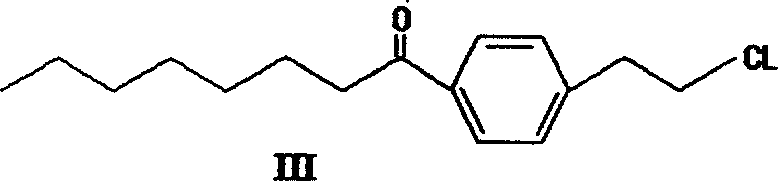
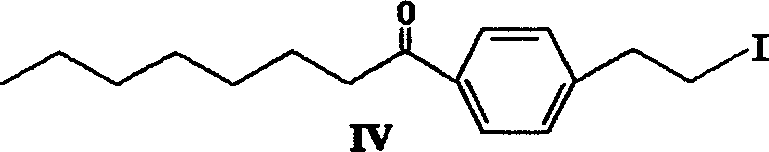
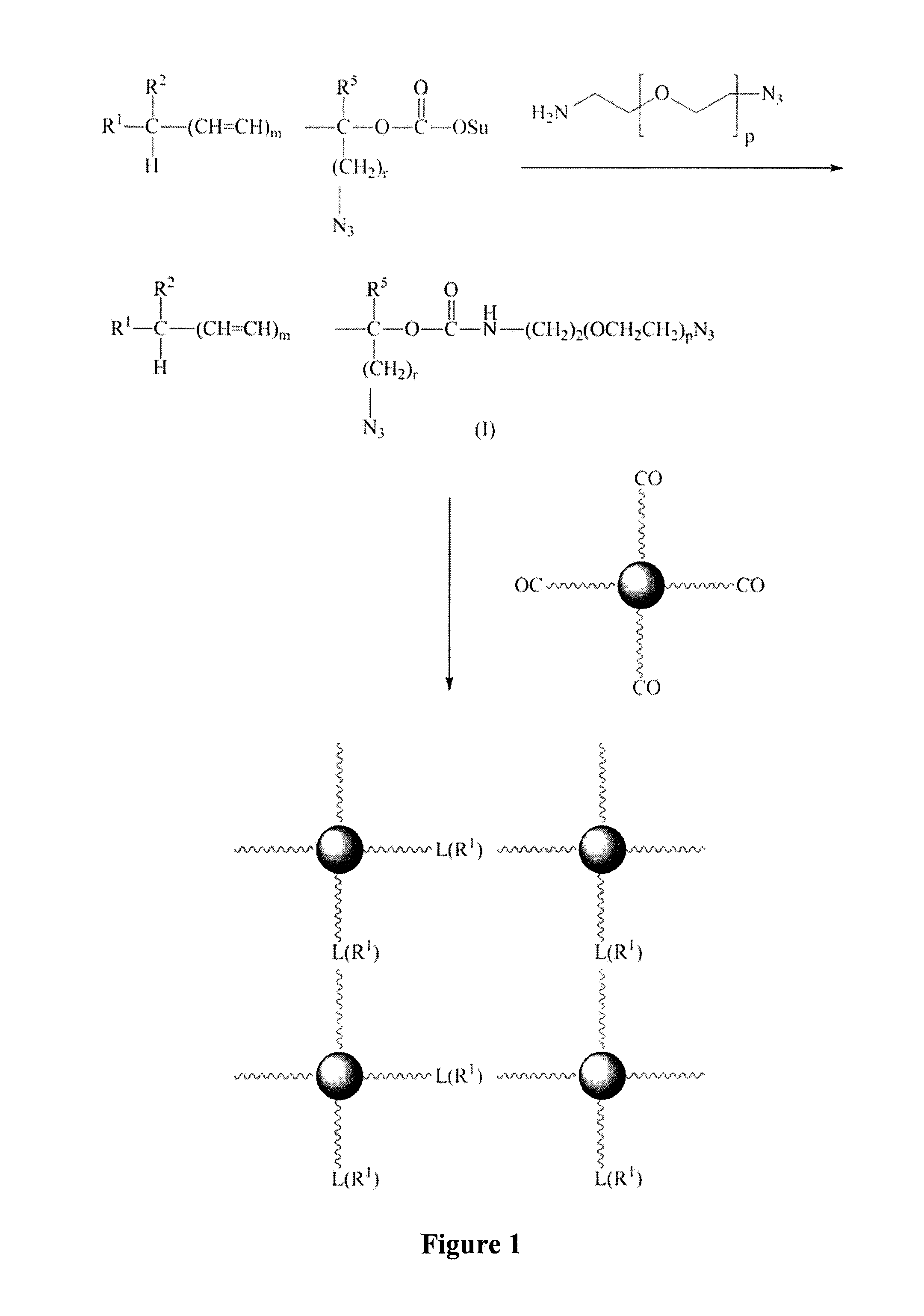
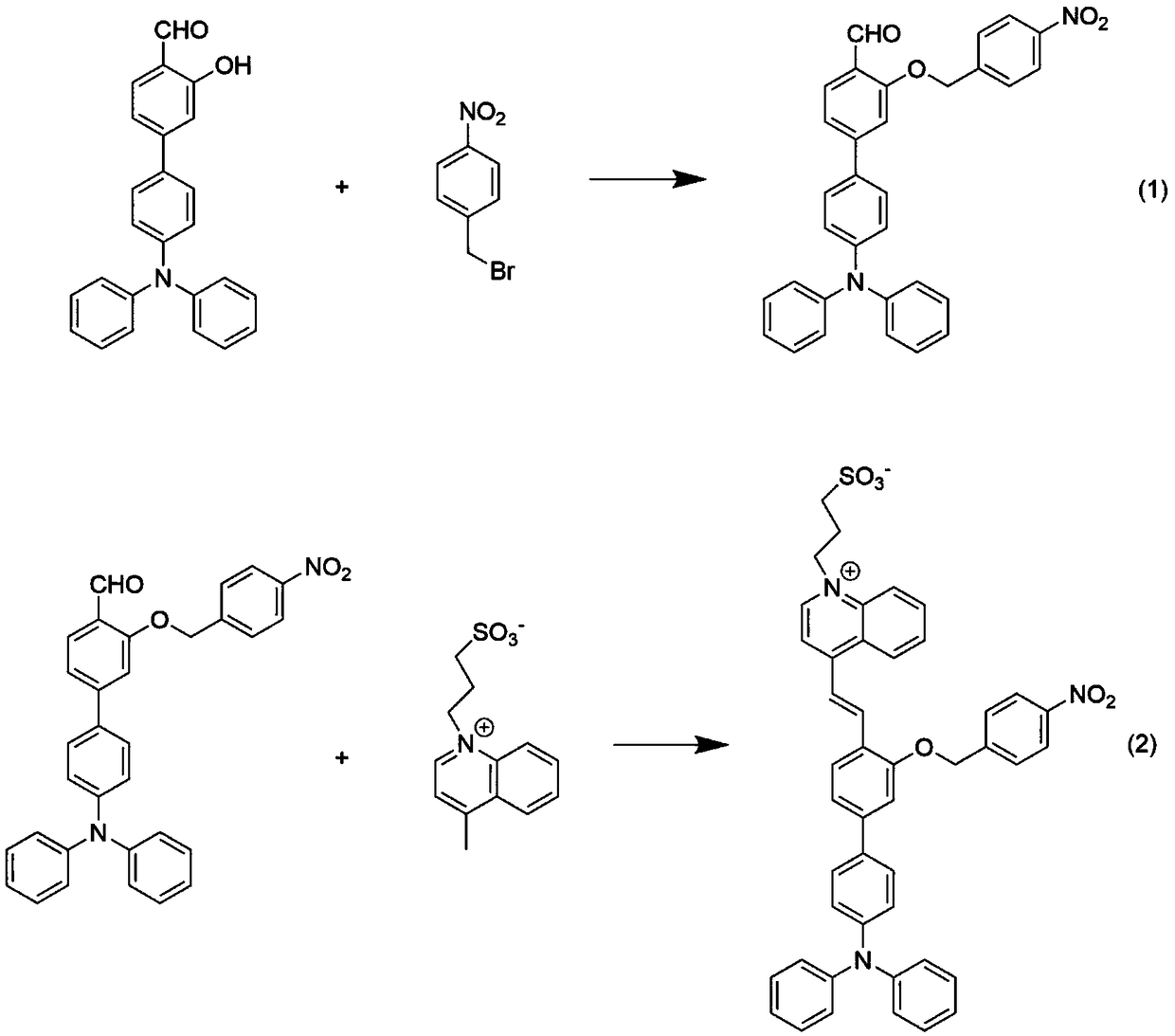
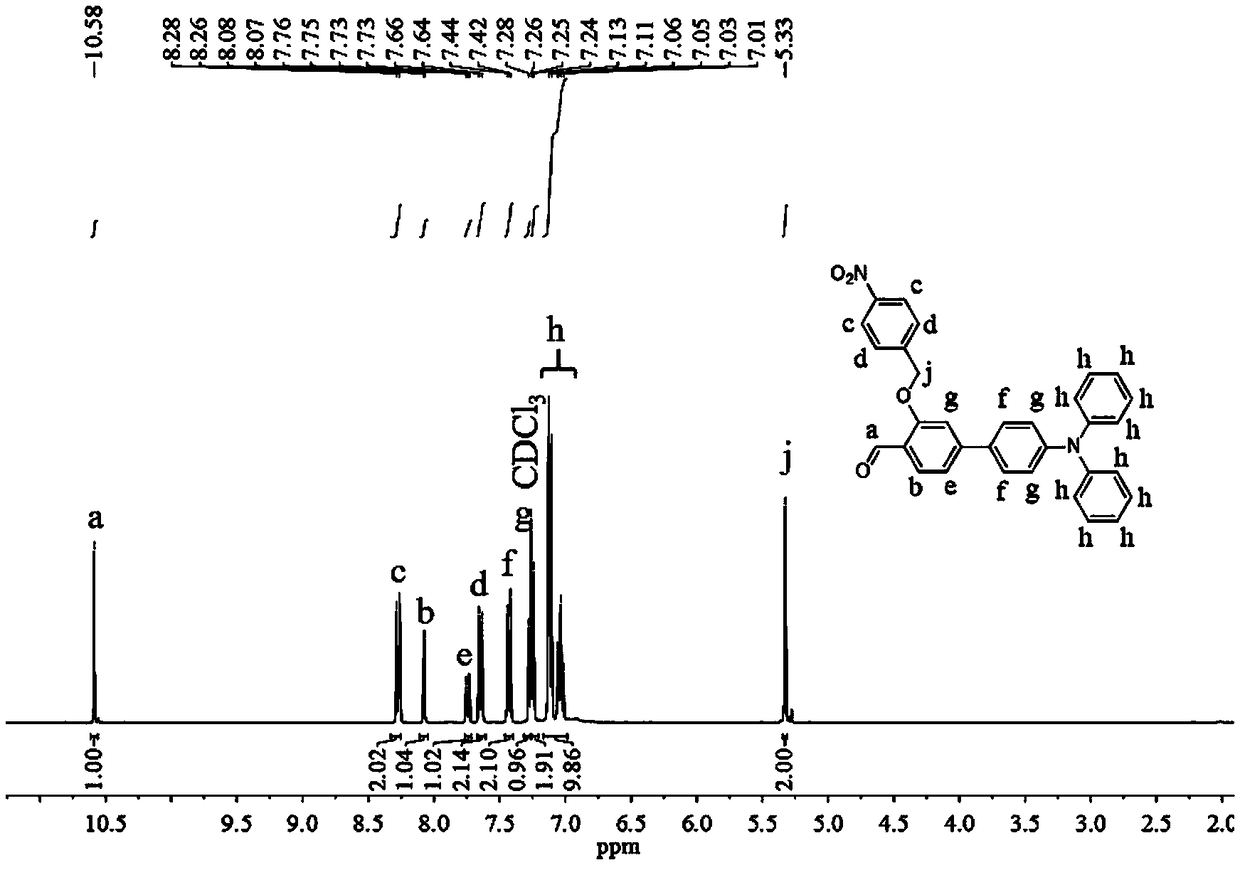
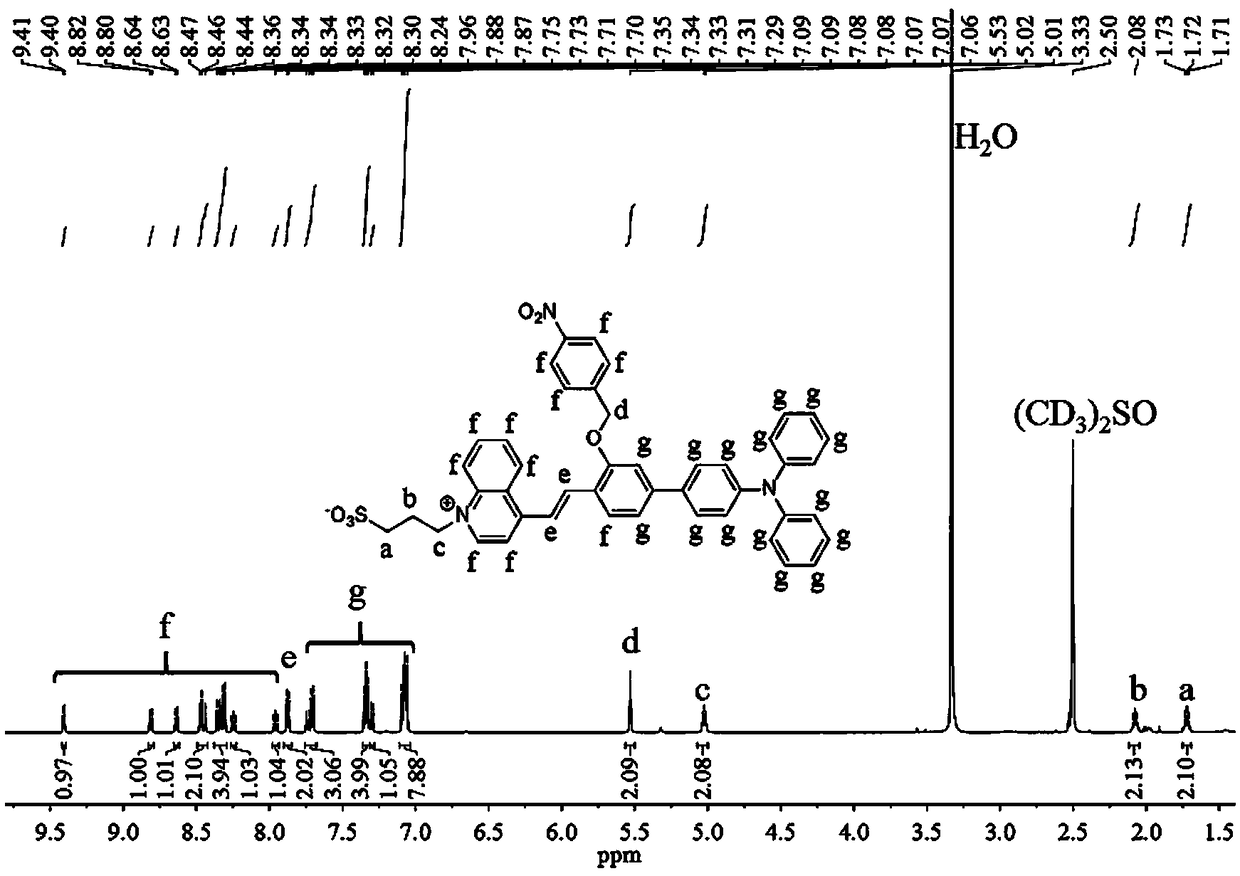
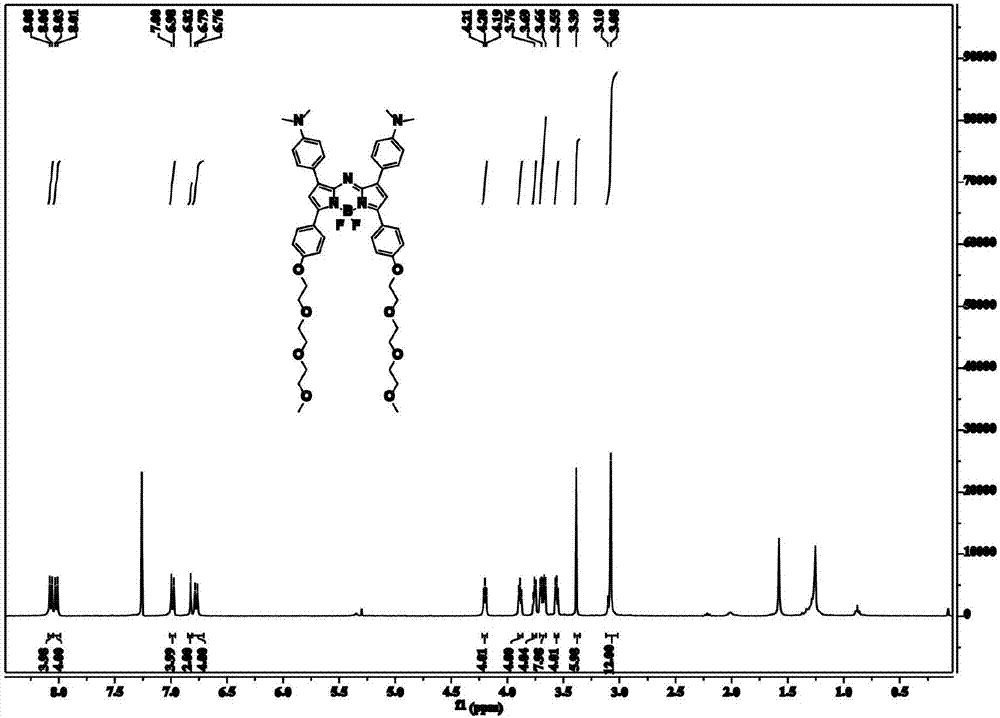
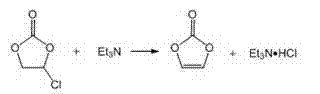
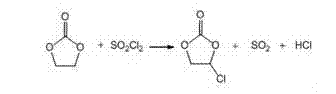
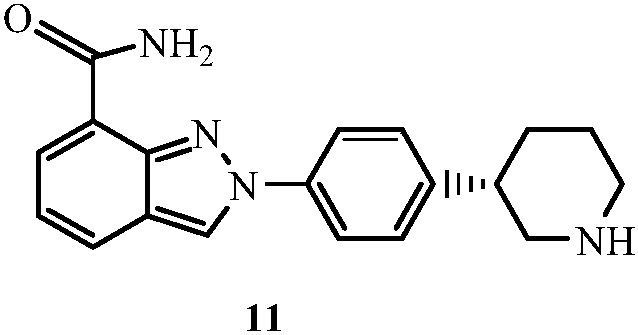
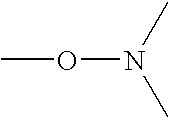Patents
Literature
574 results about "Elimination reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
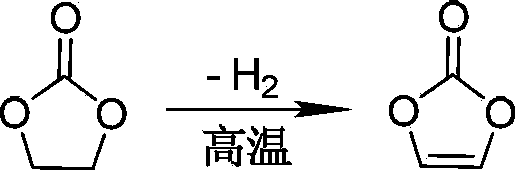
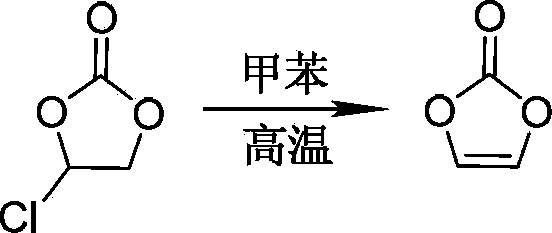
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Eᵢ mechanism.
Prepn process of hyperastable Y-type RE molecular sieve
InactiveCN1436728AExcellent anti-vanadium effectMolecular sieve catalystsFaujasite aluminosilicate zeoliteRare earthHigh activity
The present invention relates to the preparation of hyperstable Y-type RE molecular sieve for the catalytic cracking of heavy oil and containnig vanadium resisting component. The preparation uses NaY-type molecular sieve as main material and chemical aluminum-eliminating complex containing oxalic acid and / or oxalate and its mixture and through introducing RE ion in the late stage of chemical aluminum elimination reaction to from RE precipitate and hydrothermal treatment to realize hyperstabilizing and introduce RE ion and independent phase Re oxide. The formed precipitate RE precursor includes RE oxalate. Compared with conventional REY, REHY and REUSY, the molecular sieve preparing process is simple and high in RE utilization, and has homogeneously distributed aluminum, more secondary pores high hydrothermal stability, high activity and high vanadium contamination resisting capacity.
