Fluorescent probe for detecting nitroreductase and preparation method and application thereof in enzymatic reaction
A fluorescent probe, reductase technology, applied in biochemical equipment and methods, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve problems such as unfavorable use, expensive, and difficult to realize enzyme detection and analysis, and achieve obvious fluorescence enhancement. Good effect, good structural stability, easy to promote and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
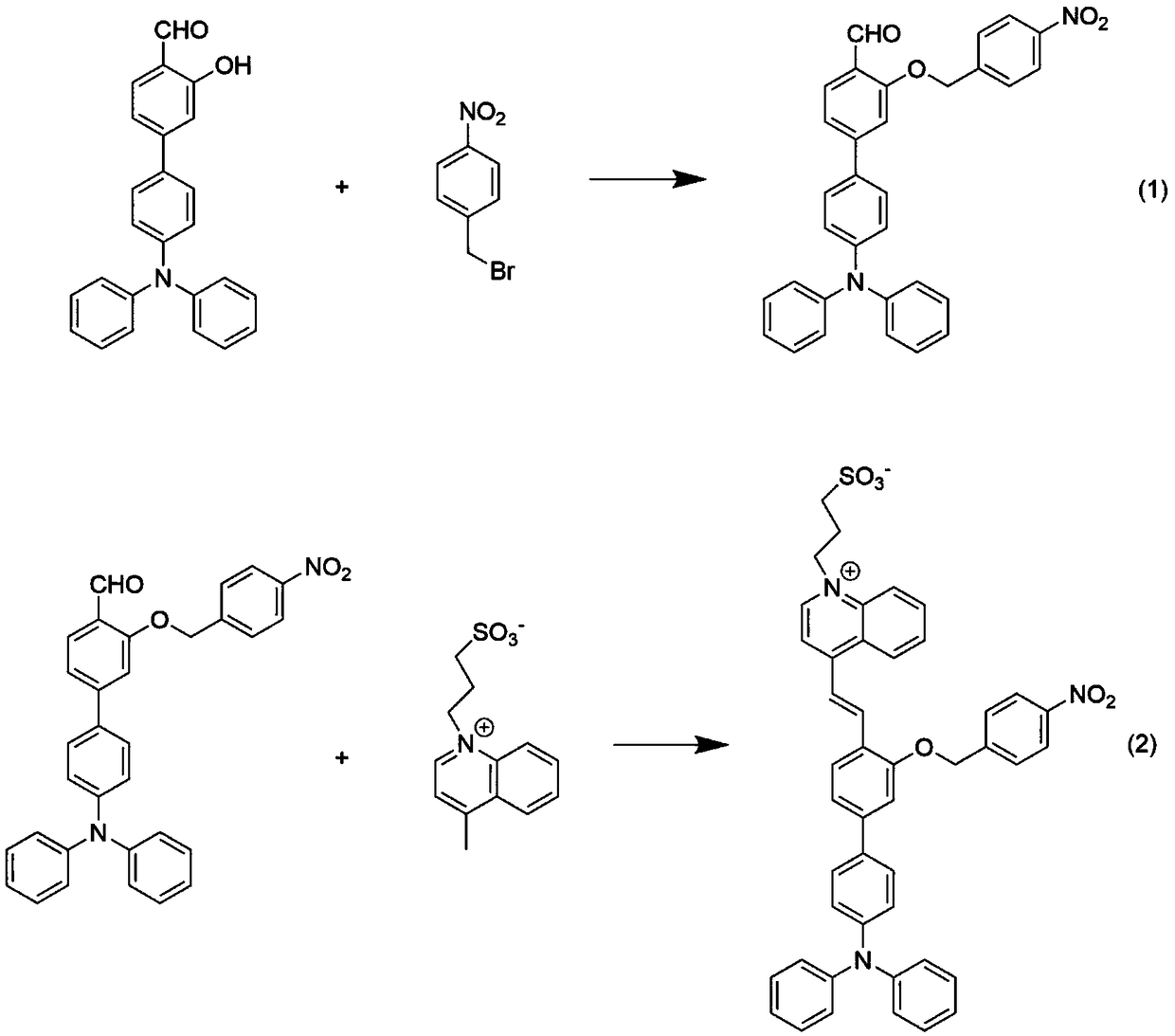
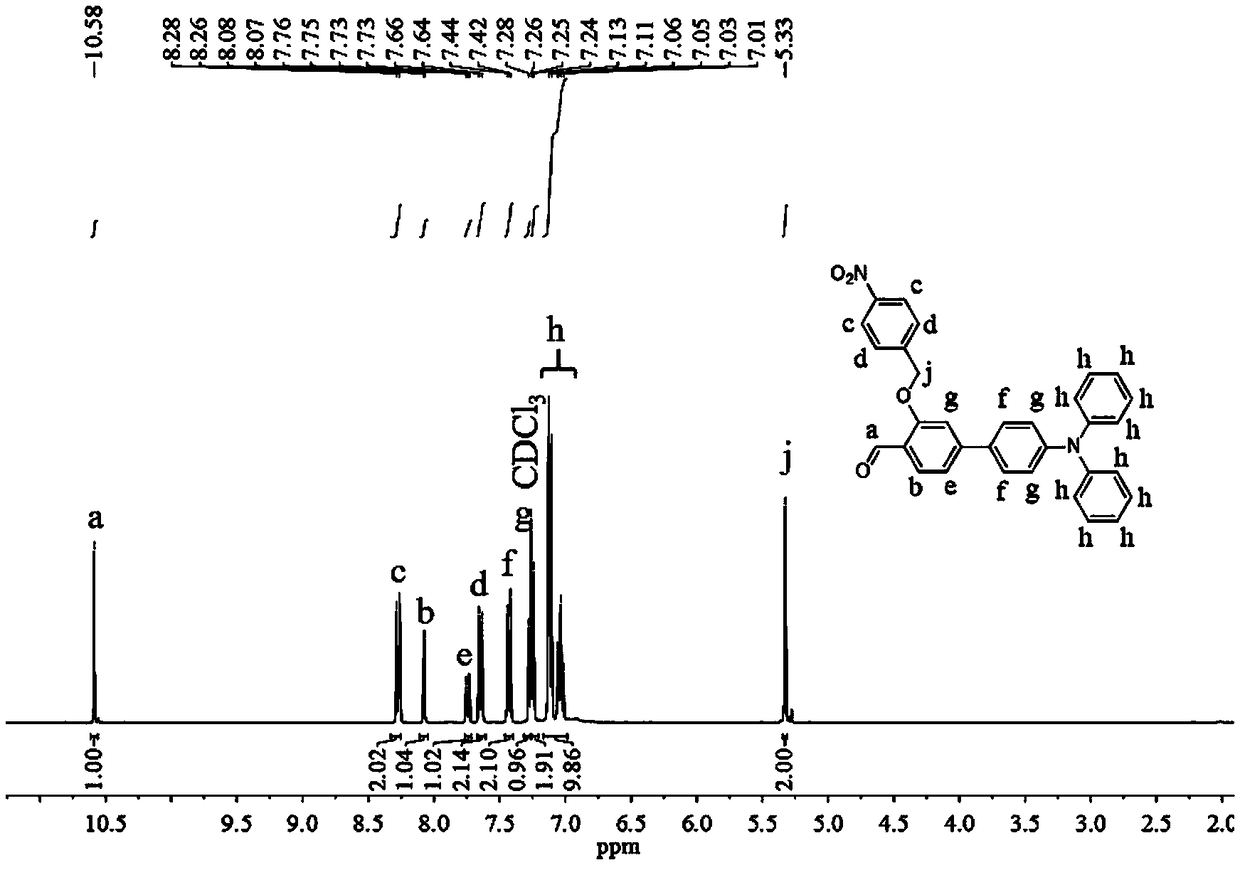
[0045] (1) Dissolve 365 mg of 4'-(diphenylamino)-3-hydroxy-[1,1'-biphenyl]-4-carbaldehyde in 10 mL of dimethyl sulfoxide, and 324 mg of p-nitrobenzyl bromide Dissolve in 10mL tetrahydrofuran, mix together after ultrasonication, and add 1.96g cesium carbonate, control the reaction temperature at 50°C, react for 5h, cool the reaction solution to room temperature, extract with dichloromethane / deionized water, collect the organic phase, and dry , filtered, and the solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel column chromatography (the eluent used was dichloromethane / petroleum ether, V / V=2:1) to obtain a yellow solid powder 4'-(diphenyl Amino)-3-((4-nitrobenzyl)oxy)-[1,1'-biphenyl]-4-carbaldehyde 405 mg (yield 81%); the product was analyzed by H NMR characterization, 1 H NMR (400MHz, CDCl 3 )δ(TMS,ppm):10.58(s,1H),8.27(d,J=8.6Hz,2H),8.07(d,J=2.4Hz,1H),7.74(dd,J=8.6,2.4Hz, 1H), 7.65(d, J=8.5Hz, 2H), 7.43(d, J=8.6Hz, 2H), 7.28(s, ...
