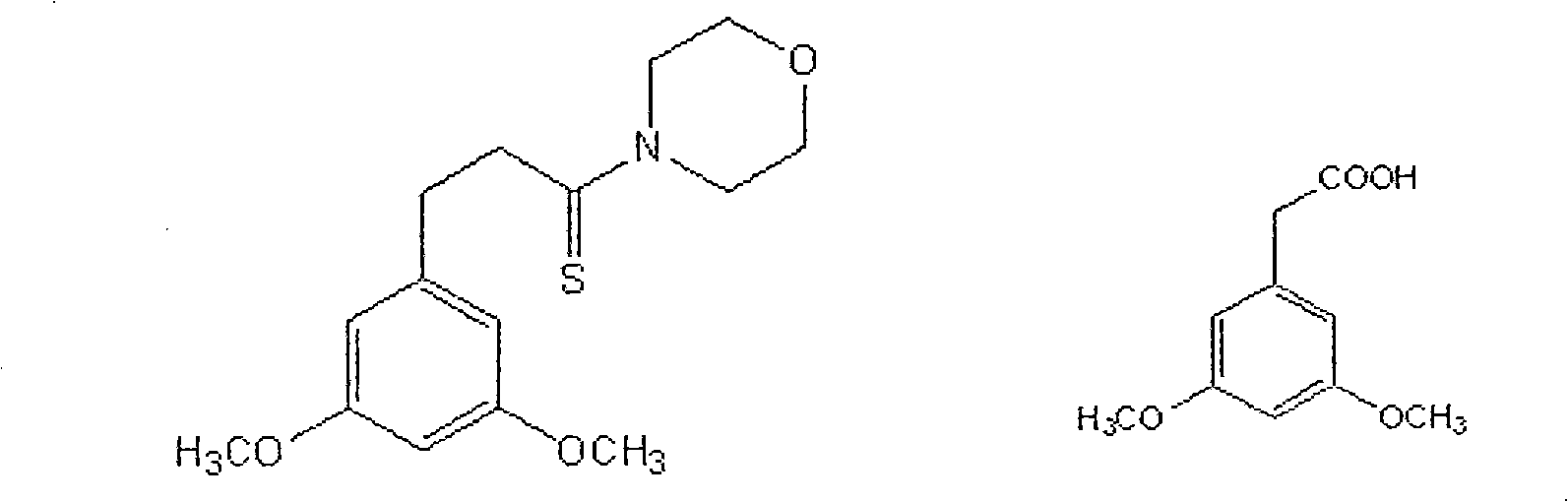
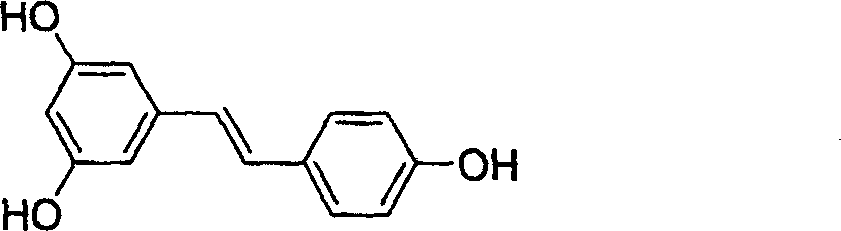
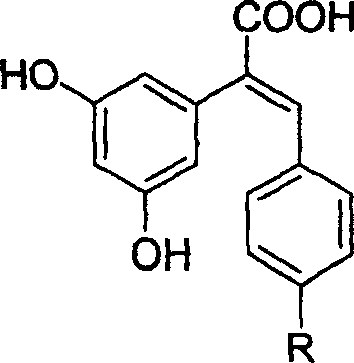
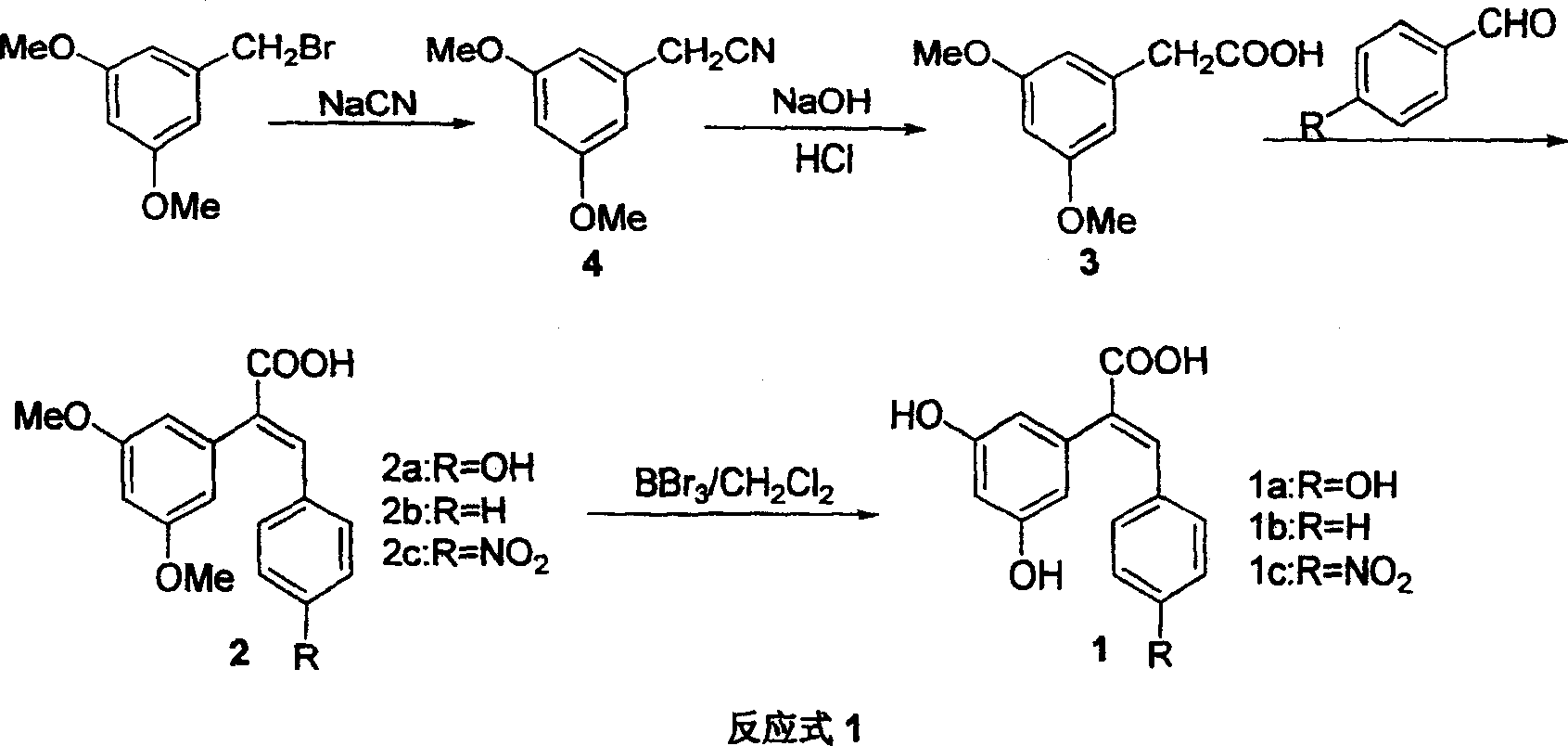
The resveratrol compound with carboxyl substituent has general formula as following, wherein, R acts for hydrogen, hydroxyl or nitryl. The opposite preparation method comprises: using Perkin reaction to the 3, 5-dimethoxylphenylacetic acid with opposite p-substituting R-benzaldehyde; removing the methoxy protection to obtain the product as the derivative of 1, 2-toluylene. This invention is similar to the resveratrol as 3, 4', 5-trihydroxy -trans-stilbene, and has wide application for antitumor and cardiovascular protection.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
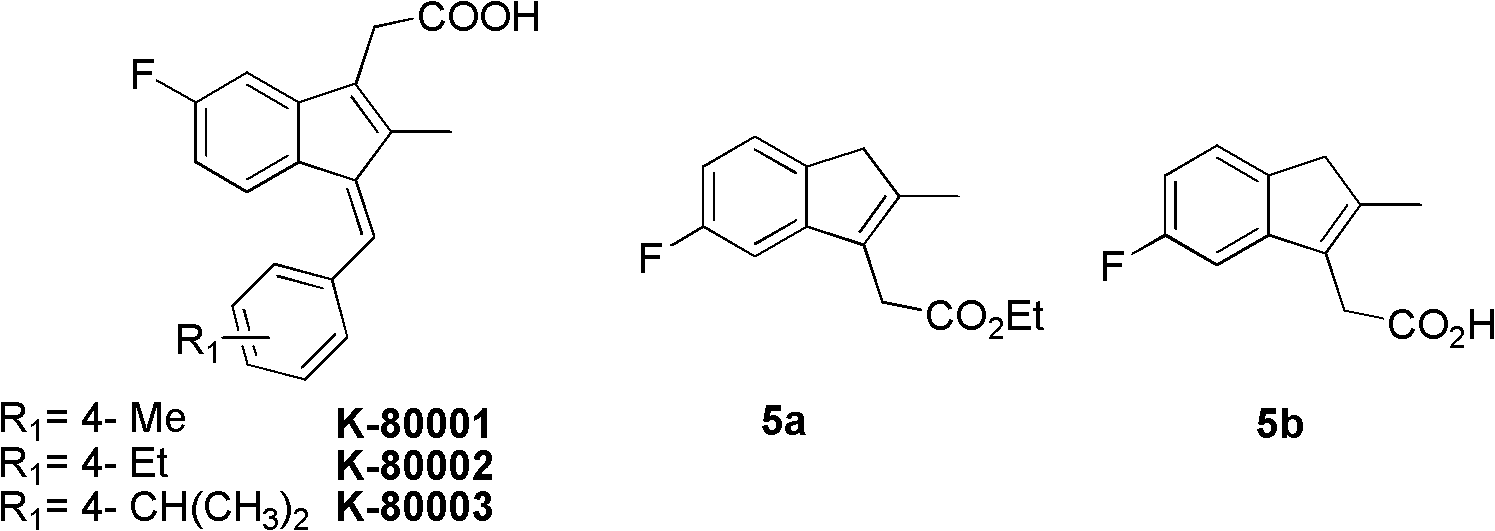
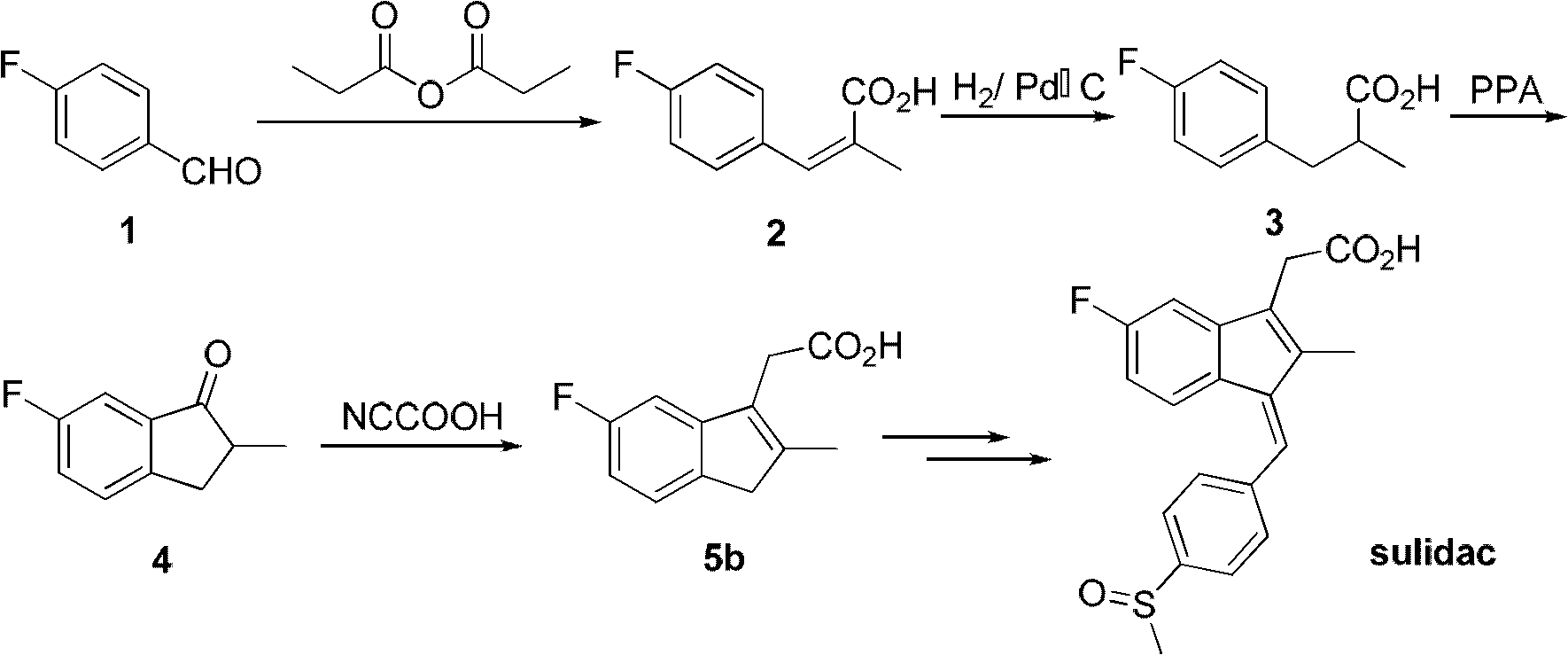
Synthesis method and application of intermediate of sulindac analogue
InactiveCN102030642AReduce dosageAvoid it happening againOrganic compound preparationCarboxylic acid esters preparationKetoneSolvent