Owner:PETROCHINA CO LTD
Laser coding
InactiveUS6888095B2Sharp contrastElectric discharge heatingPharmaceutical containersChemical compoundLaser beam guide
A method for making an object, wherein the object comprises a material including a functional group and a metal compound or acid that causes an elimination reaction on irradiation with a laser, to form a reaction product of contrasting colour, comprises directing a laser beam on to the areas of the object to be marked. For example, by using a carbohydrate and a metal salt, effective marking can be achieved on the coating of a pill or other edible material.
Owner:DATALASE
Method for preparing semaglutide
InactiveCN106928343AEasy to operateNo side effectsPeptide-nucleic acidsPeptide preparation methodsSide reactionSemaglutide
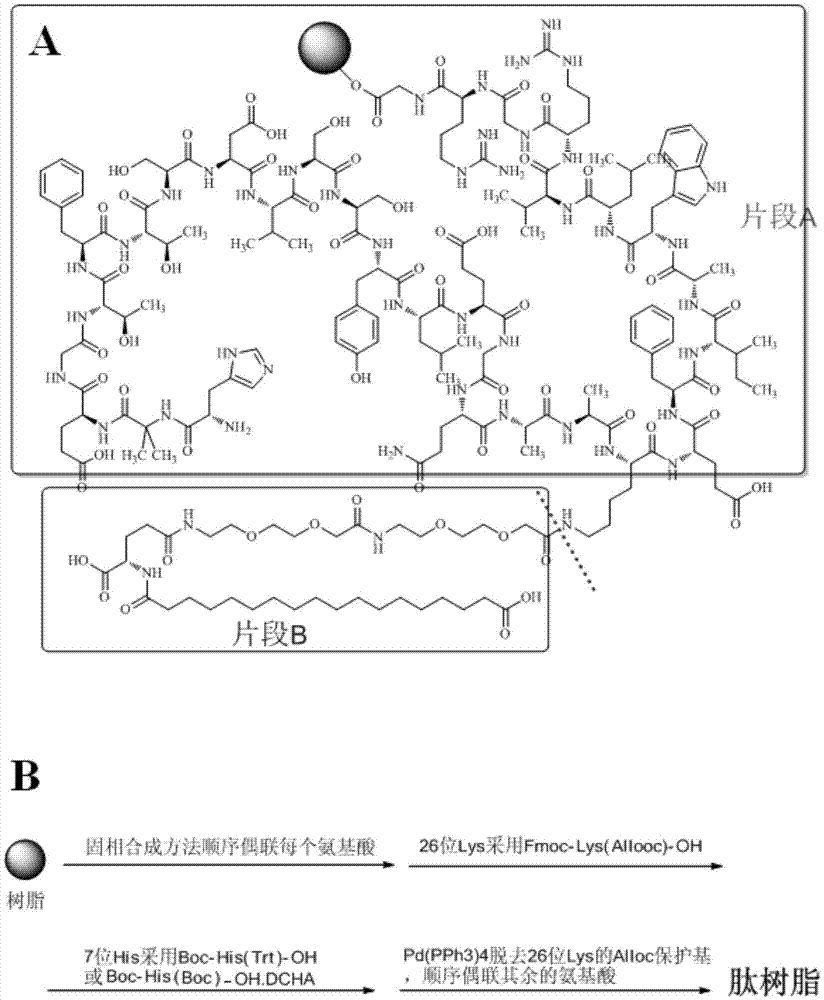
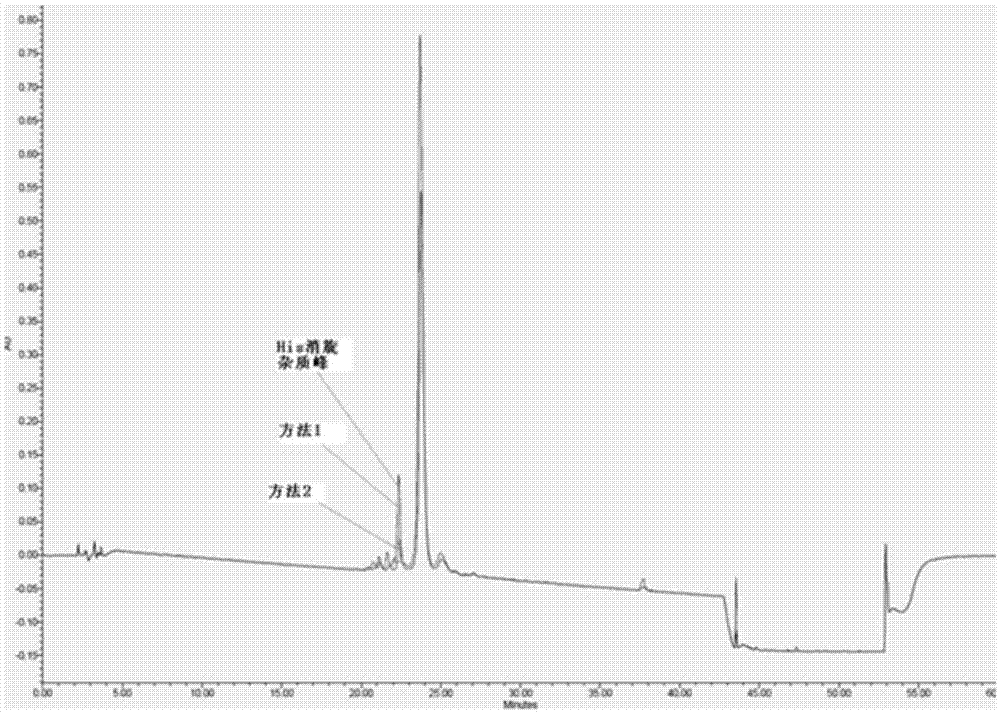
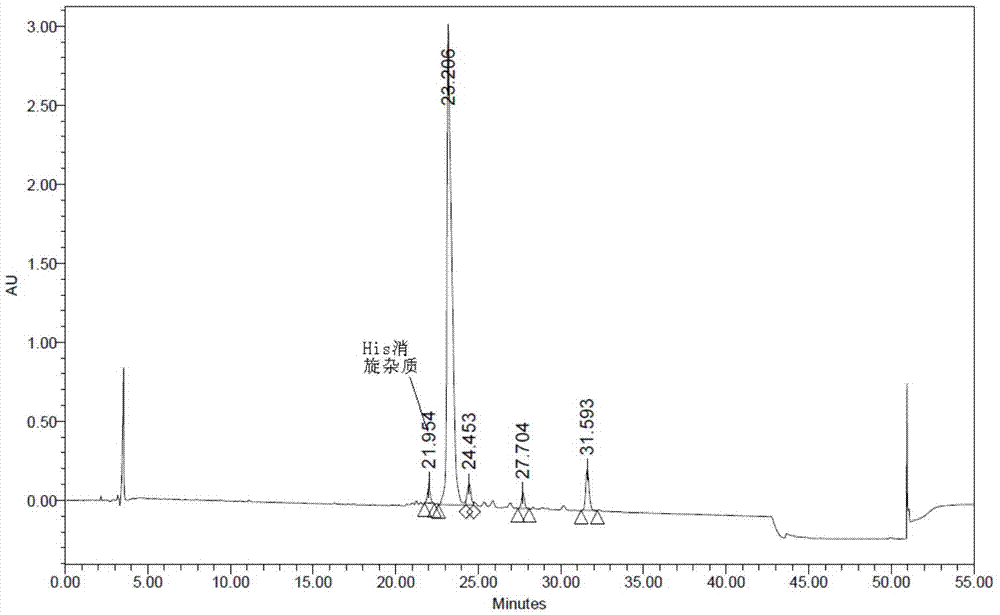
The invention relates to the field of polypeptides, in particular to a method for preparing semaglutide. The method has the advantages that Fmoc-Lys (Alloc)-OH protection amino acid is used as a raw material, de-protection is carried out by the aid of selected Pd (PPh3) 4, accordingly, operation procedures are simple, only 1-2 times of simple elimination reaction operation are required, each elimination reaction operation is carried out for 10-30 min, side reaction is prevented, the operation procedures are safe, and enlarged production can be facilitated; Boc-His (Boc)-OH. DCHA and Boc-His (Trt)-OH are used as raw materials in the procedures, and accordingly His racemization risks can be reduced to the greatest extent; special fragments are coupled, and accordingly the synthesis efficiency can be improved.
