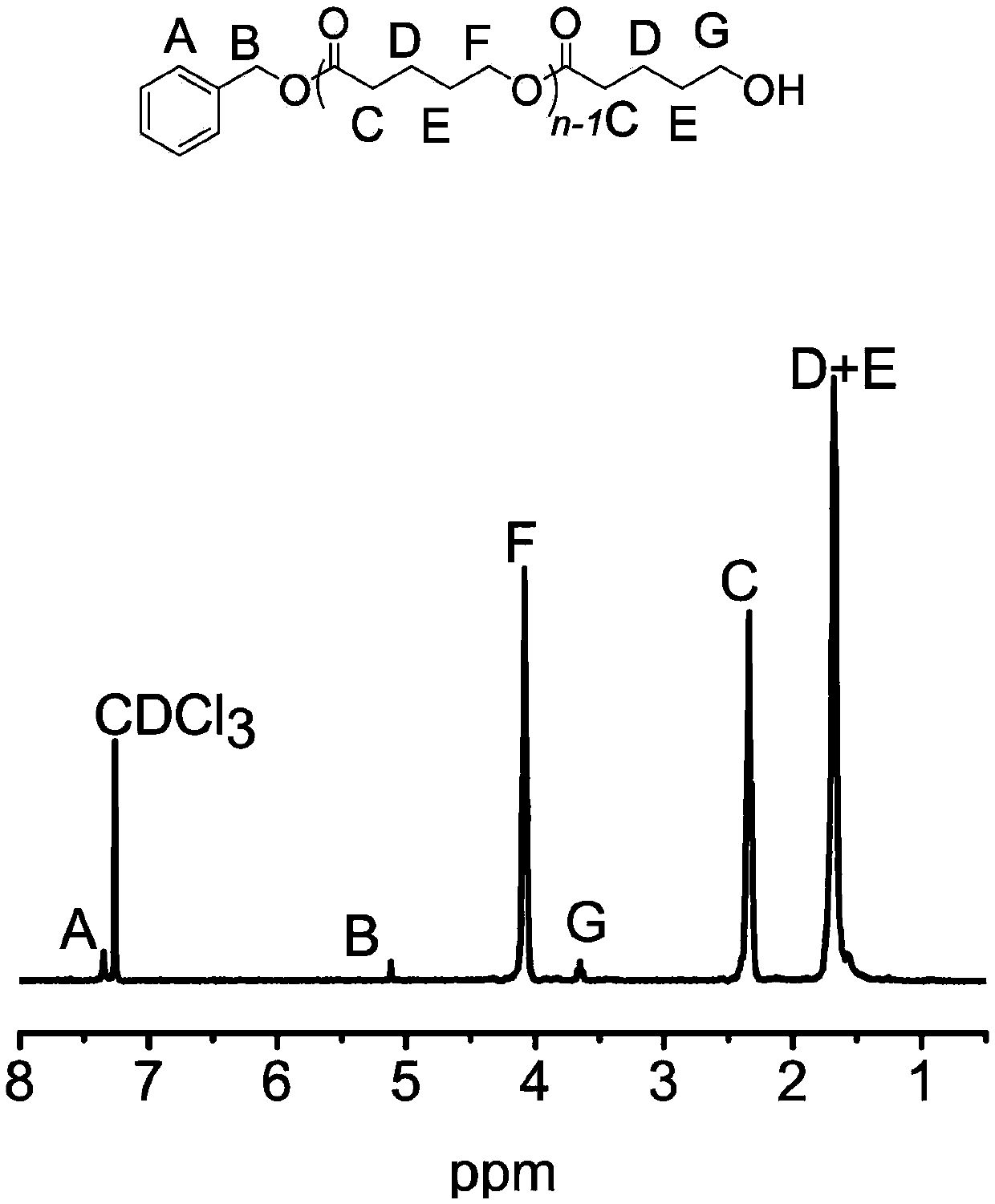