The invention discloses a synthesis method and application of intermediates of a sulindac analogue, relating to intermediates of a sulindac analogue. The intermediates are 5-fluoro-2-metyl-3-indene ethyl acetate (5a) and 5-fluoro-2-metyl-3-indene acetic acid (5b). The synthesis method comprises the following steps of: subjecting 4-fluorobenzaldehyde as an initial raw material, propionic anhydride as a solvent and propionic anhydride to a perkin reaction to obtain 4-fluoro-2-metyl-methylcinnamic acid; catalyzing the 4-fluoro-2-metyl-methylcinnamic acid with palladium carbon with the palladium content of 5-20 percent and reducing in the hydrogen gas atmosphere to obtain 3-(4-fluorine phenyl)-2-methyl propionate; subjecting the 3-(4-fluorine phenyl)-2-methyl propionate to the intramolecilar friedel-crafts acyl browning reaction under the action of polyphosphoric acid under the heating condition to form 6-fluoro-2-methyl indene ketone; and subjecting the 6-fluoro-2-methyl indene ketone and halogenated acetate to reformatsky reaction under the action of the activated zinc powder to obtain a crude product and eliminating the crude product in an acid solution to obtain 5-fluoro-2-metyl-3-indene acetate. The intermediates can be used for preparing novel sulindac analogues with anticancer activity.