Owner:HYBIO PHARMA
Laser coding
InactiveUS20050186511A1Sharp contrastPhotomechanical apparatusSemiconductor/solid-state device manufacturingCompound (substance)Irradiation
A method for marking an object, wherein the object comprises a material including a functional group and a metal compound or acid that causes an elimination reaction on irradiation with a laser, to form a reaction product of contrasting colour, comprises directing a laser beam on to the areas of the object to be marked. For example, by using a carbohydrate and a metal salt, effective marking can be achieved on the coating of a pill or other edible material.
Owner:KHAN NAZIR
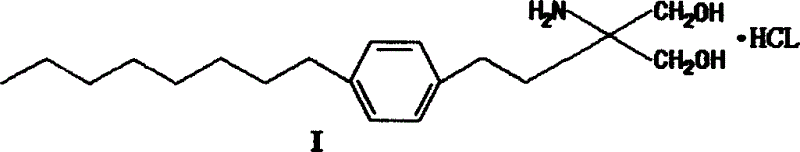
Method for preparing 2-para octylphenyl ehtyl-2-amino propanediol
InactiveCN1528738AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSodium iodidePropanediol
The invention provides a method to prepare a compound method, including the steps: ethylbenzene and capryl chloride make Friedel-Crafts reaction to generate p-capryl chloroethylbenzene; convert capryl chloroethylbenzene under the action of sodium iodide into p-capryl iodoethylbenzene; p-capryl iodoethylbenzene and acetylamino diethyl malonate condense under the action of alkali to generate 2-(p-capryl phenethyl)-2-acetylamino diethyl malonate; or p-capryl iodoethylbenzene makes elimination reaction under the action of alkali to generate p-capryl styrene, which together with acetylamino diethyl malonate condenses under the action of alkali into 2-(p-capryl phenethyl)-2- acetylamino diethyl malonate; a compound is reduced into 2-[4-(1-hydroxyoctyl) phenethyl-]2- acetylamino propylene alcohol; the other coumpound makes hydrogenolysis to obtain 2-(p-octyl phenethyl)-acetylamino propylene alcohol; make alkali hydrolyzation and then acidifies them into salt, so as to obtain it. It also provides a method to prepare intermediate in the above preparing course.
Owner:马启明
Intumescent flame retardant functional silicate and preparation method thereof
ActiveCN102757581AHigh grafting rateHigh flame retardant efficiencyPigment treatment with organosilicon compoundsNitrogenFire retardant
The invention discloses an intumescent flame retardant functional silicate and a preparation method thereof. According to the invention, the preparation method comprises the following two methods: one method comprises the steps of first preparing silane coupling agent organic functional silicate, and then preparing the intumescent flame retardant functional silicate on the basis of the former step; and the second method comprises the steps of first preparing a modified silane coupling agent containing phosphorus and nitrogen flame retardant elements, and then modifying inorganic silicate by using the modified silane coupling agent on the basis of the former step. According to the invention, the intumescent flame retardant functional silicate comprises an organic part B and an inorganic part A, wherein the organic part B is grafted to the inorganic part A in such a way that a silanol group formed by after a silane coupling agent containing amino has dehydration reaction with hydroxyls on the surface and between layers of the inorganic part A; and the organic part B is formed in such a way that a phosphoryl chloride compound has elimination reaction with an amino silane coupling agent. The intumescent flame retardant functional silicate disclosed by the invention is integrated with reinforcing, flame retardant and smoke suppressing functions.
Owner:SOUTH CHINA UNIV OF TECH +1
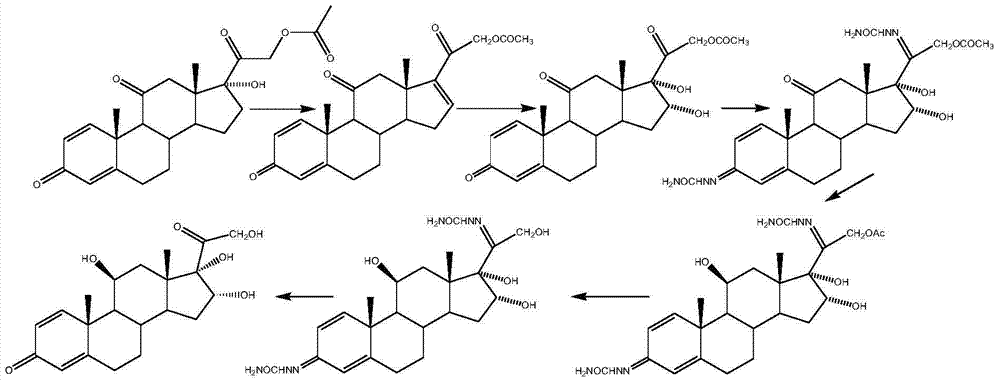
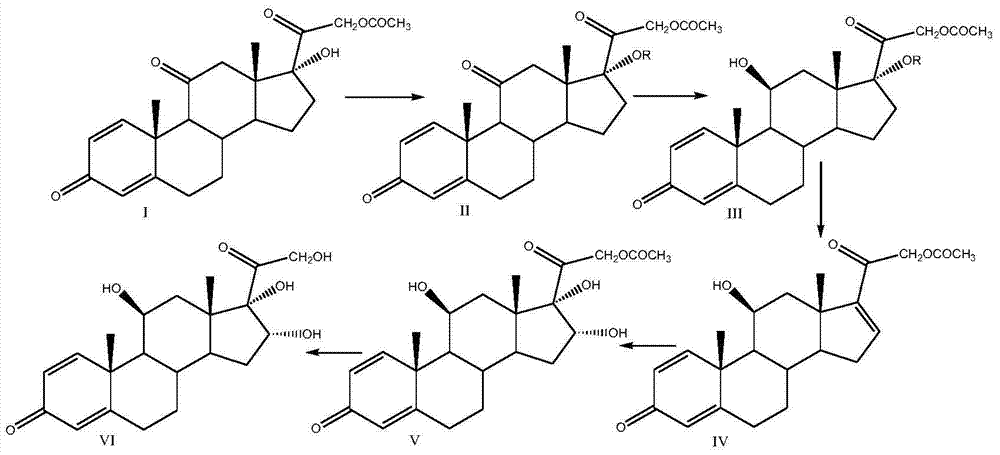
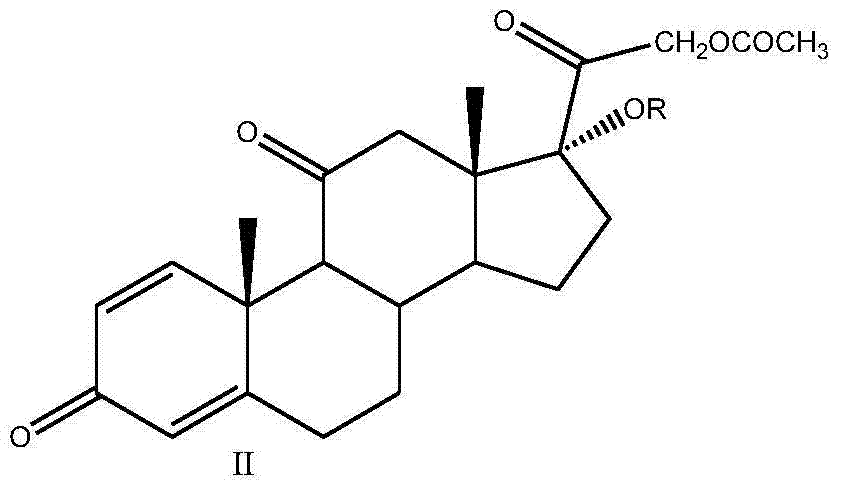
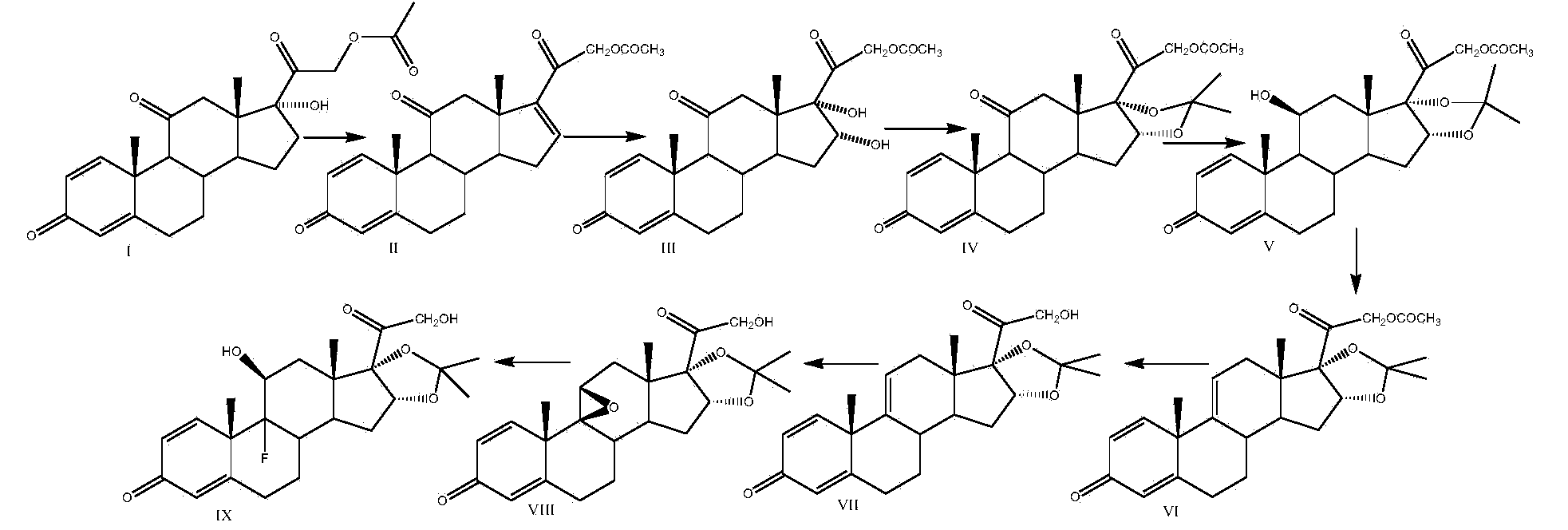
Preparation method of 16alpha-hydroxyprednisolone
The invention discloses a preparation method of 16alpha-hydroxyprednisolone, which comprises the following steps: by using a compound I prednisone acetate as an initial raw material, carrying out esterification reaction, reduction reaction, elimination reaction, oxidation reaction and hydrolysis reaction to obtain the end product VI 11beta,16alpha,17alpha,21-tetrahydroxyprogsteroid-1,4-diene-3,20-dione, i.e. 16alpha-hydroxyprednisolone. By using the cheaper initial raw material, the method has the advantages of shorter reaction steps, feasible reaction steps, higher yield and higher economy and safety for production, and is more suitable for industrial production. The method avoids using the traditional virulent product for bis-hydroxy oxidation, enhances the reaction safety and operability, simplifies the multiple protection and deprotection operation steps, greatly shortens the synthesis route, lowers the production cost, basically prevents the three-site carbonyl groups from being simultaneously reduced in the reduction process, greatly reduces the side reactions, and enhances the yield and quality of the reduction reaction.
Owner:江西赣亮医药原料有限公司
Laser coding
InactiveUS20050269304A1Sharp contrastThermographyLaser beam welding apparatusChemical compoundIrradiation
A method for marking an object, wherein the object comprises a material including a functional group and a metal compound or acid that causes an elimination reaction on irradiation with a laser, to form a reaction product of contrasting colour, comprises directing a laser beam on to the areas of the object to be marked For example, by using a carbohydrate and a metal salt, effective marking can be achieved on the coating of a pill or other edible material.
Owner:DATALASE
Use of Streptomyces hyalurolyticus enzyme in ophthalmic treatments
InactiveUS6902548B1Easy to useHigh purityPeptide/protein ingredientsPharmaceutical delivery mechanismProteinase activityStreptomyces
A highly specific and easily purified form of hyaluronidase is described for use in ophthalmic treatments. The enzyme, from Streptomyces hyalurolyticus is specific for hyaluronidase and carries out an elimination reaction that results in the production of double bonds at the nonreducing end of hyaluronic acid. Hyaluronidase from Streptomyces hyalurolyticus has a higher activity than comparable enzymes from other species. The enzyme is now capable of being purified in what is essentially a protease-free form making it applicable to medical treatments. The use of this source of hyaluronidase in ophthalmic treatments is now made possible by its high activity, specificity for hyaluronidase and purity.
