Rhodamine-based fluorescent indicator for indicating aging degree of rubber and preparation method of indicator
A technology of aging degree and indicator, which is applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems that rubber products cannot be used, aging performance damages detection objects, etc., and achieves low production cost, low price, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
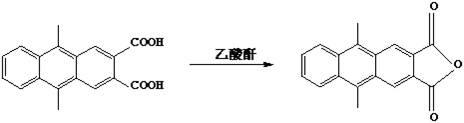
[0021] 1. Preparation of 9,10-dimethyl-2,3-anthracenedicarboxylic anhydride
[0022] Dissolve 8g of 9,10-dimethyl-2,3-anthracenedicarboxylic acid in 200mL of acetic anhydride, and react under reflux for 1 hour. After cooling to room temperature, 3.6 g of 9,10-dimethyl-2,3-anthracene dicarboxylic anhydride was obtained by filtration, with a yield of 60%. 1H NMR (CDCl 3 ): δ: 3.23 (s 6H), 7.72 7.75 (m, 2H), 8.42 8.46 (m, 2H), 9.12 (s, 2H). MS (EI ): m / z 276 (M ). Mp: 278℃ .
[0023]
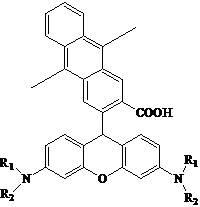
[0024] 2. Preparation of 9-[2-(3-carboxy-9,10-dimethyl)anthracene]-6-amino-3H-oxanthene-3-imine
[0025] 3g of 9,10-dimethyl-2,3-anthracene dicarboxylic anhydride and 6g of m-aminophenol were dissolved in 60mL of methanesulfonic acid, and reacted at 90°C for 9 hours under the protection of nitrogen. After cooling, the reaction solution was poured into 2000 mL of distilled water, and the precipitate was filtered out and vacuum-dried at 70°C for 20 hours to obtain a red powder. Dissolve this ...
Embodiment 2
[0028] 1. Preparation of 9,10-dimethyl-2,3-anthracenedicarboxylic anhydride
[0029] Dissolve 9g of 9,10-dimethyl-2,3-anthracenedicarboxylic acid in 300mL of acetic anhydride, and react under reflux for 2 hours. After cooling to room temperature, 4.6 g of 9,10-dimethyl-2,3-anthracene dicarboxylic anhydride was obtained by filtration, with a yield of 65%. 1H NMR (CDCl 3 ): δ: 3.23 (s 6H), 7.72 7.75 (m, 2H), 8.42 8.46 (m, 2H), 9.12 (s, 2H). MS (EI ): m / z 276 (M ). Mp: 278℃ .
[0030]
[0031] 2. Preparation of 9-[2-(3-carboxy-9,10-dimethyl)anthracene]-6-amino-3H-oxanthene-3-imine
[0032] 4g of 9,10-dimethyl-2,3-anthracene dicarboxylic anhydride and 8g of NN-diethyl-m-aminophenol were dissolved in 80mL of 98% sulfuric acid, and reacted at 70°C for 12 hours under the protection of nitrogen. After cooling, the reaction solution was poured into 2000 mL of distilled water, and the precipitate was filtered out and vacuum-dried at 65° C. for 24 hours to obtain a red powder. Di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com