Owner:XIAMEN UNIV
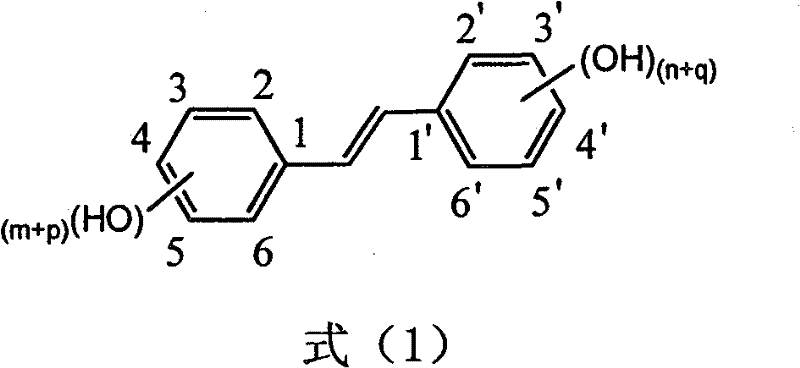
Preparation of trans-polyhydroxy diphenyl ethylene
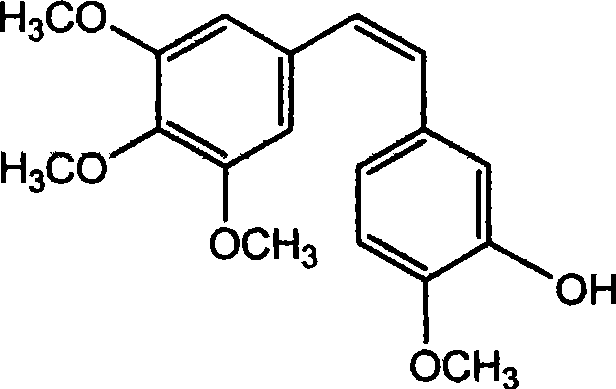
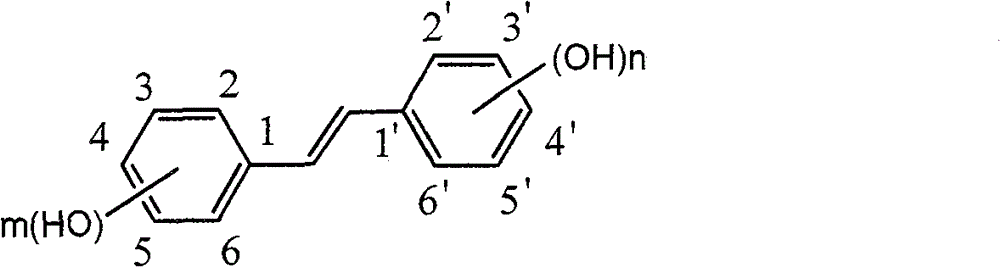
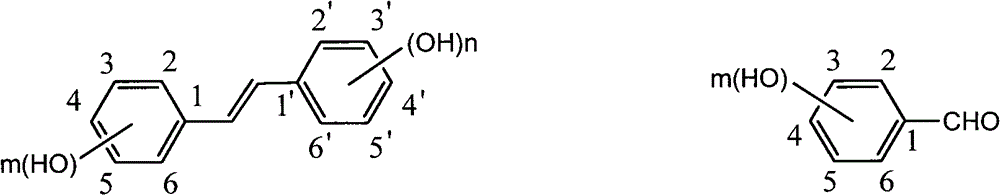
InactiveCN101440023ANo protectionEasy to operateOrganic chemistryOrganic compound preparationIsomerizationBenzaldehyde
The invention discloses a method for preparing trans-form polyhydroxy phenethylene. The method uses (bromo) hydroxy benzaldehyde and (bromo) hydroxyphenylacetic acid as raw materials, utilizes Perkin reaction to construct a syn-form diphenyl ethylene skeleton, and then obtains the trans-form polyhydroxy phenethylene through functional group conversion and decarboxylation-isomerization reaction. The method has the advantages of simple operation, mild reaction conditions, good atom economy, high trans-form selectivity, no need of hydroxyl protection, easy purification for the products, short synthetic route, high yield, low cost, and the like, and has favorable industrialized application prospect.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Scopoletin labeled by stable isotope and synthetic method for scopoletin
ActiveCN105884733AThe synthesis steps are simpleHigh yieldIsotope introduction to heterocyclic compoundsStable Isotope LabelingPhenyl acetate
The invention belongs to the synthesis field of isotope labeled compound synthesis, and particularly relates to scopoletin labeled by stable isotope and a synthetic method for the scopoletin. The synthetic method is characterized in that scopoletin labeled acetylchloride-2-13 C is taken as a raw material, and the scopoletin labeled acetylchloride-2-13C reacts with 2,4-dihydroxyl-5-methoxybenzaldehyde to generate 2-aldehyde-4-methoxy-5-hydroxyl phenyl acetate; under the action of a basic catalyst, scopoletin-2-13C is generated through Perkin reaction. The synthetic method is simple in synthesis step, is gentle in reaction condition, is simple and convenient in operation, is high in product yield being 26% or higher, is capable of effectively synthesizing an isotope labeled coumarin derivative, so that a novel way is provided for synthesizing the isotope labeled coumarin derivative. Certain innovation and unique research significance are achieved on organic synthetic method.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +1
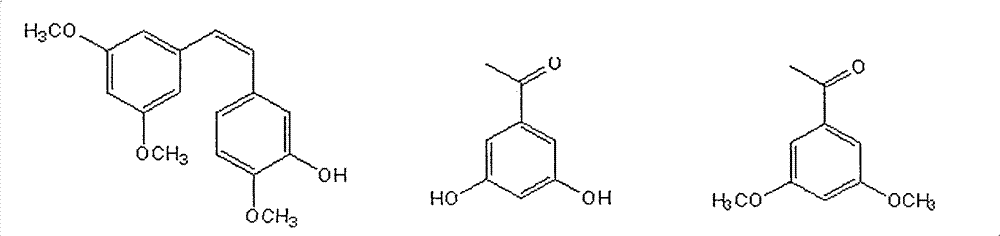