Owner:SCHULER ED +1
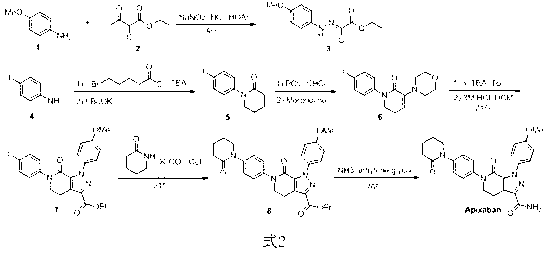
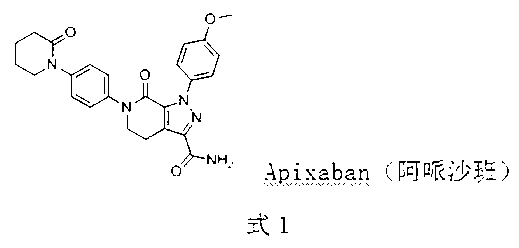
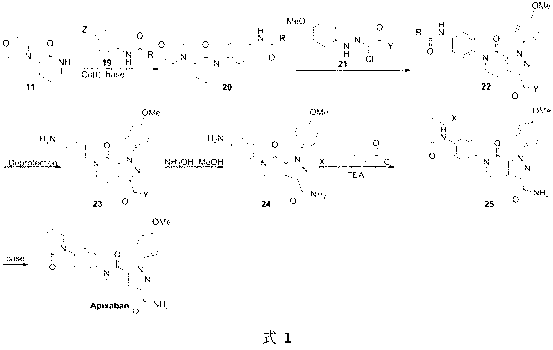
Preparation method of Apixaban as anti-thrombotic drug
ActiveCN103342704AEasy to synthesizeMethod steps are shortOrganic chemistryBulk chemical productionAntithrombotic AgentAniline
The invention discloses a preparation method of a compound Apixaban as an anti-thrombotic drug. The method disclosed by the invention comprises the following steps of: with a compound 11 shown as the formula 1 as a starting raw material, subjecting the compound 11 and amino protected paraiodoaniline 19 to coupling reaction in the existence of a cuprous reagent and an inorganic base to obtain a compound 20; subjecting the compound 20 and a compound 21 to [3+2] cyclization-elimination reaction to obtain a compound 22; removing a protecting group of the compound 22 to obtain a compound 23, or subjecting the compound 20 and the compound 21 to [3+2] cyclization reaction, directly carrying out elimination reaction under an acidic condition, and meanwhile, removing the protecting group to obtain the compound 23; subjecting the compound 23 to ammonolysis reaction to obtain a compound 24; subjecting the compound 24 and 5-chlorovaleryl halogen to amidation reaction to obtain a compound 25; and cyclizing the compound 25 under an alkaline condition to obtain the Apixaban, or subjecting the compound 24 and 5-chlorovaleryl bromine to amidation and cyclization two-step one-pot method reaction under the alkaline condition to obtain a target product, namely the Apixaban.
Owner:甘肃皓天科技股份有限公司
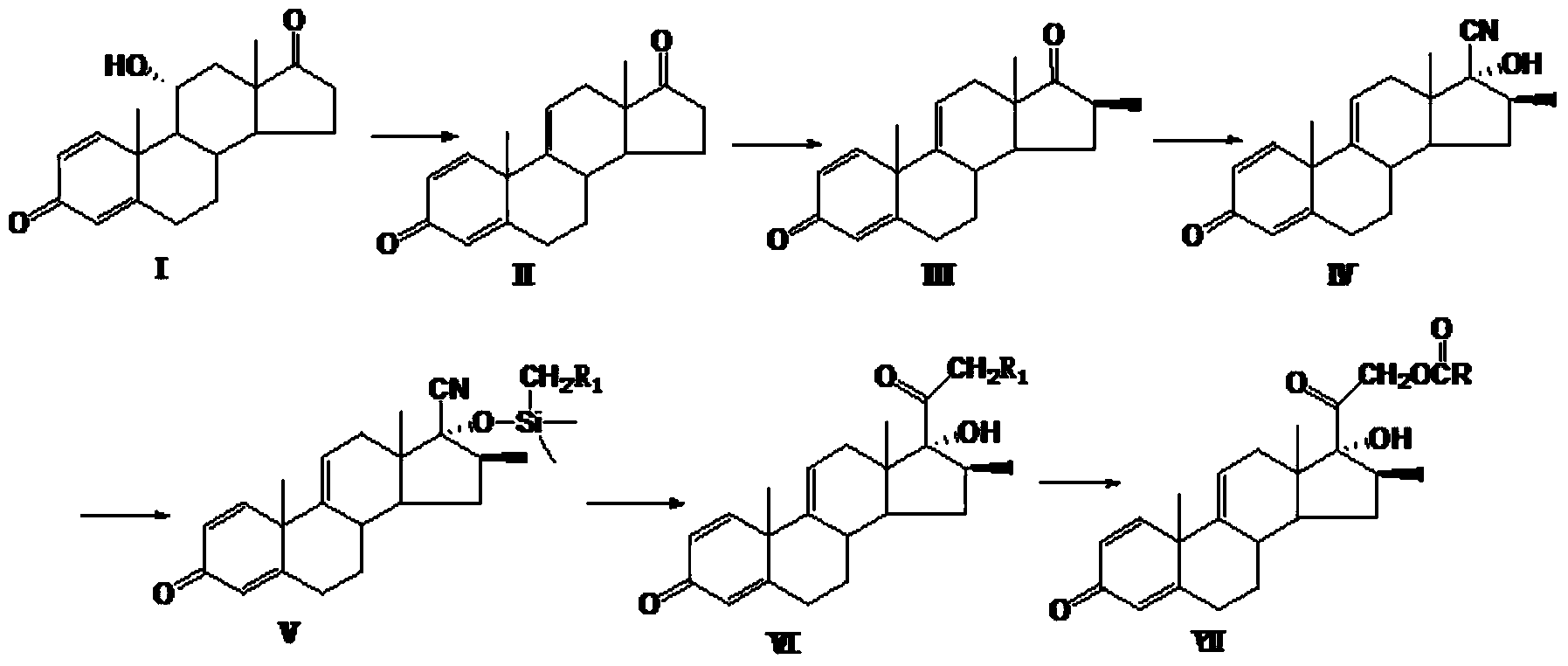
Preparation method for betamethasone intermediate or its analogue
ActiveCN103641878AStarting materials are readily availableHigh and stable yieldSteroidsSNiBetamethasone
The invention relates to a preparation method of a steroid hormone medicinal intermediate, in particular to a preparation method for a betamethasone intermediate or its analogue. The betamethasone intermediate or its analogue is prepared from a compound I by means of elimination reaction, methylation reaction, cyano-group substitution reaction, siloxy protective reaction, intramolecular nucleophilic substitution reaction and esterification reaction. The method provided by the invention has the characteristics of cheap raw materials, and high and stable yield. The reaction route is as the following, wherein R1 is Cl or Br, and R is H or hydrocarbonyl of C1-C10.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Method for synthesizing vinylene carbonate
ActiveCN101407508AAvoid the problem of crystallization clogging pipesEasy to separate by filtrationOrganic chemistryFiltrationUltraviolet lights
The invention discloses a method for synthesizing vinylene carbonate, and the method mainly comprises the following steps: (1) chlorine gas is introduced into the raw material of ethylene carbonate in ultraviolet light condition, thus synthesizing chlor-ethylene carbonate; (2) taking mixture of ester, ether and hydrocarbon as the organic solvent, the chlor-ethylene carbonate which is obtained in the step (1) produces elimination reaction with triethylamine, thus eliminating hydrogen chloride and generating the vinylene carbonate; and (3) the mixed product obtained in the step (2) is distilled and purified after filtration. By adopting the mixed solvent of ester, ether and hydrocarbon, the reaction product is easily isolated from triethylamine hydrochloride, thus increasing the yield and the production efficiency; and BHT can be used as a polymerization inhibitor in the reaction process, thus controlling the polymerization of the vinylene carbonate effectively and increasing the yield.
Owner:江苏瀚康新材料有限公司
Process for producing alpha-olefin
