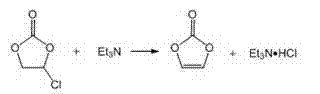
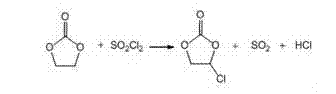
Method for continuously preparing vinylene carbonate by tubular reactor
A technology of vinylene carbonate and tubular reactor, which is applied in the field of continuous production of vinylene carbonate by using tubular reactor, which can solve the problems of inability to carry out continuous production, high production environment requirements, and unsuitability for continuous production. , to reduce equipment and production environment requirements, meet continuous production, and prevent polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) After preheating 88.06kg (1000mol) ethylene carbonate to 30~40°C, fully dissolve and mix with 202kg (1500mol) sulfuryl chloride and 880g azobisisobutyronitrile in a mixer, then use a flow rate of 0.05m / s Inject into a horizontal tubular reactor and react at 40-45°C for 100-200 minutes to replace the SO produced by the reaction 2 and HCl are absorbed using a four-stage falling film absorption tower, and the chlorinated products enter the 1.5m 3 The intermediate tank is equipped with an emptying pipe, and the volatilized sulfuryl chloride in the tank is discharged into the main emptying pipe through the emptying pipe, and then pumped into the 6.5m by a vacuum pump. 3 Carry out rectification in the rectification kettle, collect 80 ℃ / 2.66×10 respectively 3 Pa and 106-107℃ / 1.33×10 3 The cut of Pa obtains 100.9 kg of chloroethylene carbonate, the content of chloroethylene carbonate is 96.5%, and the yield is 82%.
[0030] 2) After fully dissolving and mixing 61.3kg (500...
Embodiment 2
[0032] 1) After preheating 88.06kg (1000mol) ethylene carbonate to 30~40℃, fully dissolve and mix with 202kg (1500mol) sulfuryl chloride and 220g benzoyl peroxide in the mixer, inject at a flow rate of 0.05m / s In a horizontal tubular reactor, react at 40~45°C for 200~300 minutes to replace the SO produced by the reaction 2 and HCl are absorbed using a four-stage falling film absorption tower, and the chlorinated products enter the 1.5m 3 The intermediate tank is equipped with an emptying pipe, and the volatilized sulfuryl chloride in the tank is discharged into the main emptying pipe through the emptying pipe, and then pumped into the 6.5m by a vacuum pump. 3 Carry out rectification in the rectification kettle, collect 80 ℃ / 2.66×10 respectively 3 Pa and 106-107℃ / 1.33×103 The cut of Pa obtains 88kg of chloroethylene carbonate, the content of chloroethylene carbonate is 94.5%, and the yield is 71.5%.
[0033] 2) After fully dissolving and mixing 61.3kg (500mol) chloroethylene ...
Embodiment 3
[0035] 1) After preheating 88.06kg (1000mol) ethylene carbonate to 30~40°C, fully dissolve and mix with 202kg (2500mol) sulfuryl chloride and 440g benzoyl peroxide in the mixer, inject at a flow rate of 0.1m / s In a horizontal tubular reactor, react at 50~55°C for 200~300 minutes to replace the SO produced by the reaction 2 and HCl are absorbed using a four-stage falling film absorption tower, and the chlorinated products enter the 1.5m 3 The intermediate tank is equipped with an emptying pipe, and the volatilized sulfuryl chloride in the tank is discharged into the main emptying pipe through the emptying pipe, and then pumped into the 6.5m by a vacuum pump. 3 Carry out rectification in the rectification kettle, collect 80 ℃ / 2.66×10 respectively 3 Pa and 106-107℃ / 1.33×10 3 The cut of Pa obtains 107kg of chloroethylene carbonate, the content of chloroethylene carbonate is 97.5%, and the yield is 87.3%.
[0036] 2) After fully dissolving and mixing 61.3kg (500mol) chloroethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com