Embodiment 2
[0048] (1) Dissolve 365 mg of 4'-(diphenylamino)-3-hydroxy-[1,1'-biphenyl]-4-carbaldehyde in 10 mL of dimethyl sulfoxide, and dissolve 389 mg of p-nitrobenzyl bromide Dissolve in 10mL tetrahydrofuran, mix together after ultrasonication, and add 2.64g cesium carbonate, control the reaction temperature at 100°C, react for 24h, cool the reaction solution to room temperature, extract with dichloromethane / deionized water, collect the organic phase, and dry , filtered, and the solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel column chromatography (the eluent used was dichloromethane / petroleum ether, V / V=2:1) to obtain a yellow solid powder 4'-(diphenyl Amino)-3-((4-nitrobenzyl)oxy)-[1,1'-biphenyl]-4-carbaldehyde 415 mg (yield 83%);
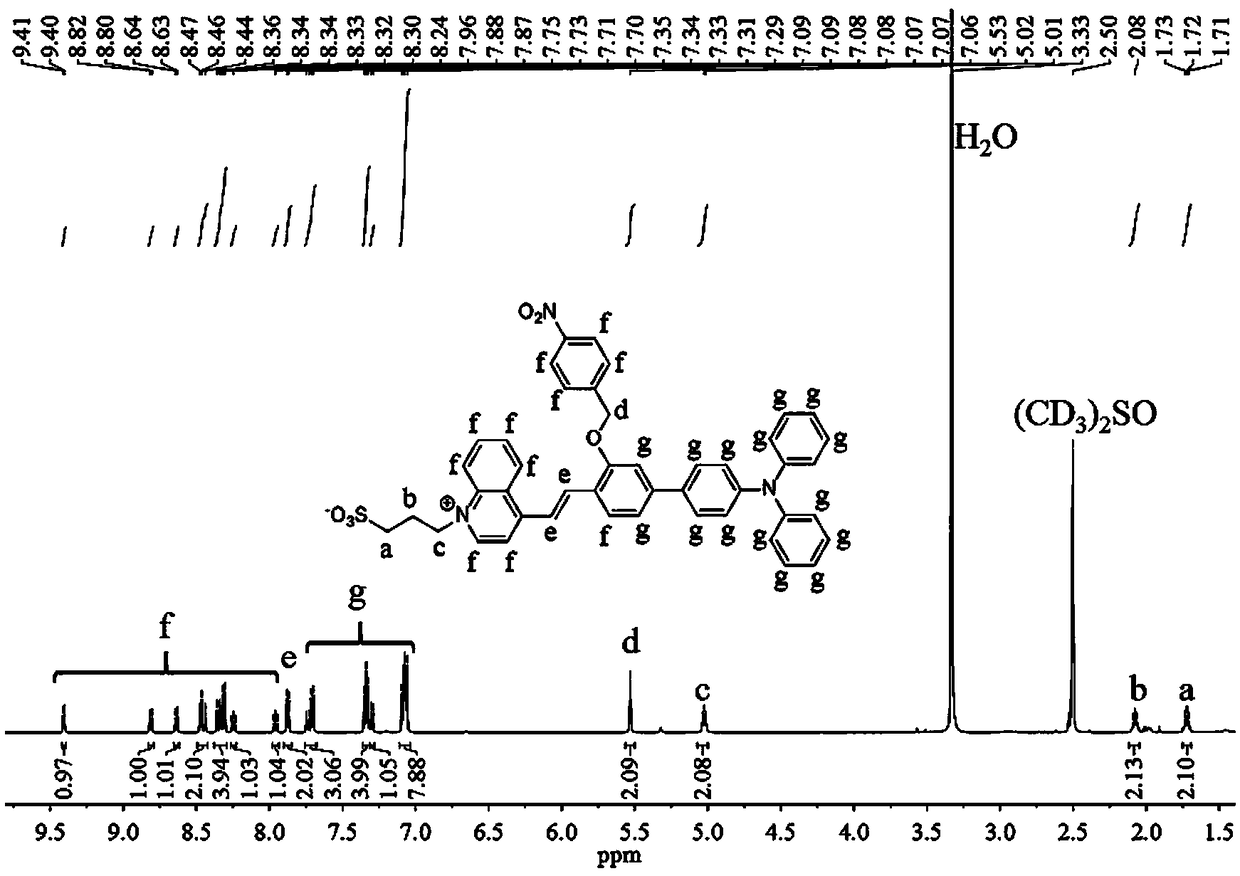
[0049] (2) Dissolve 265 mg of 3-(4-methylquinoline-1-bromo)propane-1-sulfonate in 10 mL of pyridine, then add 171 μL of acetic acid, mix well and add 750 mg of 4'-(diphenyl Amino)-3-((4-nitrobenzyl)oxy)-[1...
Embodiment 3
[0052] (1) Dissolve 365 mg of 4'-(diphenylamino)-3-hydroxy-[1,1'-biphenyl]-4-carbaldehyde in 10 mL of dimethyl sulfoxide, and 432 mg of p-nitrobenzyl bromide Dissolve in 10mL tetrahydrofuran, mix them together after ultrasonication, and add 3.26g cesium carbonate, control the reaction temperature at 150°C, react for 48h, cool the reaction solution to room temperature, extract with dichloromethane / deionized water, collect the organic phase, and dry , filtered, and the solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel column chromatography (the eluent used was dichloromethane / petroleum ether, V / V=2:1) to obtain a yellow solid powder 4'-(diphenyl Amino)-3-((4-nitrobenzyl)oxy)-[1,1'-biphenyl]-4-carbaldehyde 430 mg (yield 86%);
[0053](2) Dissolve 265 mg of 3-(4-methylquinoline-1-bromo)propane-1-sulfonate in 10 mL of pyridine, then add 228 μL of acetic acid, mix thoroughly and add 1000 mg of 4'-(diphenyl Amino)-3-((4-nitrobenzyl)oxy)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com