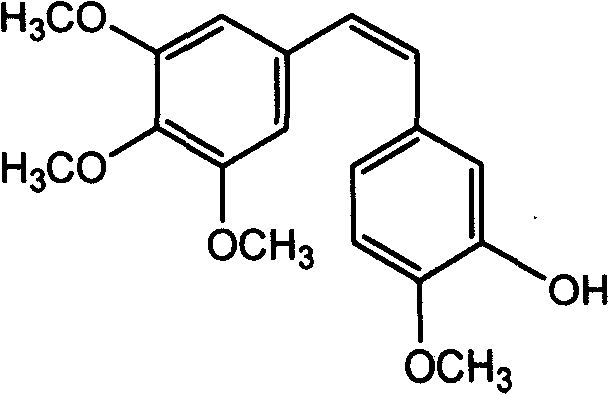
Method for preparing (Z)-3'-hydroxy-3,4',5-trimethoxy diphenylethene
InactiveCN101665419ALow priceLow costOrganic chemistryOrganic compound preparationHydrolysisMedicinal chemistry
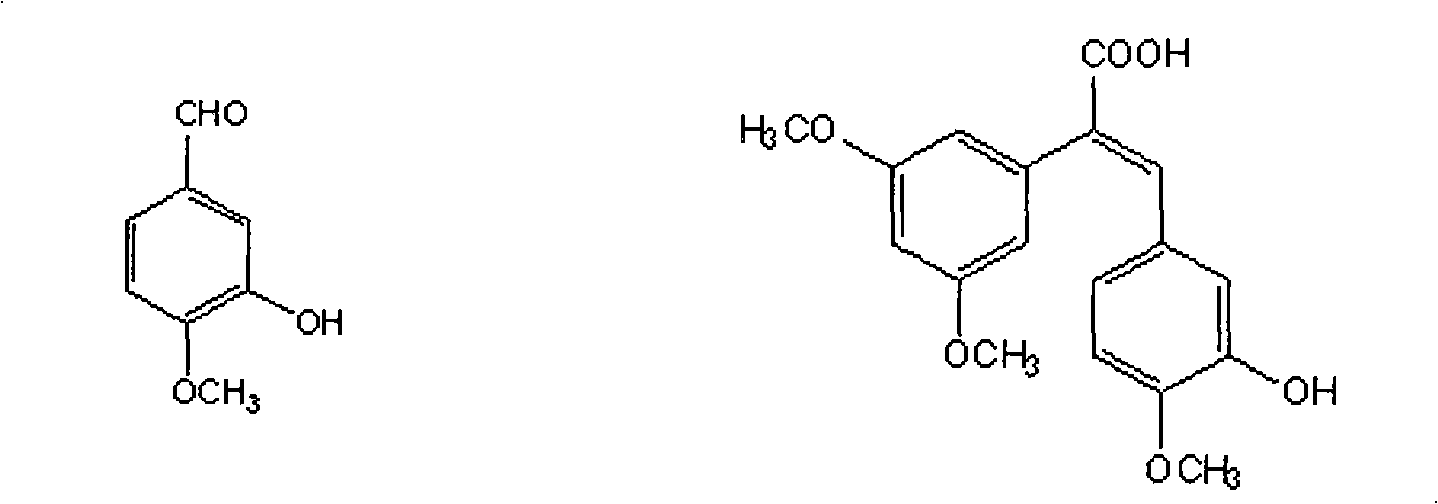
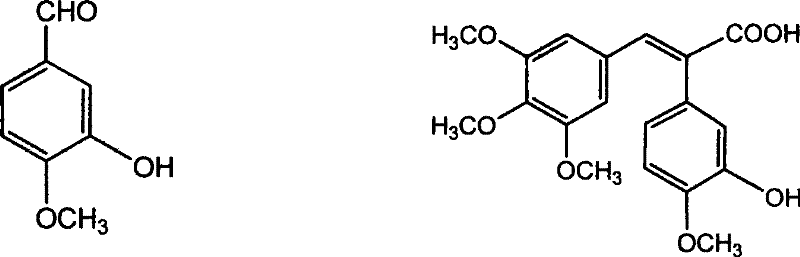
The invention discloses a method for preparing (Z)-3'-hydroxy-3,4',5-trimethoxy diphenylethene, comprising the following steps: taking 3,5-dihydroxyacetophenone as raw material, and then obtaining 3,5-dimethoxy hypnone by methylation reaction under the alkaline condition; then obtaining 3,5-dimethoxyphenylacetic acid by Willgerodt-Kindler rearrangement and hydrolysis reaction; enabling the 3,5-dimethoxyphenylacetic acid and isovanillin to carry out Perkin reaction to obtain (E)-2-(3',5'-dimethoxyphenyl)-3-(3'-hydroxy-4'-methoxyphenyl)crylic acid, and then obtaining the target compound, i.e. the (Z)-3'-oxhydryl-3,4',5-trimethoxy diphenylethene, by decarboxylic reaction. The raw materials of 3,5-dihydroxyacetophenone and isovanillin adopted by the invention have low price and are easy to obtain. The technical process has the advantages of simple operation, good cis-selectivity and high yield.
Owner:中科检测技术服务(广州)股份有限公司
Method for preparing trans polyhydroxystilbene compounds
InactiveCN101774894AReduce pollutionSimple post-processingOrganic chemistryOrganic compound preparationAcetic acidIsomerization
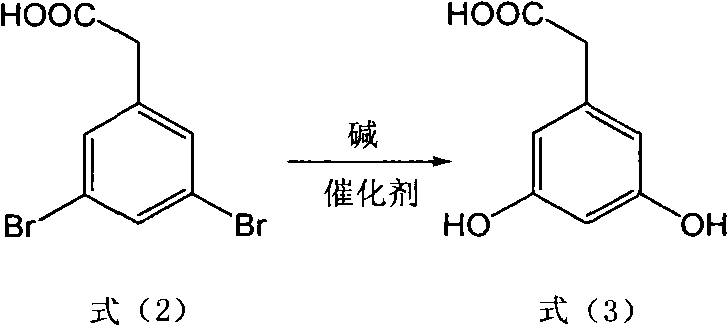
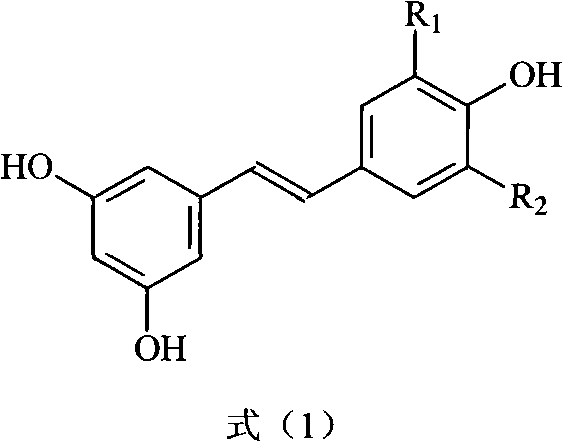
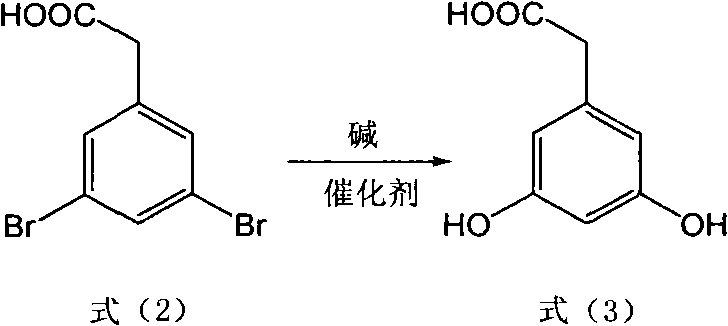
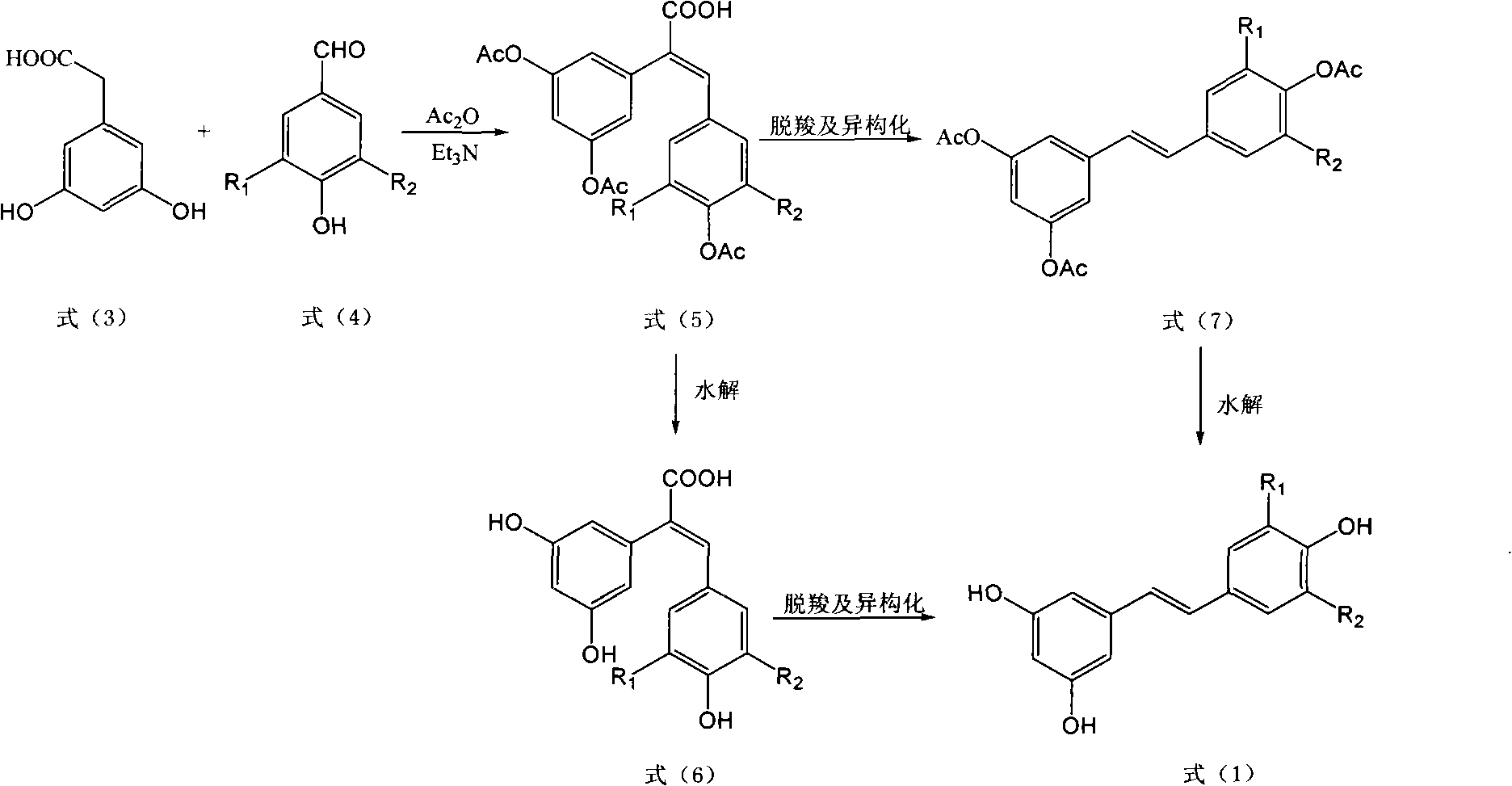
The invention discloses a method for preparing trans polyhydroxystilbene compounds. The method comprises the following steps: 3,5-dibromophenylacetic acid is converted into 3,5-dihydroxyphenyl acetic acid under alkali conditions;3,5-dihydroxyphenyl acetic acid and 4-hydroxybenzaldehyde compounds are subject to a Perkin reaction to obtain E-2-(3,5-diacetyl phenyl)-3-(4'-acetoxyl phenyl)acrylic acid compounds; and the E-2-(3,5-diacetyl phenyl)-3-(4'- acetoxyl phenyl)acrylic acid compounds are subject to decarboxylation isomerization and hydrolysis reaction, or hydrolysis and decarboxylation isomerization reaction to obtain the trans polyhydroxystilbene compounds. The all-trans products obtained by using the method have the advantages of favorable atom economy, simple synthetic route, simpleaftertreatment, low cost, high yield and the like, and can easily realize large-scale preparation.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Preparation method of Sarpogrelate intermediate 2-((3-methoxy) phenethyl) phenol
