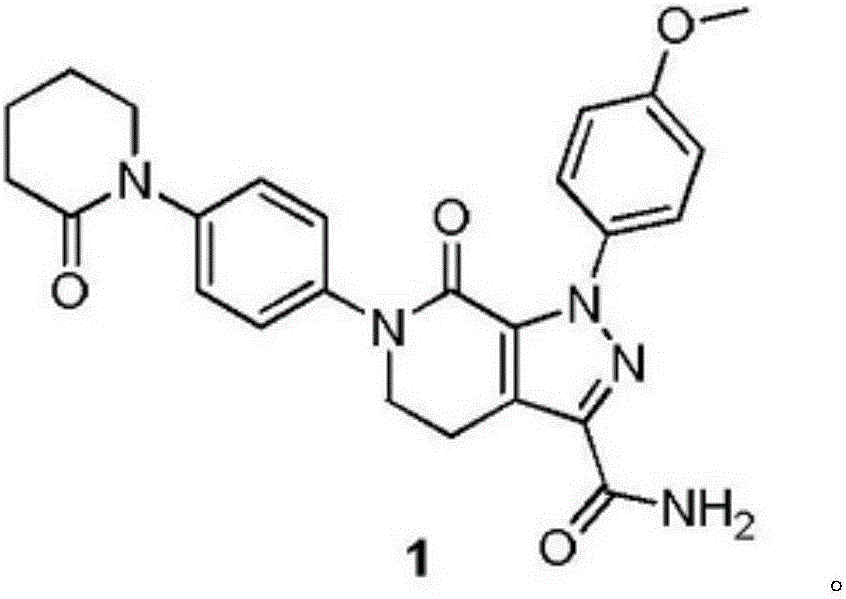
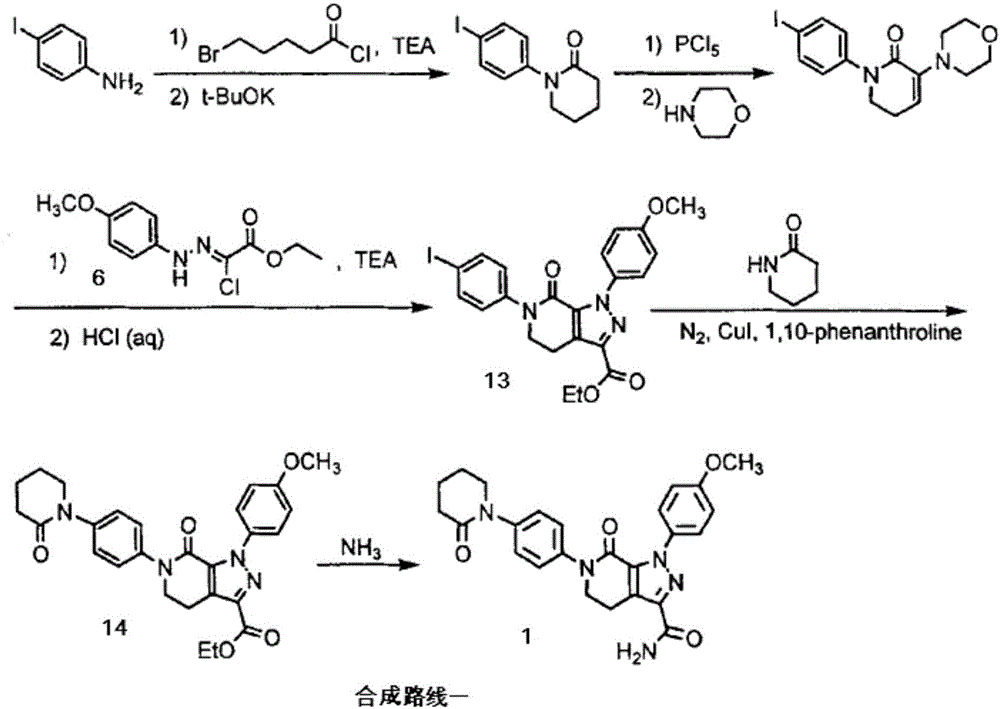
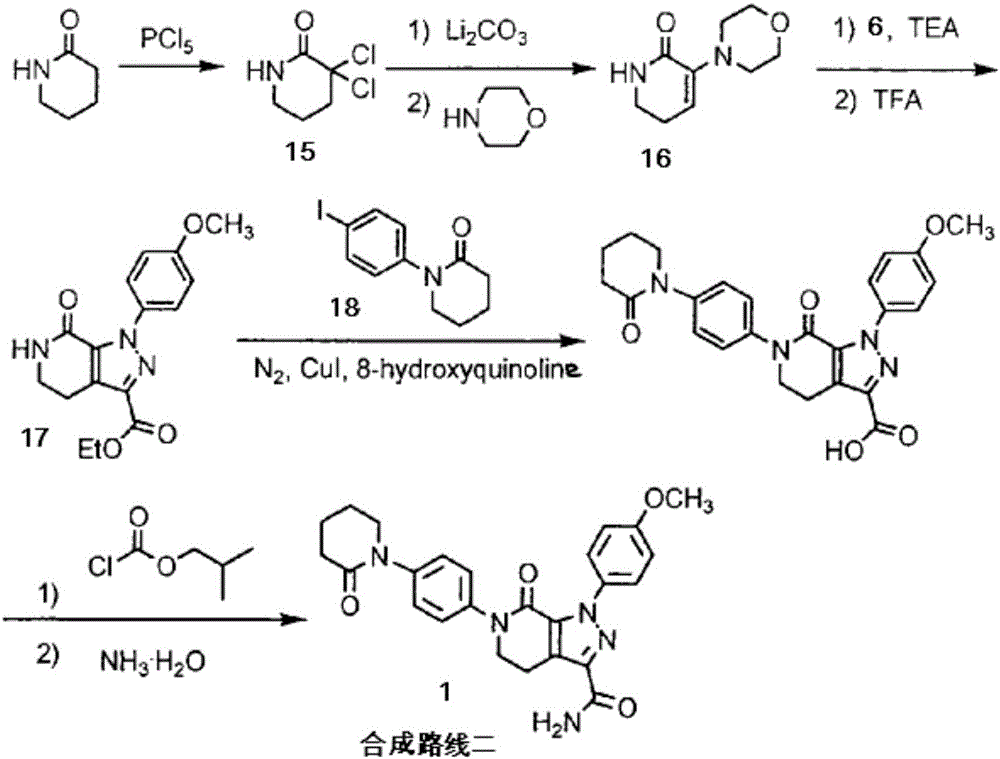
Preparation method of apixaban
A technology for apixaban and compounds, applied in the field of preparation of apixaban, can solve the problems of difficult monitoring of the reaction process, harsh reaction conditions, and high equipment requirements, and achieve optimization of reaction routes and conditions, mild reaction conditions, and simplified reactions The effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0050] Embodiment 1-1: Synthesis of compound 10
[0051] Prepare a 2M trimethylaluminum hexane solution at 5°C for later use; add 1000 mL of dichloromethane and 69.1 g of p-nitroaniline (compound 11, 0.5 mol) in sequence in the reaction flask, and add trimethylaluminum hexane with a concentration of 2M 500 mL (1 mol) of a hexane solution of aluminum base, stirred at room temperature for 2 hours, then added 50.1 g of δ-valerolactone (compound 12, 0.5 mol), heated to 40 ° C, and reacted for 20 hours. TLC monitored the completion of the reaction and cooled to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic layers, wash with anhydrous MgSO 4 After drying, filtering, and concentrating under reduced pressure, 110.7 g of compound 10 was obtained. The HPLC purity was 98.5%, and the yield was 91.5%.
Embodiment 1-2
[0052] Embodiment 1-2: the synthesis of compound 10