A production process for alpha-olefins making use of elimination reaction of a primary alcohol or an ether is characterized by use of an alumina catalyst in the presence of an amine. The production process for alpha-olefins according to the present invention can yield an alpha-olefin at a high proportion with respect to the entire olefin products and can maintain the reactivity of the catalyst and selectivity of the products for a prolonged time. In the process, unreacted material and ether intermediate byproduct produced in the process can be used in the reaction with the same catalyst. This allows industrially and environmentally favorable production process of alpha-olefins.
Owner:KURARAY CO LTD
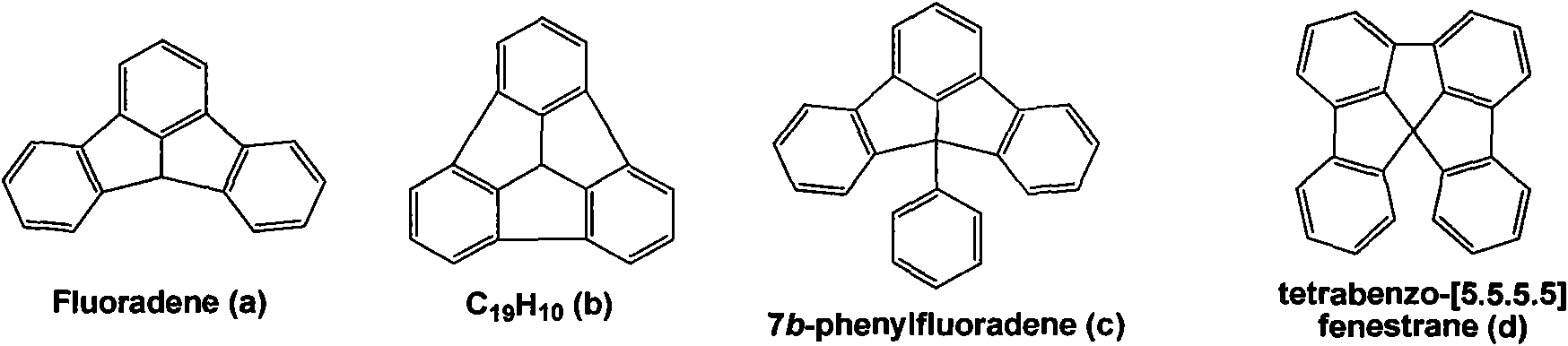
Fluoradene derivative and preparation method thereof
InactiveCN102030701ASpecific LuminescenceImprove thermal stabilityAmino preparation from aminesLuminescent compositionsHydrogen halideCarbazole
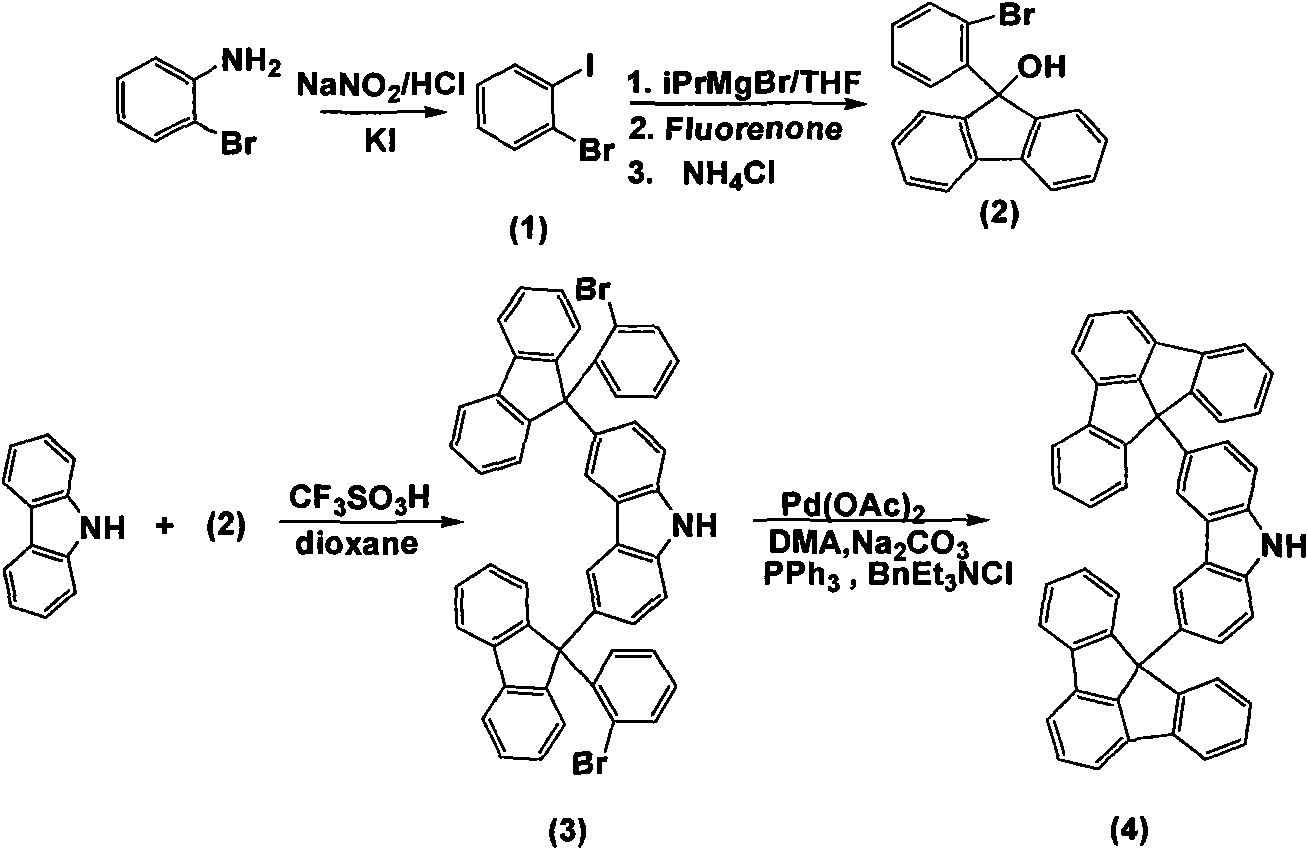
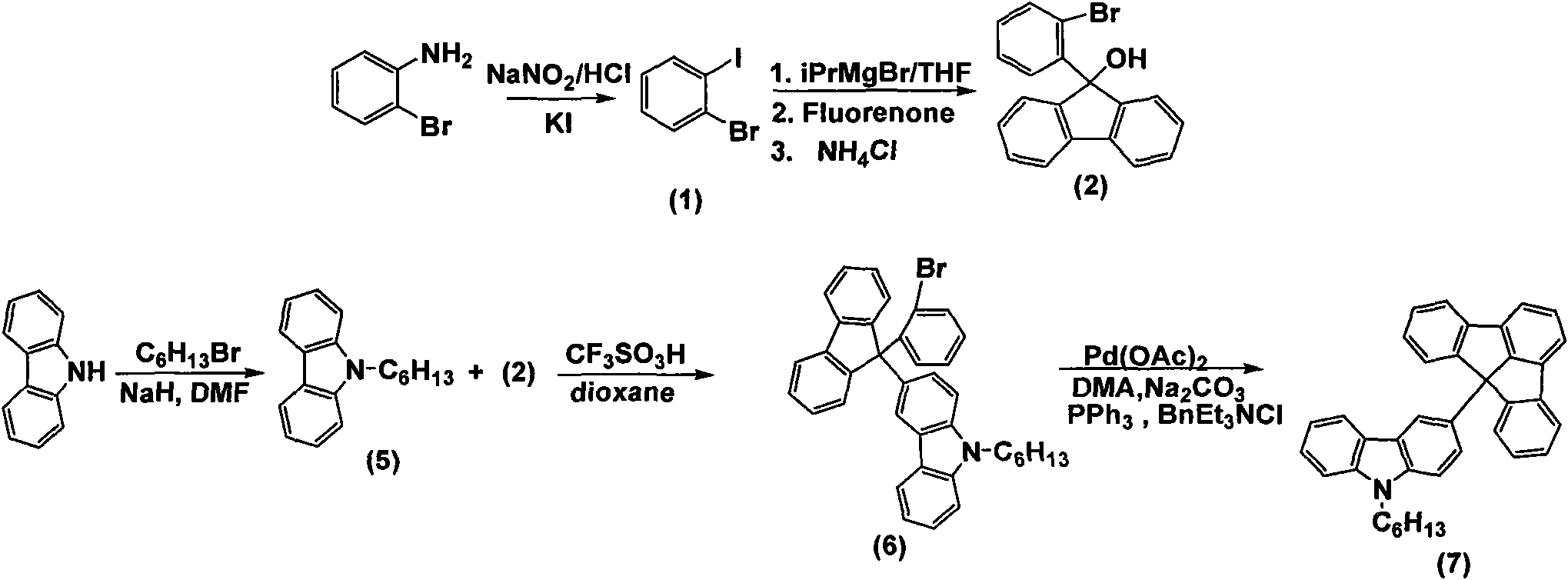
The invention discloses a fluoradene derivative and a preparation method thereof. The preparation method comprises the following steps of: introducing fluoradene rings into compounds, namely carbazole, hexyl carbazole, triphenylamine and hexyl triindole; and catalyzing intramolecular carbon-hydrogen bond activation and hydrogen halide elimination reaction through palladium and efficiently constructing a carbon ring or a heterocyclic compound, particularly constructing the carbon ring with a novel structure. The invention aims to synthesize and characterize a fluoradene structural unit-containing large-tension cyclic compound; and the novel fluoradene structural unit-containing compound is expected to be used in the field of organic photoelectric materials.
Owner:EAST CHINA NORMAL UNIV
Method for preparing DMC, DPC, ortho-carbonate, ortho-formate, dimethyl ether, and the like by using Na2CO3 or sodium formate or CO2 or CO
InactiveCN103254084AOrganic compound preparationPreparation from ortho-estersSynthesis methodsProcess synthesis
The invention relates to a recently discovered universal synthesis method of tandem-substitution-rearrangement-elimination reaction (TSRE reaction) of elemental organic chemistry, production process synthesis routes of two products of dimethyl carbonate (DMC) and diphenyl carbonate (DPC) and traditional products of ortho-carbonate, ortho-formate, dimethyl ether, and the like through CO2 and CO direct synthesis method are redesigned. Compared with prior arts for producing the products, with the preparation method provided by the invention, energy can be greatly saved, emission can be greatly reduced, and cost can be greatly reduced.
Owner:李坚
Organic compound having functional groups different in elimination reactivity at both terminals, organic thin film, organic device and method of producing the same
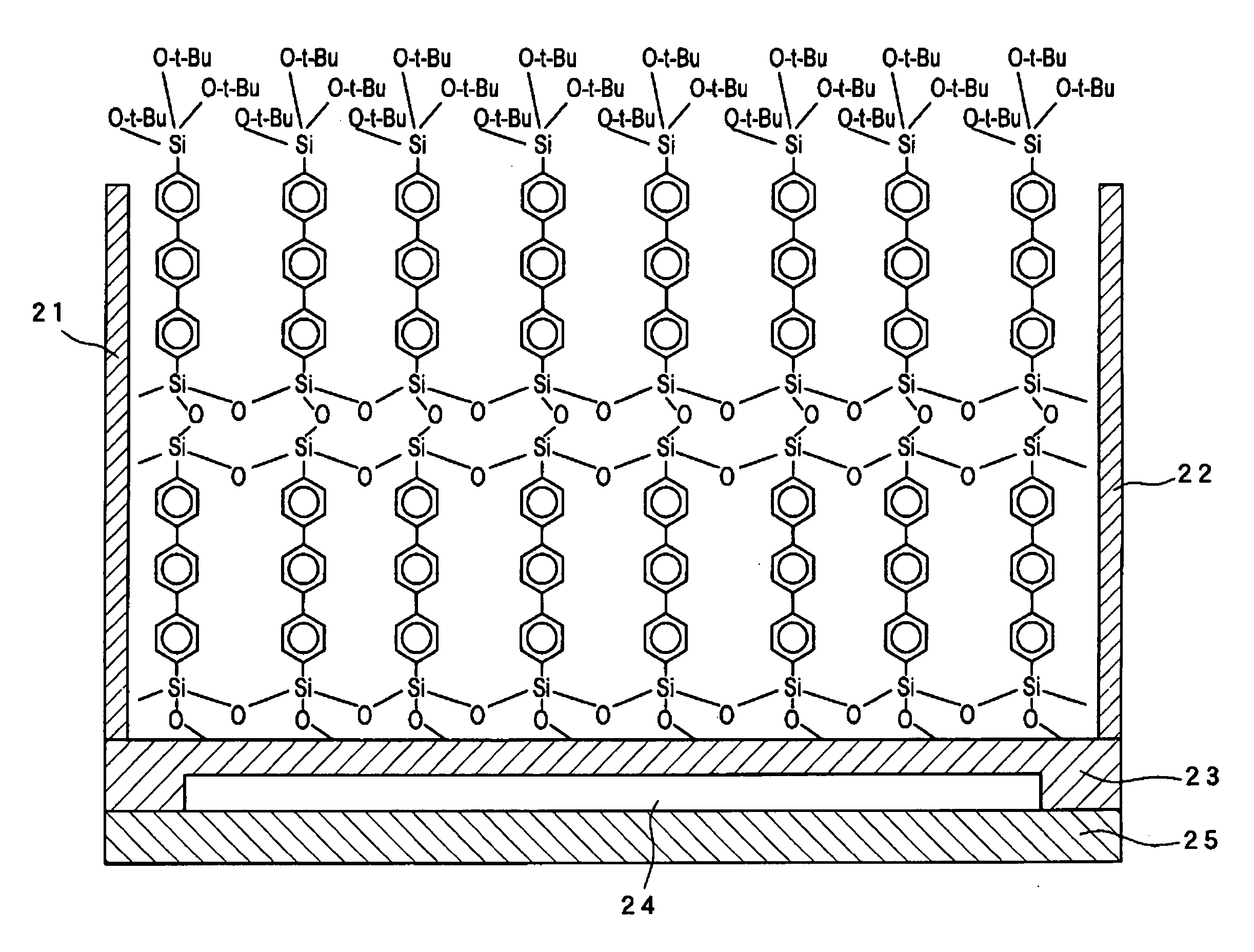
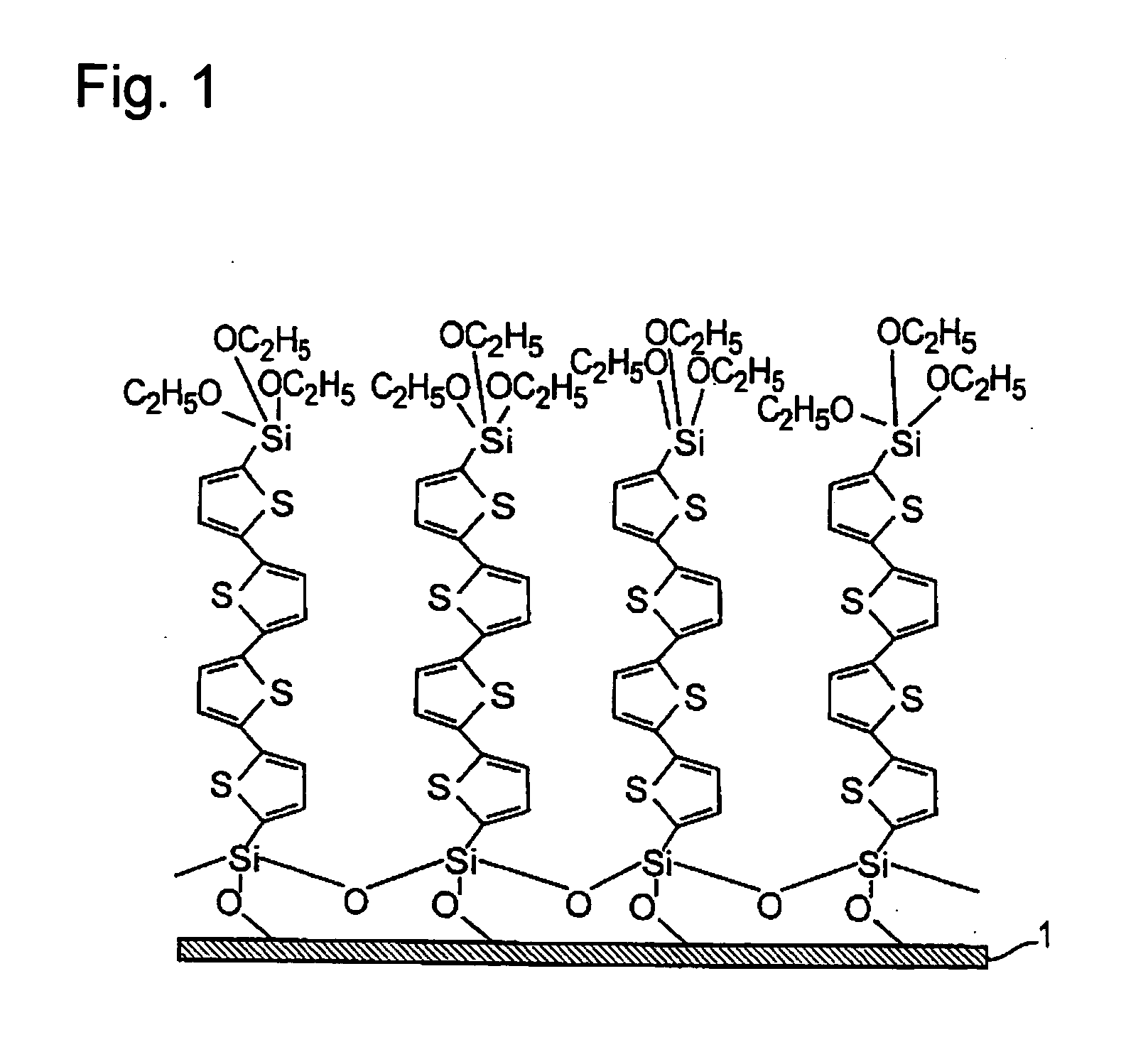
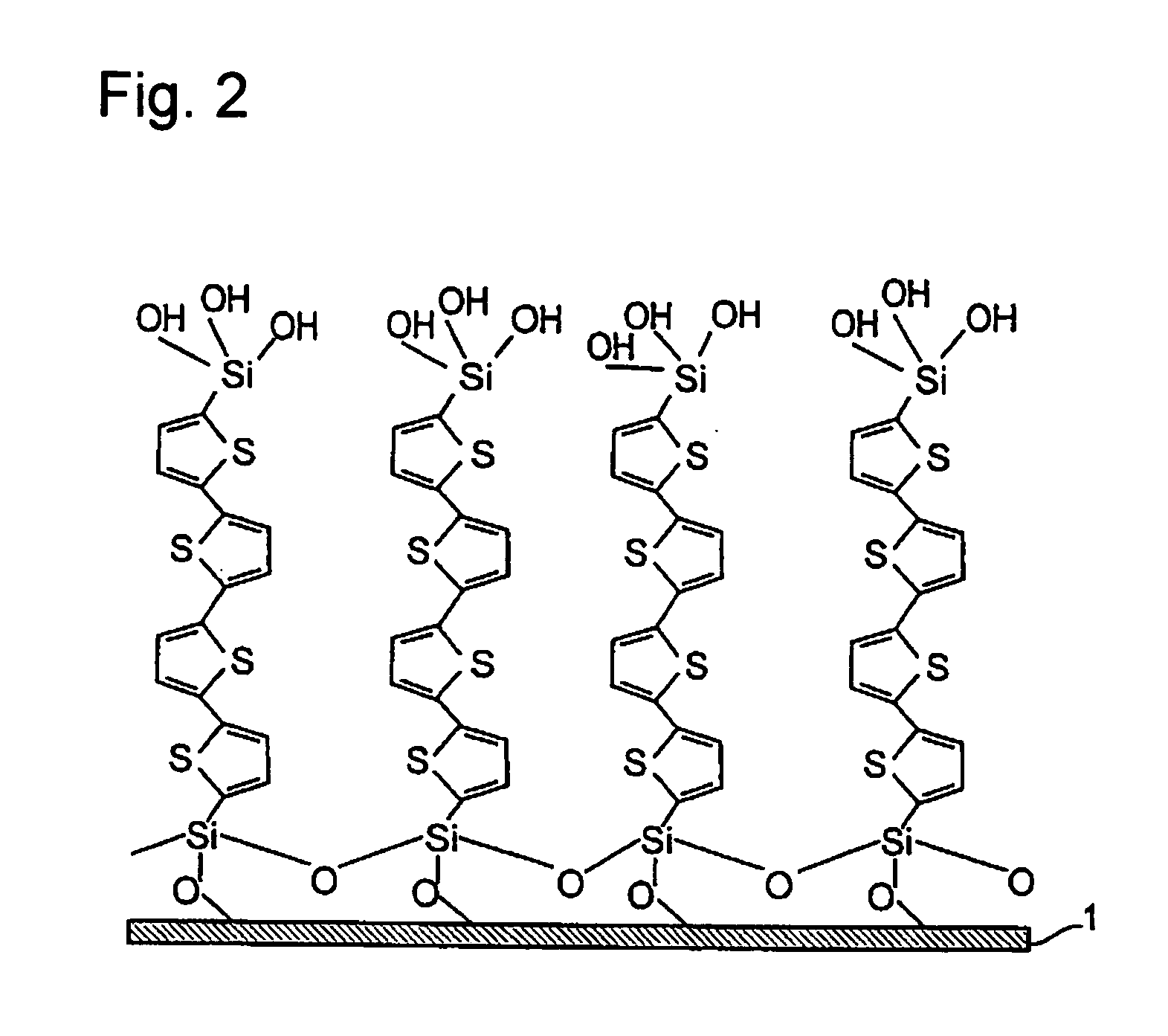
InactiveUS20070195576A1Prevents physical exfoliationImprove stabilityTransistorSilicon organic compoundsSilyleneHalogen
Provided are a single monomolecular film uniform in film thickness and highly ordered in molecule alignment and its multilayer film, an organic compound allowing production of such films at high reproducibility, an organic device superior in electroconductive properties and a method of producing the same. An organic compound represented by Formula:Si(A1)(A2)(A3)-B—Si(A4)(A5)(A6)(A1 to A6 each represent a hydrogen atom, a halogen atom, an alkoxy group or an alkyl group and satisfy the relationship in elimination reactivity of: A1 to A3>A4 to A6; and B represents a bivalent organic group), an organic thin film using the compound, and an organic device having the thin film; A method of producing an organic thin film and organic device, comprising a step of forming a single monomolecular film by allowing the silyl group having A1 to A3 in the organic compound to react with the substrate surface; a step of removing unreacted organic compounds by using a non-aqueous solvent; and a step of forming an additional monomolecular film of the organic compound by using the unreacted silyl groups present on the film surface side of the monomolecular film obtained as the sites for adsorption reaction.
