3,4-dihydro-4-aryl coumarin compounds as well as preparation method and application thereof
A technology of aryl coumarin and phenolic compounds, which is applied in 3 fields, can solve the problems of severe reaction conditions, low yield, and use restrictions, and achieve the effect of high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 3, the preparation of 4-dimethoxyphenylacrylic acid:
[0029] Add 2.08g (0.02mol) malonic acid, 2.8g (0.017mol) 3,4-dimethoxybenzaldehyde, 3ml (0.037mol) pyridine, 0.3ml (0.003mol) six Hydropyridine, heated to 95°C and refluxed for 3 hours, the reaction was completed, cooled for 10 minutes, added 50ml of 3mol / L hydrochloric acid solution, left for 2 hours, filtered with suction, washed with 200ml of water to obtain the crude product; the crude product was recrystallized with absolute ethanol to obtain the pure product 3.1 g of 3,4-dimethoxyphenylacrylic acid, yield 88.3%.
Embodiment 2
[0031] Preparation of 3-hydroxy-4-methoxyphenylacrylic acid:
[0032] Add 12.98g (0.125mol) malonic acid, 12.65g (0.083mol) 3-hydroxyl-4-methoxybenzaldehyde, 15ml (0.168mol) pyridine, 1.5ml (0.015mol) in a 100ml double-necked reaction flask Hexahydropyridine, heated to 95°C and refluxed for 3 hours, after the reaction was completed, cooled for 10 minutes, added 50ml of 3mol / L hydrochloric acid solution, stood for 2 hours, filtered with suction, washed the precipitate with 1000ml of water, and obtained the crude product; the crude product was recrystallized with absolute ethanol to obtain pure Product 3-hydroxy-4-methoxyphenylacrylic acid 14.63g, yield 90.6%.
Embodiment 3
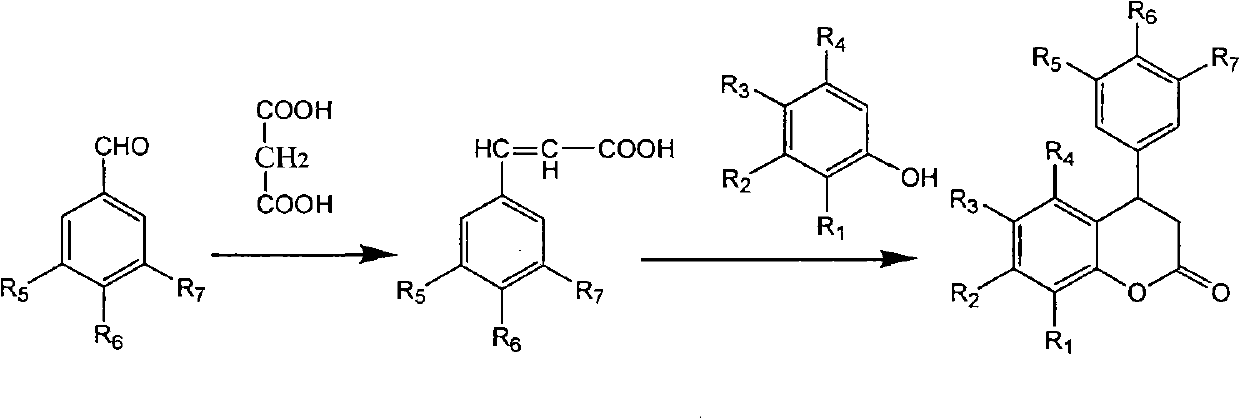
[0034] Preparation of 4-(3',4'-dimethoxyphenyl)-7-hydroxyl-3,4-dihydrocoumarin:
[0035] Place a 100ml double-necked reaction flask in an ice bath, then add 2.08g (0.01mol) 3,4-dimethoxyphenylacrylic acid, 1.1g (0.01mol) resorcinol, 3g (0.02mol) tris Phosphorus oxychloride, 5.6g (0.04mol) boron trifluoride ether, stir evenly, remove the ice bath after 10min, stir at room temperature (15-30°C), TLC monitors the reaction process (developing agent: petroleum ether: ethyl acetate = 2 : 1), after 12h, the reaction was stopped, poured into ice water, extracted with ether, spin-dried to remove the ether to obtain crude product; recrystallized with acetone and water to obtain pure product 4-(3', 4'-dimethoxyphenyl )-7-hydroxy-3,4-dihydrocoumarin 1.82g, yield 60.7%.
[0036] IR: 3429(OH), 1761(CO), 1626, 1597, 1516, 1462, 1419, 1335, 1271, 1244, 1159, 1103, 1024, 991, 847 and 812cm -1
[0037] 1HNMR (400MHz, CDCl 3 )δ: 2.98(2H, m, C-3H), 3.79and 3.84(6H, 2s, 3H and 3H each, 2×OCH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com