InactiveCN102516043ASolve pollutionSimple processOrganic chemistryOrganic compound preparationAcetic anhydrideSalicylaldehyde
The invention aims to provide a preparation method of Sarpogrelate intermediate 2-((3-methoxy) phenethyl) phenol (I), which comprises the following steps of: 1) carrying out perkin reaction on 3-methoxyphenylacetic acid (II), salicylic aldehyde (III), organic base and acetic anhydride; 2) further obtaining 2-(3-methoxyphenyl)-3-(2-acetoxy-phenyl) acrylic acid (IV); 3) decarboxylating a compound IV under the existence of quinoline and metal copper to obtain 3-methoxy-2'-acetoxy stilbene (V); 4) hydrolyzing the compound IV to obtain 2-((3-methoxy) styryl) phenol (VI); and 5) carrying out catalytic hydrogenation on the compound IV to obtain a compound I. The technical scheme disclosed by the invention has the characteristics of simplicity in operation, mild reaction condition, no hydroxyl protection or deprotection required, easiness for product purification, short synthetic route, easiness for material obtaining, high yield, low cost and the like and has a wide industrial application prospect.
Owner:PKU HEALTHCARE CORP LTD
Method for preparing caffeic acid derivative
ActiveCN106146296AHigh yieldReduce manufacturing costOrganic compound preparationCarbonyl compound preparationAcetic anhydrideCaffeic acid
The invention discloses a method for preparing a caffeic acid derivative. A compound shown as the formula II serves as a raw material to be subjected to a substitution reaction with haloalkane to prepare a compound shown as the formula III, and the compound shown as the formula III and acetic anhydride are subjected to a Perkin reaction to prepare a compound shown as the formula I, namely, the target object caffeic acid derivative, wherein the structures of the compounds shown as the formula I, the formula II and the formula III are shown in the specification, wherein R represents methyl or cyclopropyl cyclopropyl. According to the method, the synthetic route is short, the product yield is high, adopted reagents are low in toxicity and safe, the production cost is low, and the method is suitable for large-scale industrial production.