[0053] Prepare a toluene solution of trimethylaluminum with a concentration of 2M at 5°C for subsequent use; add 1000 mL of toluene and 138.1 g of p-nitroaniline (compound 11, 1 mol) in sequence in the reaction flask, and add a solution of trimethylaluminum with a concentration of 2M 750 mL (1.5 mol) of toluene solution was stirred at room temperature for 2 hours, then 50.1 g of δ-valerolactone (compound 12, 0.5 mol) was added, heated to 60 ° C, and reacted for 10 hours. TLC monitored the completion of the reaction and cooled to room temperature. Add water to quench the reaction, extract with ethyl acetate, combine the organic layers, wash with anhydrous MgSO 4 After drying, filtering, and concentrating under reduced pressure, 114.9 g of compound 10 was obtained. The HPLC purity was 99.3%, and the yield was 95.8%.
Embodiment 2-1
[0054] Embodiment 2-1: Synthesis of Compound 8
[0055] Add 96.7g of compound 10 (prepared in Example 1-1, 0.4mol), 72.4g of diisopropylethylamine (0.56mol), 3000mL of dichloromethane into the reaction flask, add 100.2g of methanesulfonyl chloride dropwise at 5°C (0.52mol), after adding, react at this temperature for 1-2 hours, TLC follows the reaction until the reaction is complete. Continue to add 68.1 g of sodium ethoxide (1.0 mol) to the reaction flask, raise the temperature to 50° C. and react for 2 hours, and follow the reaction by TLC until the reaction is complete. Add 2000mL of saturated ammonium chloride solution and 2000mL of ethyl acetate to the reaction solution, extract in layers, dry the organic layer with anhydrous sodium sulfate for 4h, filter, concentrate the filtrate to dryness in vacuo, and recrystallize from ethanol to obtain 76.5g of compound 8, molar yield Yield 92.3%, HPLC purity 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com