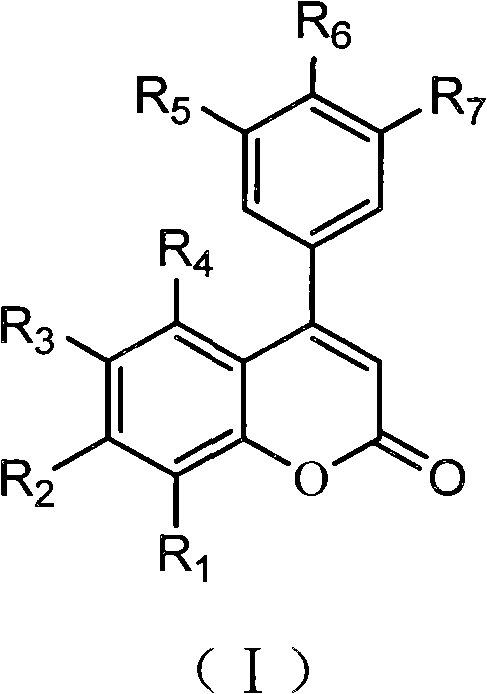
4-aryl coumarin compound and preparation method and application thereof
A technology of aryl coumarin and compound is applied in the field of 4-aryl coumarin compound and its preparation, and can solve problems such as just starting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
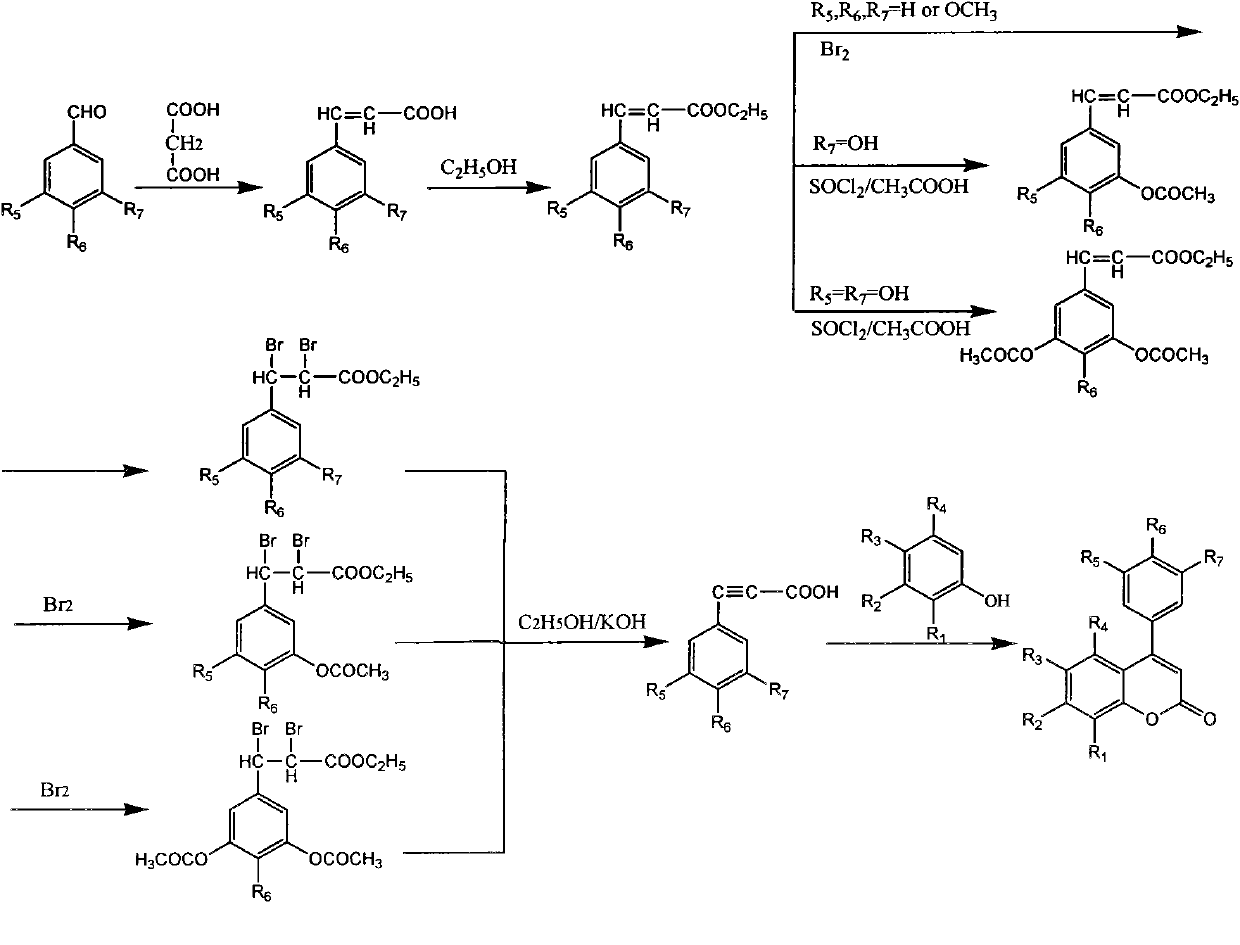
[0037] Preparation of 3,4-dimethoxyphenylacrylic acid
[0038] Add 2.08g (0.02mol) of malonic acid, 2.8g (0.017mol) of 3,4-dimethoxybenzaldehyde, 3mL of pyridine, and 0.3mL of hexahydropyridine into a 100mL double-necked reaction flask, and heat to 95°C Reflux for 3 hours, after the reaction is complete, cool for 10 minutes, add 50 mL of 10 mol / L hydrochloric acid solution, let stand for 2 hours, filter with suction, and wash the precipitate with 200 mL of water to obtain crude 3,4-dimethoxyphenylacrylic acid. Then recrystallized with absolute ethanol to obtain 3.1 g of pure 3,4-dimethoxyphenylacrylic acid, with a yield of 88.3%.
Embodiment 2
[0040] Preparation of 3-Hydroxy-4-Methoxyphenyl Acrylic Acid
[0041]Add 12.98g (0.125mol) of malonic acid, 12.65g (0.083mol) of 3-hydroxy-4-methoxybenzaldehyde, 15mL of pyridine, and 0.5mL of hexahydropyridine into a 100mL double-necked reaction flask, and heat to 95°C After reflux for 3 hours, the reaction was completed, cooled for 60 minutes, added 