Owner:KPC PHARM INC
Drug for preventing and treating pulmonary artery hypertension, synthesis and applications thereof
InactiveCN103819483AImprove stabilityImprove bioavailabilityOrganic chemistryRespiratory disorderBenzoic acidAcetic anhydride
The invention discloses a drug for preventing and treating pulmonary artery hypertension, synthesis and applications thereof. The drug has a structural formula (I) which is represented in the description, wherein the R1 and R2 are independently selected from -CH3 and -CH2CH3. The compound is prepared by the following steps: making phenylacetic acid derivatives and benzoic acid derivatives carry out a Perkin reaction in the presence of acetic anhydride and an alkaline catalyst so as to obtain an intermediate, and then making the intermediate carry out condensation reactions with isosorbide mononitrate so as to obtain the compound represented by the structural formula (I). The structure of a primer namely iso-resveratrol is modified so as to strengthen the stability, thus the biological utilization degree of the iso-resveratrol is increased and the drug has a long-acting effect; furthermore, the shortages of easy oxidation and difficult storage of resveratrol with hydroxyl structure are overcome.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
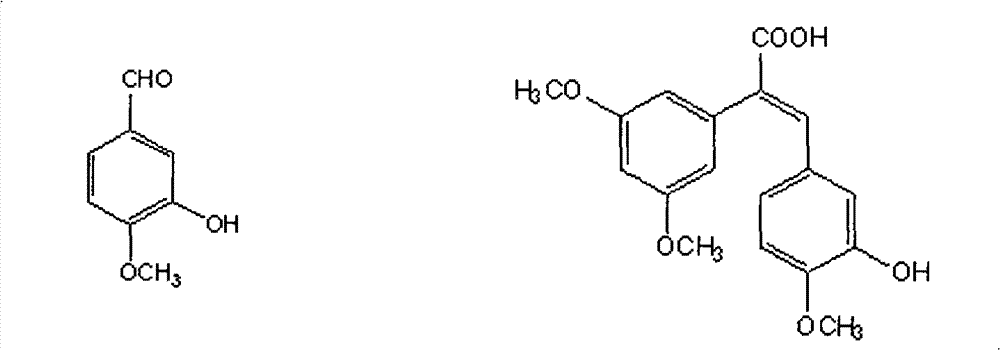
Preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene
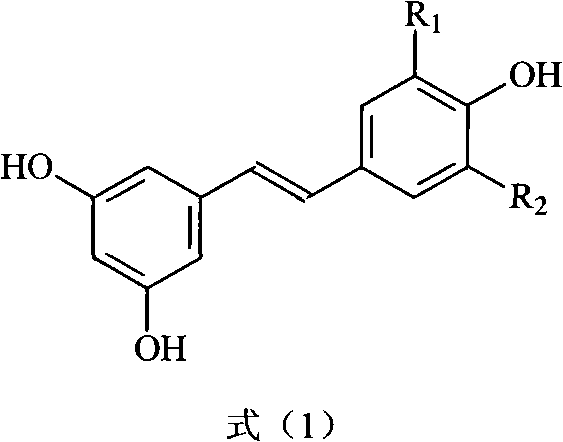
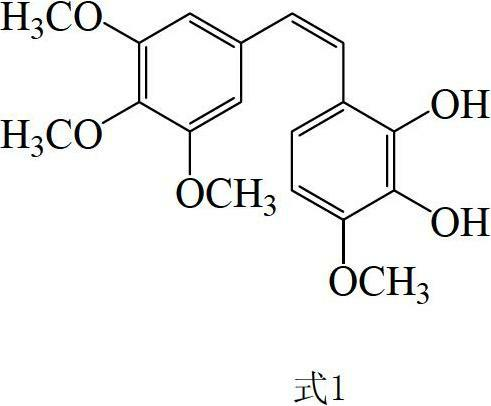
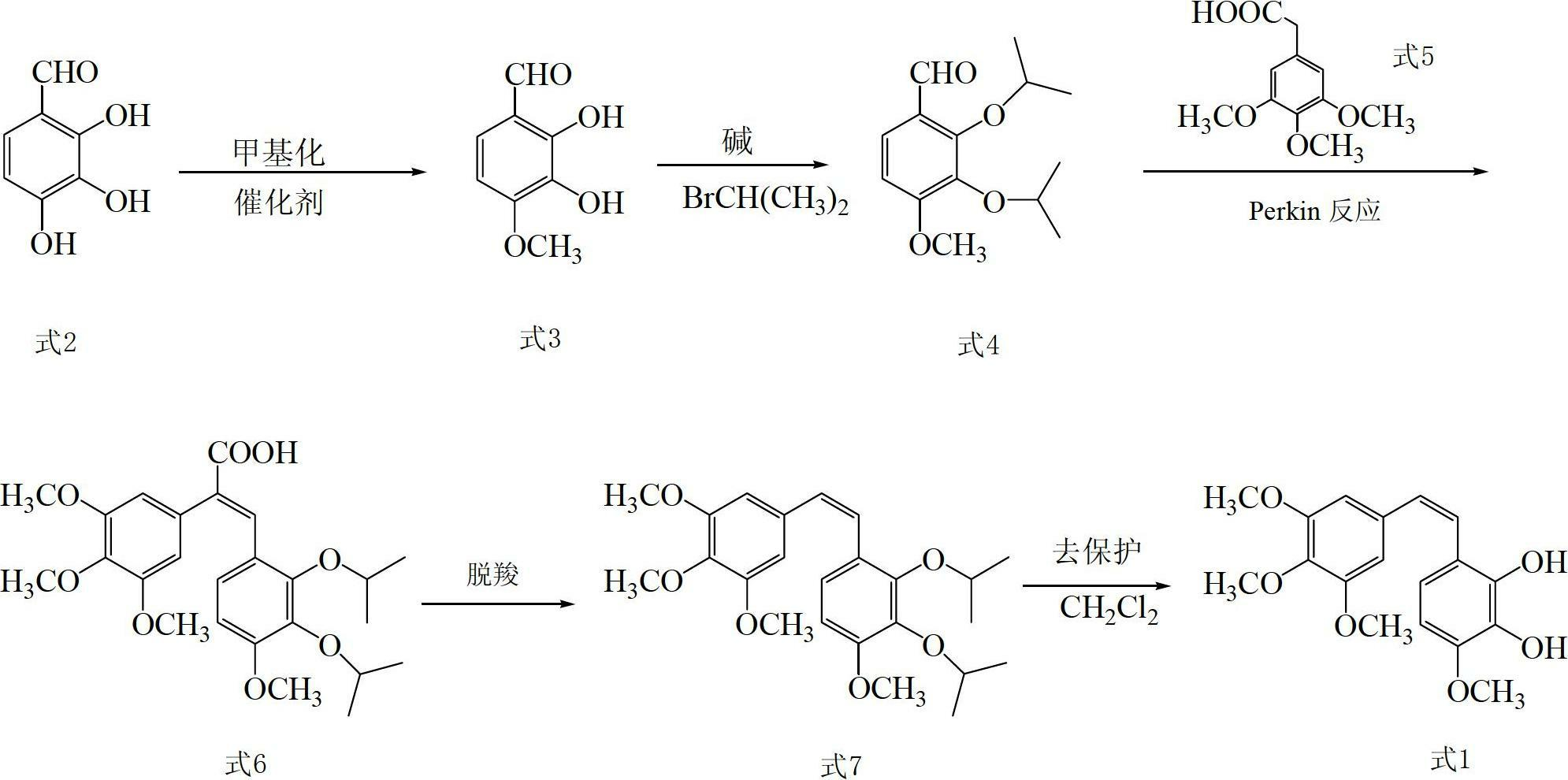
The invention discloses a preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene. The preparation method comprises the following steps of: taking 2,3,4-trihydroxy phenylfluorone as a raw material; carrying out single methylation on the 2,3,4-trihydroxy phenylfluorone; carrying out substitution reaction on the methylated 2,3,4-trihydroxy phenylfluorone with bromopropane so as to obtain 2,3-diisopropoxy-4-methoxybenzaldehyde; carrying out Perkin reaction on 2,3-diisopropoxy-4-methoxybenzaldehyde with 3,4,5-trimethoxyphenylacetic acid so as to obtain E-2-(3,4,5-trimethoxyphenyl)-3-(2',3'-diisopropoxy-4'-methoxyphenyl)acrylic acid; carrying out decarboxylation to obtain Z-3,4,4',5-tetramethoxy-2',3'-diisopropoxy diphenylethylene; and finally obtaining Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene after carrying out deprotection. The synthetic route provided by the invention is simple and direct, cis-selectivity is higher, and the yield of the Perkin reaction as well as the total yield of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene are both higher. The preparation method disclosed by the invention has the characteristics of simpleness and feasibility for operation, low price and easy obtainment of used raw materials and reagents, small pollution on environment, good atom economy, low cost and capability of being applied to industrialized production.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
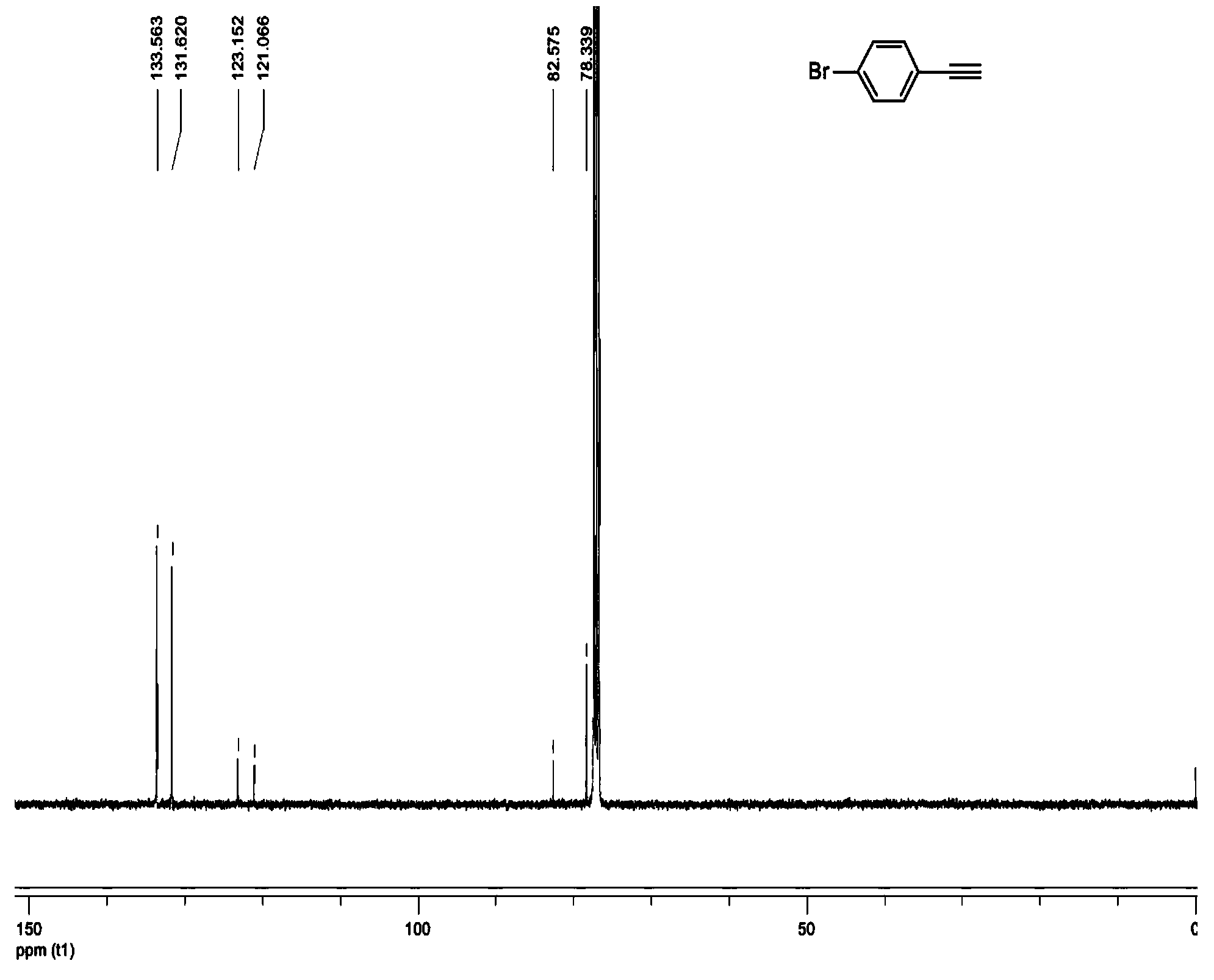
New synthetic method of 4-halogenated phenylacetylene
InactiveCN104262085AHigh purityThe synthetic method is green and environmentally friendlyPreparation by hydrogen halide split-offOrganic compound preparationAcetic anhydrideBenzaldehyde