Owner:SHARP KK
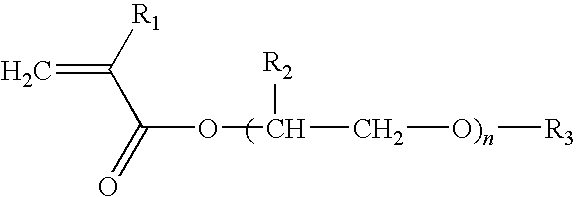
Biodiesel cold flow improver
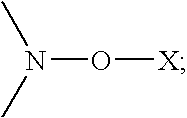
The present invention is directed to the use of alkyl (meth)acrylate polymers or copolymers of the formula (I)In-Poly-(E)y (I)as cold flow improvers in biodiesel fuel (or bio-fuel) and biodiesel compositions incorporating said polymers or copolymers, obtained by nitroxyl mediated controlled free radical polymerization,whereinIn is the initiator fragment starting the polymerization reaction;E is an end group bearing at least one stable free nitroxyl radical, which is bound via an oxygen atom to the polymer or copolymer; or a group which results from a substitution or elimination reaction of the attached stable free nitroxyl radical;Poly is any polymer or copolymer formed from ethylenically unsaturated monomer(s); andy is a number 1 or greater than 1 indicating the average number of end groups E attached to Poly.
Owner:BASF AG
Hydrogels with biodegradable crosslinking
ActiveUS20140288190A1Small degradationCosmetic preparationsImpression capsBiodegradable hydrogelsAbsorbent material
Hydrogels that degrade under appropriate conditions of pH and temperature by virtue of crosslinking compounds that cleave through an elimination reaction are described. The hydrogels may be used for delivery of various agents, such as pharmaceuticals. This invention provides hydrogels that degrade to smaller, soluble components in a non-enzymatic process upon exposure to physiological conditions and to methods to prepare them. The hydrogels are prepared from crosslinking agents that undergo elimination reactions under physiological conditions, thus cleaving the crosslinking agent from the backbone of the hydrogel. The invention also relates to the crosslinking agents themselves and intermediates in forming the hydro gels of the invention. The biodegradable hydro gels prepared according to the methods of the invention may be of use in diverse fields, including biomedical engineering, absorbent materials, and as carriers for drug delivery.
Owner:PROLYNX LLC
Fluorescent probe for detecting nitroreductase and preparation method and application thereof in enzymatic reaction
ActiveCN109456264AHigh sensitivityNo quenchingOrganic chemistryMicrobiological testing/measurementHigh concentrationSulfonate
The invention discloses a fluorescent probe for detecting NTR (nitroreductase) and a preparation method and application thereof in enzymatic reaction, and belongs to the technical field of industrialanalysis detection. The fluorescent probe is a 3-(4-(2-(4'-(diphenylamino)-3-((4-nitrobenzyl)oxy)-[1,1'-biphenyl]-4-yl)vinyl)quinoline-1-bromo)propane-1-sulfonate. The fluorescent probe compound has the advantages that by leading hydrophilic group sulfonate and quinoline salt, the hydrophilia is enhanced; under the catalyzing function of NTR, the 1,6-rearrangement and elimination reaction is performed, the hydroxyl is produced, and the detection and analysis of NTR in the enzymatic reaction are realized by the fluorescence change caused by ICT (intramolecular charge transfer) effect. The preparation method has the advantages including that the preparation is simple and convenient, and the yield is high; the preparation method is suitable for detection of content of high-concentration enzyme in the enzymatic reaction, and the great application prospect is realized in the field of detection of enzyme in the enzymatic reaction system in the chemical engineering field.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of phosphonitrile flame retardant
InactiveCN102603800AEnvironmentally friendlyAvoid hydrolysisGroup 5/15 element organic compoundsOrganic solventChloride
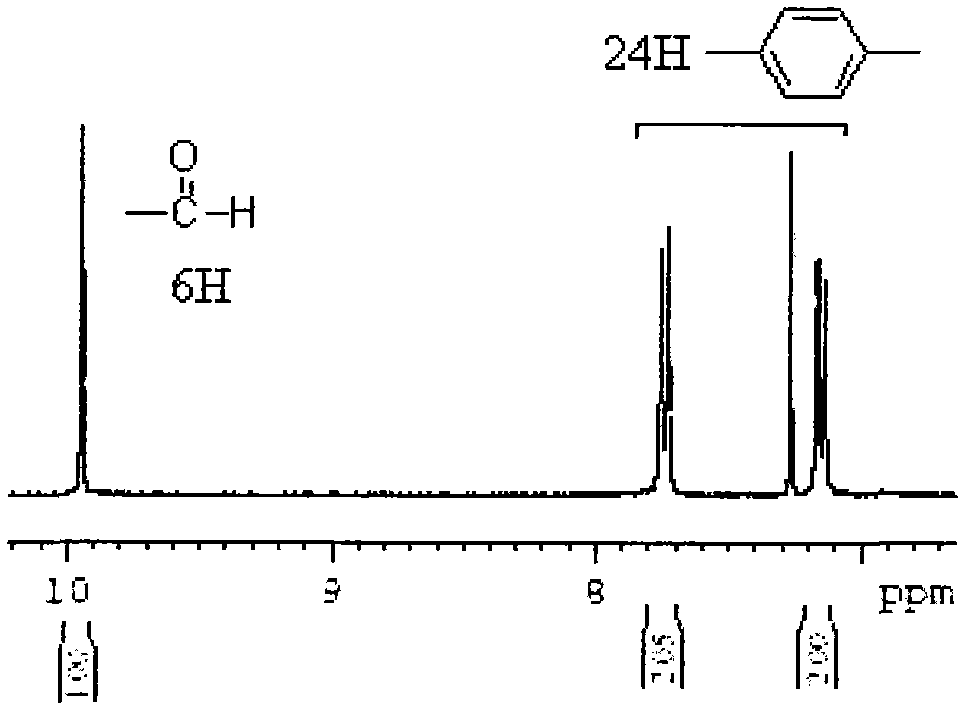
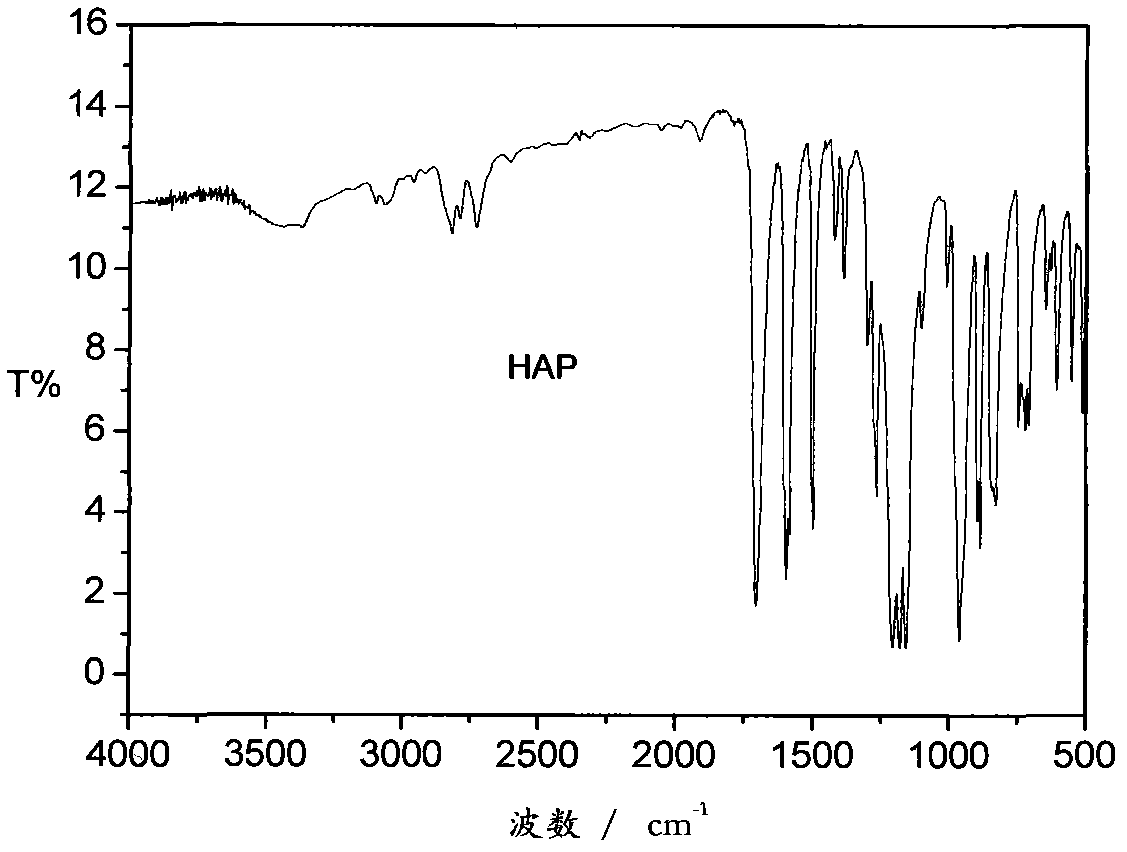
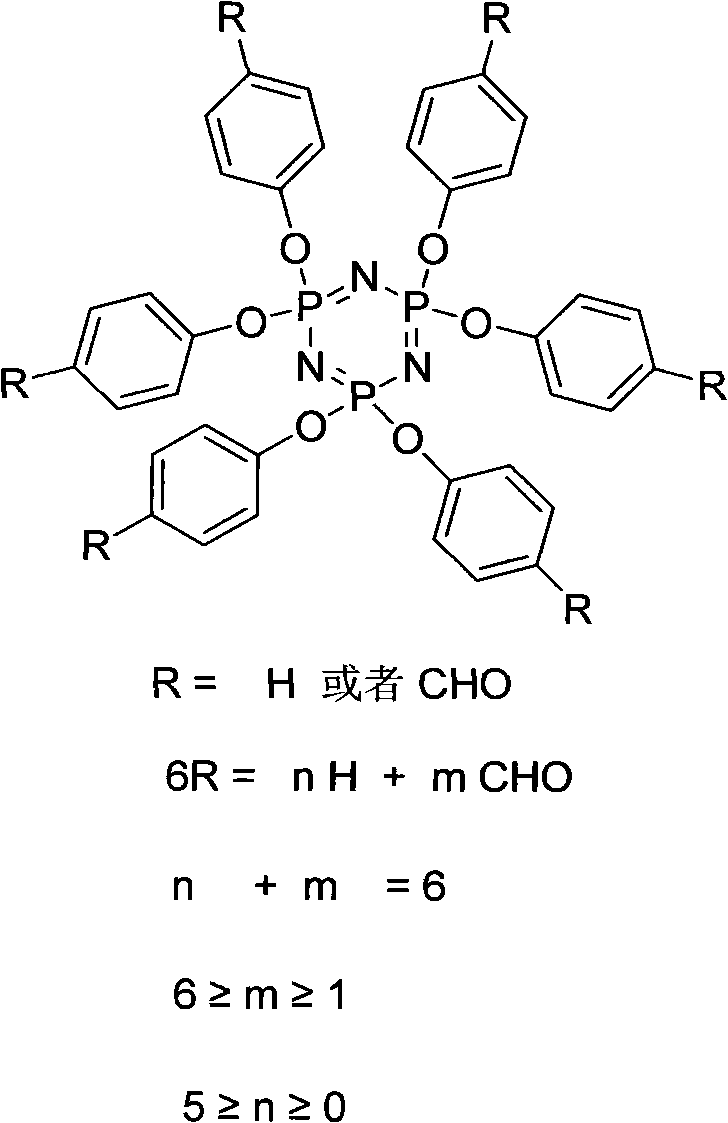
The invention relates to a synthesis preparation method of an aldehyde phenoxyl substituted cyclotriphosphazene flame retardant. According to the preparation method, phosphonitrilic chloride trimer, p-hydroxybenzaldehyde, phenol and an ammonium catalyst are adopted as raw materials, the removal of chlorine hydride is realized by an elimination reaction under the catalytic action of ammonium in an organic solvent, aldehyde phenoxyl cyclotriphosphazene (HAP) can be synthetized with a high yield without using an acid-binding agent, the excessive raw materials and the catalyst are removed by washing, and the solvent is removed to obtain aldehyde phenoxyl cyclotriphosphazene.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY +1