50 mL of 3mol / L hydrochloric acid solution, left for 10 hours, filtered with suction, and washed the solid with water to obtain crude 3-hydroxy-4-methoxyphenylacrylic acid. Then recrystallized from absolute ethanol to obtain 14.63 g of pure 3-hydroxy-4-methoxyphenylacrylic acid, with a yield of 90.6%.
Embodiment 3
[0043] Preparation of 3-Hydroxy-4-methoxyphenylpropiolic acid
[0044] Dissolve 1.94g (10mmol) of 3-hydroxy-4-methoxyphenylacrylic acid obtained in Example 2 in 30mL of absolute ethanol, add 1mL of SOCl 2 Heat to reflux for 2 hours, stop heating, concentrate the reaction solution, add water, the product precipitates, place it, and filter with suction to obtain crude ethyl 3-hydroxy-4-methoxyphenyl acrylate. Dissolve the crude ethyl 3-hydroxy-4-methoxyacrylate in 30 mL of acetic acid, add 1 mL of SOCl 2 Heat to reflux. After reacting for 2 hours, the heating was stopped, and water was added, and the product precipitated naturally. After standing overnight, the product solidified and was filtered by suction to obtain crude ethyl 3-hydroxy-4-acetoxyphenyl acrylate. Dissolve ethyl 3-hydroxy-4-acetoxyphenyl acrylate in 20 mL of dichloromethane, add 0.5 mL of Br dropwise in ice bath 2 (9.8mmol), 5min added, stirred for 3h, washed the reaction solution, spin-dried, left overnight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com