The invention discloses a new synthetic method of 4-halogenated phenylacetylene, which comprises the following steps: taking 4-halogenated benzaldehyde and acetic anhydride which are cheap and are easy to obtain as raw materials and obtaining the 4-halogenated benzaldehyde which is a final target product through Perkin reaction, bromination reaction and debromination, decarboxylation and elimination reaction. The new synthetic method has the benefits that as the 4-halogenated benzaldehyde and the acetic anhydride are taken as substrates, and an environmental protection solvent with low toxicity is adopted in the reaction process, the new synthetic method has the characteristics of greenness and environmental protection, high efficiency, low cost, reasonable yield, mild reaction conditions and easiness in control; through the optimization of the reaction conditions and a post-processing method, in particular to an innovative application of a sublimation method to product purification, the new synthetic method disclosed by the invention also has the characteristic of high product purity.
Owner:NANJING XIAOZHUANG UNIV
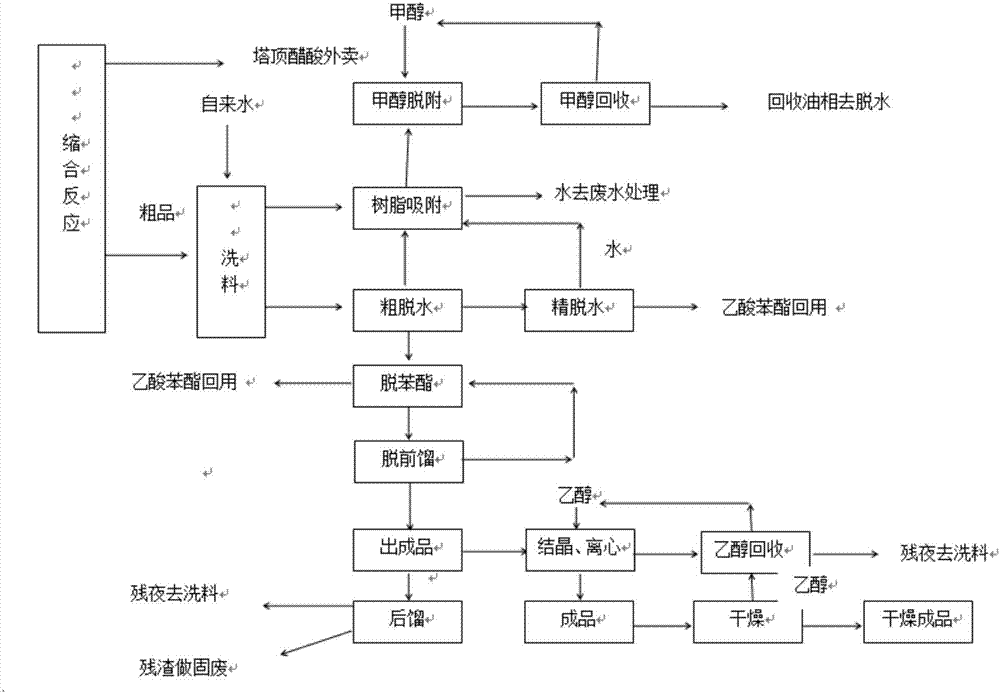
Refining method of coumarin crude product
InactiveCN106866598AQuality assuranceEfficient yieldOrganic chemistryPurification methodsProcess conditions
The invention provides a refining method of a coumarin crude product. The refining method comprises the following steps of first mixing the coumarin crude product prepared through a perkin reaction and water, so as to obtain a water-containing coumarin crude product; afterwards, subjecting the water-containing coumarin crude product to a coarse water removal process, a phenyl ester removal process, before-removal rectification process and a finished product outputting process in sequence through continuous rectification, so as to obtain a coumarin pure product. According to the refining method, through controlling process conditions in the coarse water removal process, the phenyl ester removal process, the before-removal rectification process and the finished product outputting process, a purification method provided by the invention can be used for realizing the continuous rectification; not only are the quality and the yield of a coumarin rectified product guaranteed, but also the high efficiency and the energy conservation of the rectification are guaranteed.
Owner:成都建中香料香精有限公司
Method for preparing trans polyhydroxystilbene compounds
InactiveCN101774894BReduce pollutionSimple post-processingOrganic chemistryOrganic compound preparationPhenyl acetic acidIsomerization
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Preparation method of gliclazide
PendingCN113527155AMild route conditionsReduce manufacturing costSulfonic acid amide preparationNitrosoMannich reaction
The invention relates to the field of medicine synthesis, in particular to a preparation method of gliclazide and an intermediate of gliclazide. The preparation method comprises a Mannich reaction, an amino protection reaction, a Perkin reaction, an epoxidation reaction, a hydrolysis reaction, a decarboxylation reaction and a reductive amination reaction. The preparation method is mild in reaction condition, green and environment-friendly, few three wastes are generated, a high-risk reducing agent does not need to be used, nitrosation reaction does not need to be carried out, and introduction of nitroso-nitroso impurities is successfully avoided.