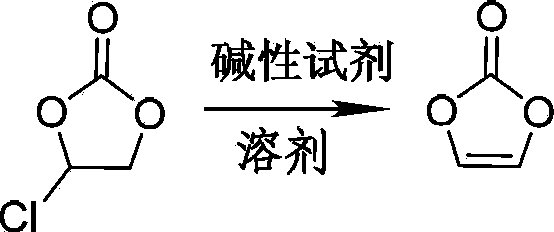
Preparation method of vinylene carbonate
The invention relates to a preparation method of vinylene carbonate. In the preparation method, monochloroethylene carbonate directly contacts with excessive triethylamine in the protection of inert gas for reaction, and no solvent is involved. In the preparation method of the present invention, triethylamine has two functions: the first one is that triethylamine is used as a dehydrochlorination reagent to participate in elimination reaction; the second one is that triethylamine is used as a solvent of dehydrochlorination reaction. The vinylene carbonate prepared by the preparation method of the invention has a yield of up to 93%, is easy to separate and purify, and has purity of up to more than 99%. The preparation method of the invention does not involve any other reaction solvent, and realizes full contact reaction of monochloroethylene carbonate with triethylamine; therefore, compared with traditional method, the preparation method of the invention is high in reaction speed, high in product yield, and suitable for large-scale industrial production. The vinylene carbonate prepared by the preparation method of the invention is applicable to various fields.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
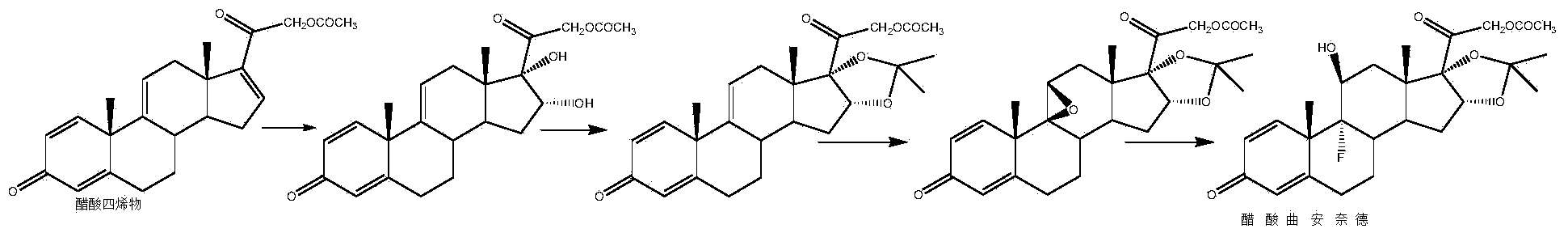
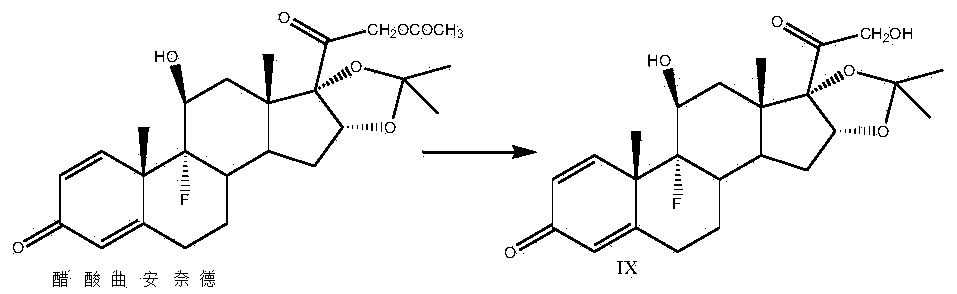
Preparation method of triamcinolone acetonide
The invention discloses a preparation method of triamcinolone acetonide. A compound I prednisone acetate which is taken as a starting material is subjected to a primary elimination reaction, an oxidation reaction, a condensation reaction, a reduction reaction, a secondary elimination reaction, a hydrolysis reaction, an epoxidation reaction and a fluorination reaction, so that an end-product IX, 9-fluoro-11beta, 21-dihydroxyl-16a,17-[(1-methyl ethylidene) bi(oxo)]-pregnen steroid-1,4-diene-3, 20-diketone, namely triamcinolone acetonide, is obtained. The preparation method of the triamcinolone acetonide has the advantages that a relatively cheap starting raw material is adopted, each reaction step can be relatively easily realized, and yield is relatively high, so that production is more economic and safer, and the triamcinolone acetonide is more applicable to industrial production; a multi-step protection and deprotection operation is simplified, a synthetic route is greatly shortened, and production cost is reduced; possibility that a three-position carbonyl group is reduced in a reduction process is almost zero, so that side effects are greatly reduced, and yield and quality of the reduction reaction are improved.
Owner:江西赣亮医药原料有限公司
Gas and solid radial reactor for moving-bed
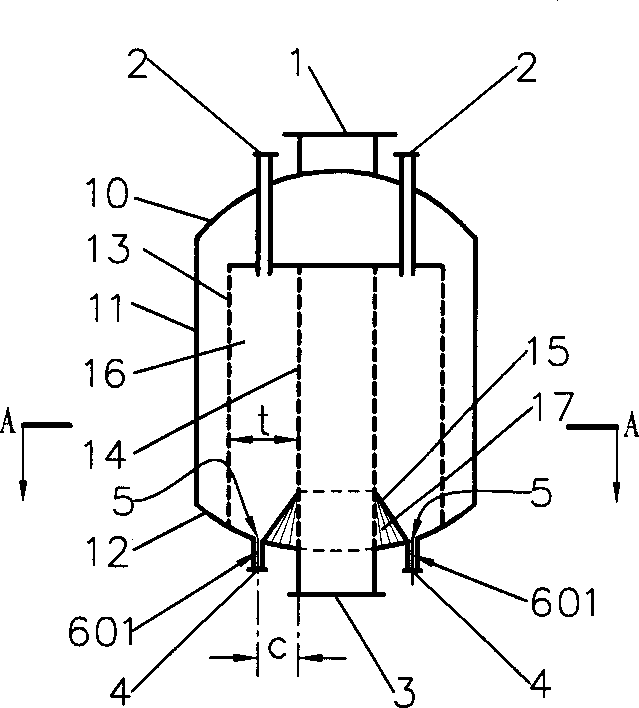
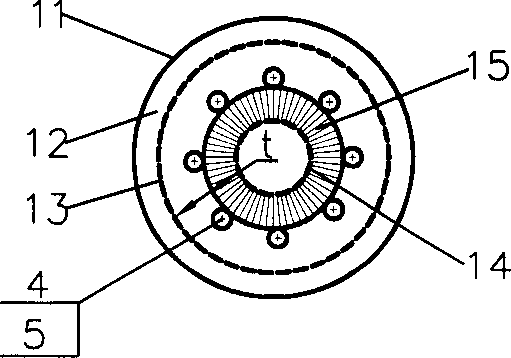
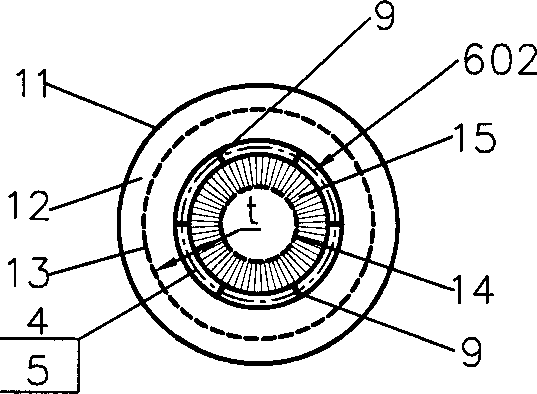
InactiveCN1333084ASimple structureReduce or eliminate flow dead zonesChemical/physical processesChemical industryOil processing
The present invention discloses a gas-solid radial reactor of moving bed for chemical industry and petroleum processing industry, and is characterized by that the lower portion of inner not of said reactor is equipped with a skirt, and the external surface of said skirt is inclined from top to bottom along the direction from inner net toward outer net, its upper edge is connected with inner net, and its lower edge is connected with internal surface of closure head of the bottom, and the radial position of the lower edge is positioned in internal side of radial position of catalyst outlet pipe, and the external surface of said skirt is generally made into circular truncated cone side face or frustum side face. Said invention can effectively reduce or eliminate flow dead zone of catalyst inthe reactor, at the same time the structure of said skirt is simple, and easy to implement. It is specially applicable to hydrocarbon conversion reaction and catforming reaction.
Owner:LUOYANG PETROCHEMICAL ENG CORP SINOPEC
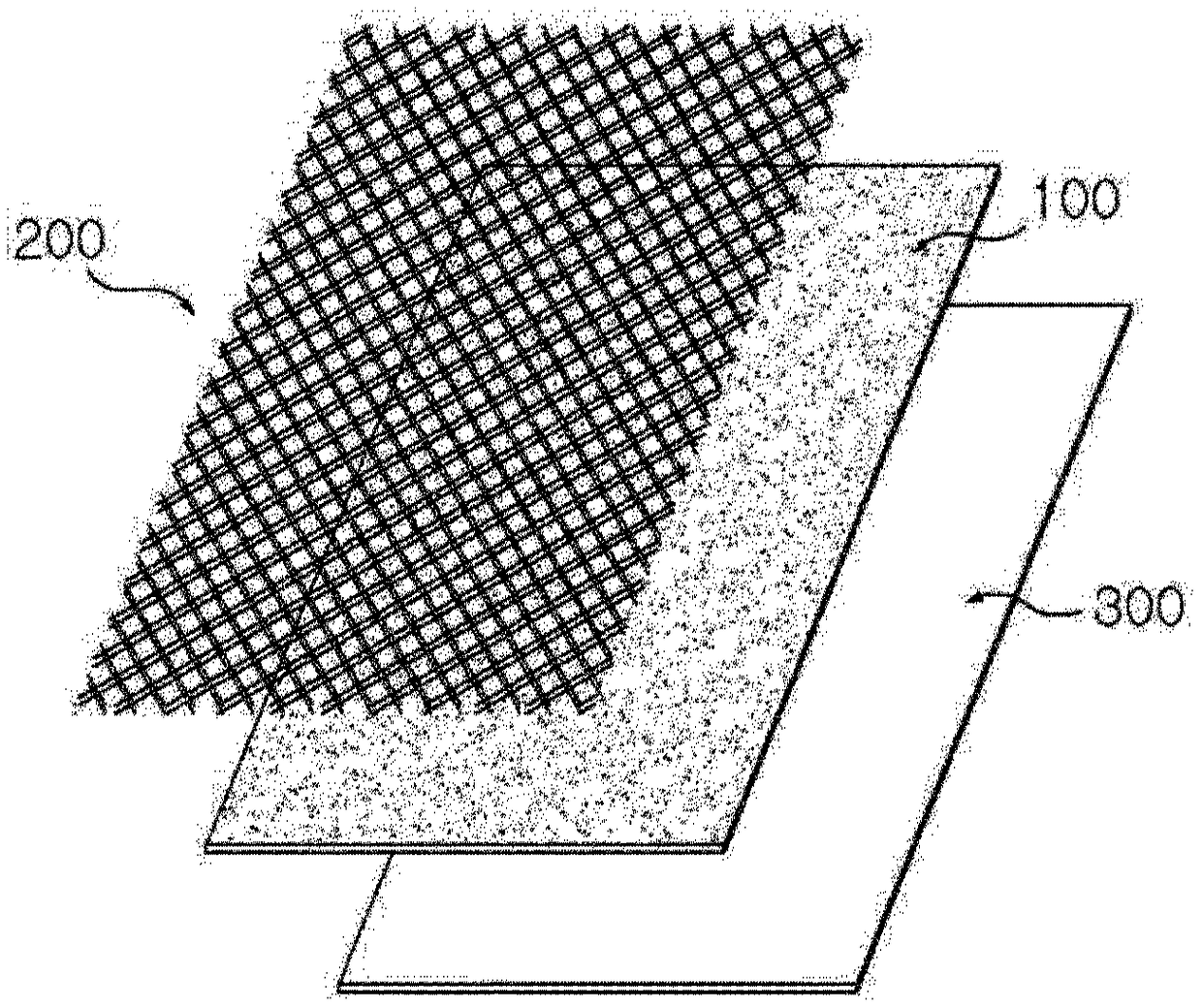
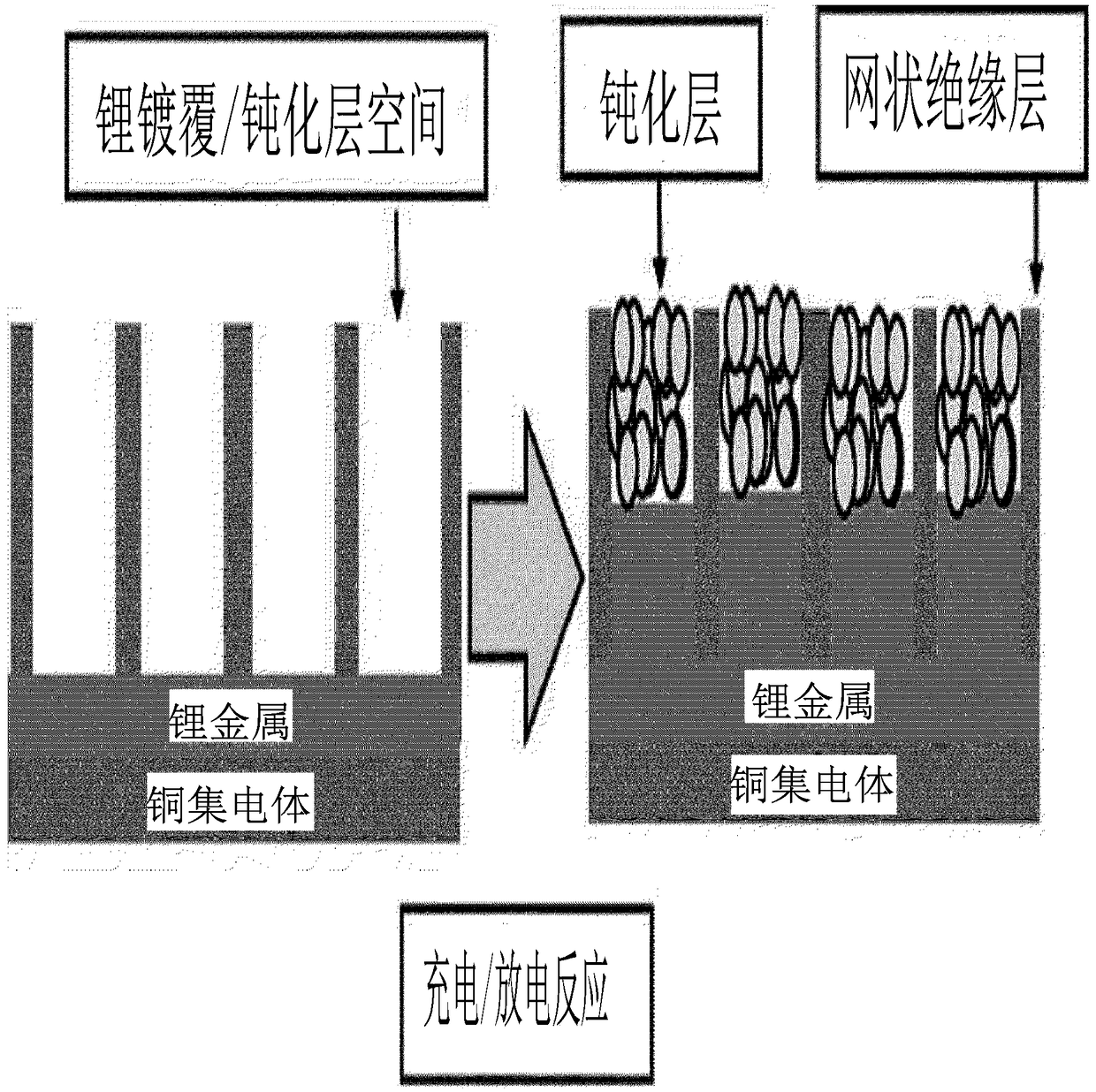
Anode for lithium secondary battery comprising mesh-shaped insulating layer, and lithium secondary battery comprising same
ActiveCN108886139AInhibition of volume expansionForm evenlyElectrode carriers/collectorsNegative electrodesDesorptionElectrical battery
The present invention relates to an anode for a lithium secondary battery comprising a mesh-type insulating layer and a lithium secondary battery comprising the same and, more particularly, to an anode for a lithium secondary battery comprising a mesh-type insulating layer which is formed on one surface of a lithium metal layer and has pores, and a lithium secondary battery comprising the same. Alithium secondary battery to which the anode according to the present invention is applied induces the precipitation and elimination reactions of lithium dendrite inside the pores of the insulating layer to suppress the local formation of the lithium dendrite on a lithium metal surface and form a uniform surface, thereby suppressing a volume expansion of a cell. In addition, the lithium secondarybattery forms a support layer on an initially formed passivation film to prevent desorption and collapse of the passivation film, such that an additional side reaction with an electrolyte is suppressed and dead lithium is minimized, thereby improving the lifetime properties of the battery.
Owner:LG ENERGY SOLUTION LTD
Near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application
InactiveCN107501313AExcellent photothermal performanceGood photoacoustic imagingEnergy modified materialsEchographic/ultrasound-imaging preparationsKetoneNitromethane
The invention discloses a near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application. The photo-thermal dye comprises an electron-donating group containing lone pair electrons and a basic aza-fluoro borane skeleton. The near-infrared photo-thermal dye based on aza-fluoro borane is prepared through steps as follows: (1), ketene synthesis: ketene is prepared from aldehyde ketone by an addition-elimination reaction under the alkaline condition; (2), an addition reaction of ketene and nitromethane; (3), a cyclization reaction; (4), a coordination reaction: a product obtained in the step (3) coordinates with boron difluoride, and a target product is obtained. The photo-thermal dye can be applied to the technical fields of photodynamic therapy and photo-thermal therapy under the guidance of photo-thermal imaging and photo-acoustic imaging, bio-marker and detection and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
Method for continuously preparing vinylene carbonate by tubular reactor
ActiveCN104844556AIncrease production capacityAdapt to capacity needsOrganic chemistryChlorosulfuric acidEthyl Chloride
The invention provides a method for continuously preparing vinylene carbonate by a tubular reactor. The method includes using chlorosulfuric acid and ethylene carbonate as raw materials to be synthesized into chloroethylene carbonate in the tubular reactor under the action of initiator, rectifying the chloroethylene carbonate and subjecting the rectified chloroethylene carbonate to elimination reaction with trimethylamine in the tubular reactor with existence of organic solvent and generating the vinylene carbonate. By the method for continuously preparing vinylene carbonate by the tubular reactor, existing intermittent production methods are changed, reaction speed is greatly increased, reaction period is reduced, yield is increased and productivity can be effectively improved.
Owner:RONGCHENG QINGMU CHEM MATERIALS
Process for preparing nitriles by elimination reactions
ActiveUS7939688B2Readily water-solubleSimple phase separationOrganic compound preparationPreparation by carboxylic acid amide dehydrationArylOrganic solvent
Process for preparing nitriles by reacting N-alkylcarboxamides (RCO—NHR1) or ammonium salts of carboxylic acids (RCOO—NH3R1+) or carboxylic acids in the presence of alkylamines or ammonium salts (RCOOH+NH2R1, RCOOH+NH3R1+), respectively, R being an arbitrarily substituted linear or branched C1-C12-alkyl radical, a C3-C12 cycloalkyl radical or an alkenyl, alkynyl or aryl or heteroaryl radical and R1 being an arbitrary substituted, linear or branched C2-C1 alkyl radical, a C3-C12 cycloalkyl radical or an alkenyl or alkynyl radical, with phosphonic anhydrides in the presence of an optional base in an organic solvent at a temperature in the range from −30 to 180° C. In advantageous embodiments, the phosphonic anhydride is a 2,4,6-substituted 1,3,5,2,4,6-trioxatriphosphinane 2,4,6-trioxide of the formula (I)
Owner:EUTICALS
Synthesis method for (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method for chiral intermediate of niraparib
InactiveCN108203404AHigh reaction yieldMild reaction conditionsOrganic chemistry methodsSynthesis methodsOrganic synthesis
The invention belongs to the technical field of organic synthesis. The synthesis method firstly provided by the invention takes benzyl-4-oxopiperidine as a starting material, and the starting materialis subjected to Grignard reaction, elimination reaction, hydrogenation reduction reaction and chiral resolution in sequence to successfully obtain a target product (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine. The synthesis method sencondly provided by the invention takes the same starting raw material for Grignard reaction, organic silicon reagent is used for removing a hydroxide radical, and benzyl is removed by catalytic hydrogenation reaction; finally, the chiral resolution is carried out to obtain a target product. The (S)-3-phenylpiperidine can be synthesized according to the synthesis method. (S)-3-p-aminosalicylic phenylpiperidine can be synthesized according to the third aspect; or according to the fourth aspect, (S)-3-p-bromophenyl piperidine is synthesized to serve asthe key intermediate for preparing the niraparib. According to the synthesis method for (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine and the synthesis method for chiral intermediate of niraparib, production cost is obviously lowered, and the synthesis methods are favorable for the large-scale industrial production of a niraparib medicine.
Owner:SHANGHAI BIOBOND PHARMA
Preparation method of apixaban
ActiveCN105732622AAdaptable to conditionsFast aminolysisOrganic chemistryP-NitroanilineNitro reduction
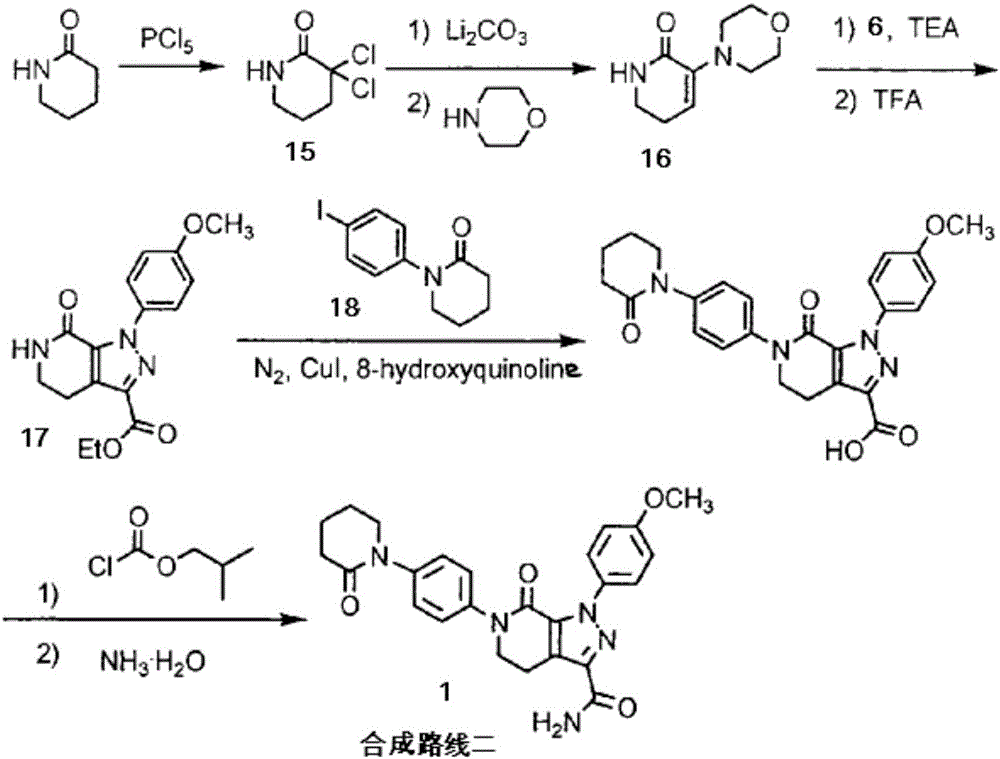
The invention discloses a preparation method of apixaban.The method comprises the steps that paranitroaniline serves as the raw material, paranitroaniline and delta-valerolactone are subjected to amidation ring-opening, substituting and ring-closing reactions under the action of AlMe3, and a compound 8 is obtained; the compound 8 is subjected to alpha-position dichloro substituting and condensation-elimination reactions, and a compound 7 is obtained; the compound 7 and a compound 6 are subjected to [3+2] cyclization-elimination and nitro reduction, and a compound 4 is obtained; the compound 4 sequentially reacts with delta-valerolactone and ammonium chloride under the action of AlMe3, and a compound 3 is obtained; the compound 3 is subjected to substituting and ring closing, and apixaban is obtained.According to the preparation method of apixaban, paranitroaniline and delta-valerolactone which are low in price are adopted to serve as the raw materials, operation of the whole route is simple, conditions of each reaction are mild, and the synthesizing method is easy to operate, high in yield and purity and suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com