Owner:浙江四维医药科技有限公司
Drugs for prevention and treatment of pulmonary arterial hypertension and their synthesis and application
InactiveCN103819483BImprove stabilityImprove bioavailabilityOrganic chemistryRespiratory disorderBenzoic acidAcetic anhydride
The invention discloses a drug for preventing and treating pulmonary artery hypertension, synthesis and applications thereof. The drug has a structural formula (I) which is represented in the description, wherein the R1 and R2 are independently selected from -CH3 and -CH2CH3. The compound is prepared by the following steps: making phenylacetic acid derivatives and benzoic acid derivatives carry out a Perkin reaction in the presence of acetic anhydride and an alkaline catalyst so as to obtain an intermediate, and then making the intermediate carry out condensation reactions with isosorbide mononitrate so as to obtain the compound represented by the structural formula (I). The structure of a primer namely iso-resveratrol is modified so as to strengthen the stability, thus the biological utilization degree of the iso-resveratrol is increased and the drug has a long-acting effect; furthermore, the shortages of easy oxidation and difficult storage of resveratrol with hydroxyl structure are overcome.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene
The invention discloses a preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene. The preparation method comprises the following steps of: taking 2,3,4-trihydroxy phenylfluorone as a raw material; carrying out single methylation on the 2,3,4-trihydroxy phenylfluorone; carrying out substitution reaction on the methylated 2,3,4-trihydroxy phenylfluorone with bromopropane so as to obtain 2,3-diisopropoxy-4-methoxybenzaldehyde; carrying out Perkin reaction on 2,3-diisopropoxy-4-methoxybenzaldehyde with 3,4,5-trimethoxyphenylacetic acid so as to obtain E-2-(3,4,5-trimethoxyphenyl)-3-(2',3'-diisopropoxy-4'-methoxyphenyl)acrylic acid; carrying out decarboxylation to obtain Z-3,4,4',5-tetramethoxy-2',3'-diisopropoxy diphenylethylene; and finally obtaining Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene after carrying out deprotection. The synthetic route provided by the invention is simple and direct, cis-selectivity is higher, and the yield of the Perkin reaction as well as the total yield of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene are both higher. The preparation method disclosed by the invention has the characteristics of simpleness and feasibility for operation, low price and easy obtainment of used raw materials and reagents, small pollution on environment, good atom economy, low cost and capability of being applied to industrialized production.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
4-aryl coumarin compound and preparation method and application thereof
InactiveCN101967135BHigh yieldSimple and fast operationAntibacterial agentsOrganic chemistryBenzaldehydeElimination reaction
The invention discloses a 4-aryl coumarin compound and a preparation method and application thereof. The 4-aryl coumarin compound has a structure shown in a formula (I). The preparation method comprises the following steps: taking substituted benzaldehyde and propandioic acid as raw materials, heating under condition of existing pyridine and piperidine, and generating Perkin reaction and decarboxylic reaction to obtain a series of substituted phenylacrylic acid compounds; carrying out bromination and elimination reaction on the substituted phenylacrylic acid compounds or acetylation to protect a phenolic hydroxyl group, and then carrying out bromination and elimination reaction to obtain substituted phenylpropiolic acid compounds; and enabling the substituted phenyl propargylic acid compounds and phenolic compounds to react under the catalysis of boron trifluoride etherate and phosphorus oxychloride or trifluoroacetic acid to obtain a series of 4-aryl coumarin compounds. The compounds can be used for preparing antineoplastic medicine, anti-abnormal-angiogenesis medicine, antimicrobial medicine, antioxidation medicine and antimalarial medicine.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Preparation of trans-polyhydroxy diphenyl ethylene
InactiveCN101440023BNo protectionEasy to operateOrganic chemistryOrganic compound preparationIsomerizationBenzaldehyde
The invention discloses a method for preparing trans-form polyhydroxy phenethylene. The method uses (bromo) hydroxy benzaldehyde and (bromo) hydroxyphenylacetic acid as raw materials, utilizes Perkin reaction to construct a syn-form diphenyl ethylene skeleton, and then obtains the trans-form polyhydroxy phenethylene through functional group conversion and decarboxylation-isomerization reaction. The method has the advantages of simple operation, mild reaction conditions, good atom economy, high trans-form selectivity, no need of hydroxyl protection, easy purification for the products, short synthetic route, high yield, low cost, and the like, and has favorable industrialized application prospect.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Method for preparing (Z)-3'-hydroxy-3,4',5-trimethoxy diphenylethene
InactiveCN101665419BLow priceLow costOrganic chemistryOrganic compound preparationHydrolysisMedicinal chemistry
Owner:中科检测技术服务(广州)股份有限公司
Carboxyl substituted resveratrol analog compound and its preparation method
The resveratrol compound with carboxyl substituent has general formula as following, wherein, R acts for hydrogen, hydroxyl or nitryl. The opposite preparation method comprises: using Perkin reaction to the 3, 5-dimethoxylphenylacetic acid with opposite p-substituting R-benzaldehyde; removing the methoxy protection to obtain the product as the derivative of 1, 2-toluylene. This invention is similar to the resveratrol as 3, 4', 5-trihydroxy -trans-stilbene, and has wide application for antitumor and cardiovascular protection.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Method of preparing (Z)-3'-hydroxyl-3,4,4',5-tetramethoxy toluylene
InactiveCN101402555BGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationNatural sourceMandelic acid
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Method for preparing (Z)-3'-hydroxy-3,4,4',5-tetramethoxy diphenyl ethylene from regenerative natural plant resource
InactiveCN101353296BGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
The invention discloses a preparation method for (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. A diphenyl ethylene framework structure is built by the Perkin reaction method, and natural aniseed fat-soluble components and propenyl anisole (anethole) are taken as raw materials, and oxidized to obtain anisaldehyde; dichlorocarbene insertion reaction is carried out on the anisaldehyde toobtain p-methoxyl-mandelic acid which is reduced to obtain methoxyl-phenylacetic acid, the methoxyl-phenylacetic acid is brominized to obtain 3-bromo-4-methoxyl-phenylacetic acid. The compound and the natural 3,4,5-trimethoxybenzaldehyde (nutgall extract derivative) carry out Perkin reaction to build a cis-form diphenyl ethylene framework which is further converted and decarboxylated by functional groups to obtain the (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. The raw materials of the invention are reproducible natural resources-anethole which are rich in China and 3,4,5-trimethoxybenzaldehyde, and replace non-renewable petrochemical materials which are used by the prior art and become less and less so that the method has the advantages of good sustainable development capability as well as remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Preparation of trans-polyhydroxy diphenyl ethylene
InactiveCN101481300BEasy to operateMild reaction conditionsOrganic chemistryOrganic compound preparationIsomerizationPhenylacetic acid
The invention discloses a preparation method of trans-polyhydroxy diphenyl ethylene. The method comprises the following steps: taking hydroxy-substituted benzaldehyde and hydroxy-substituted phenylacetic acid as raw materials, building up a cis-diphenyl ethylene skeleton by a Perkin reaction, and obtaining the trans-polyhydroxy diphenyl ethylene by decarboxylation and isomerization reaction. The preparation method has the advantages of simple operation, mild reaction condition, good atom economy, high trans-form selectivity, no need of hydroxyl protection and deprotection, easy purification of products, short synthetic route, higher yield, low cost and the like, so the method has good industrialized application prospect.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com