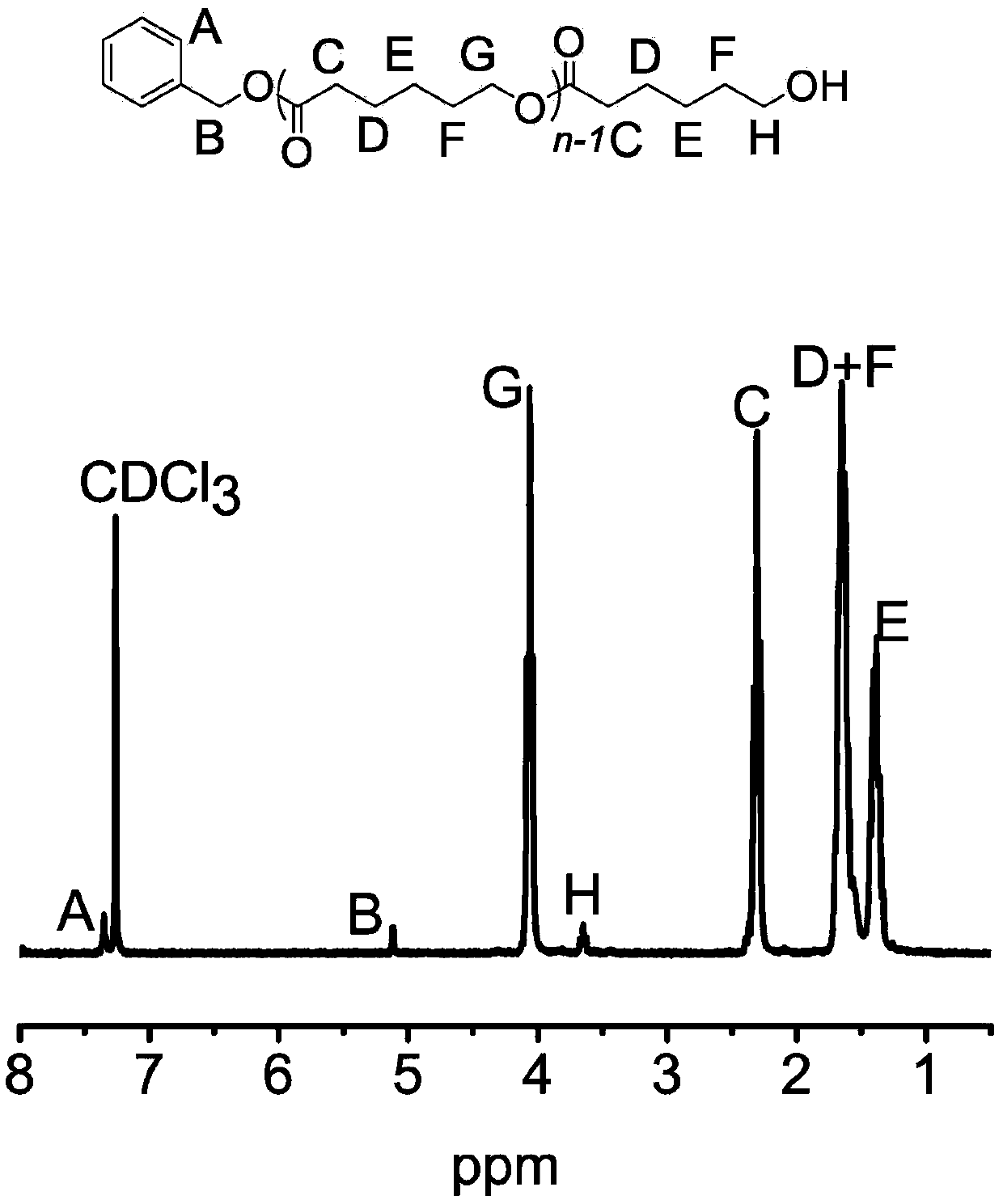
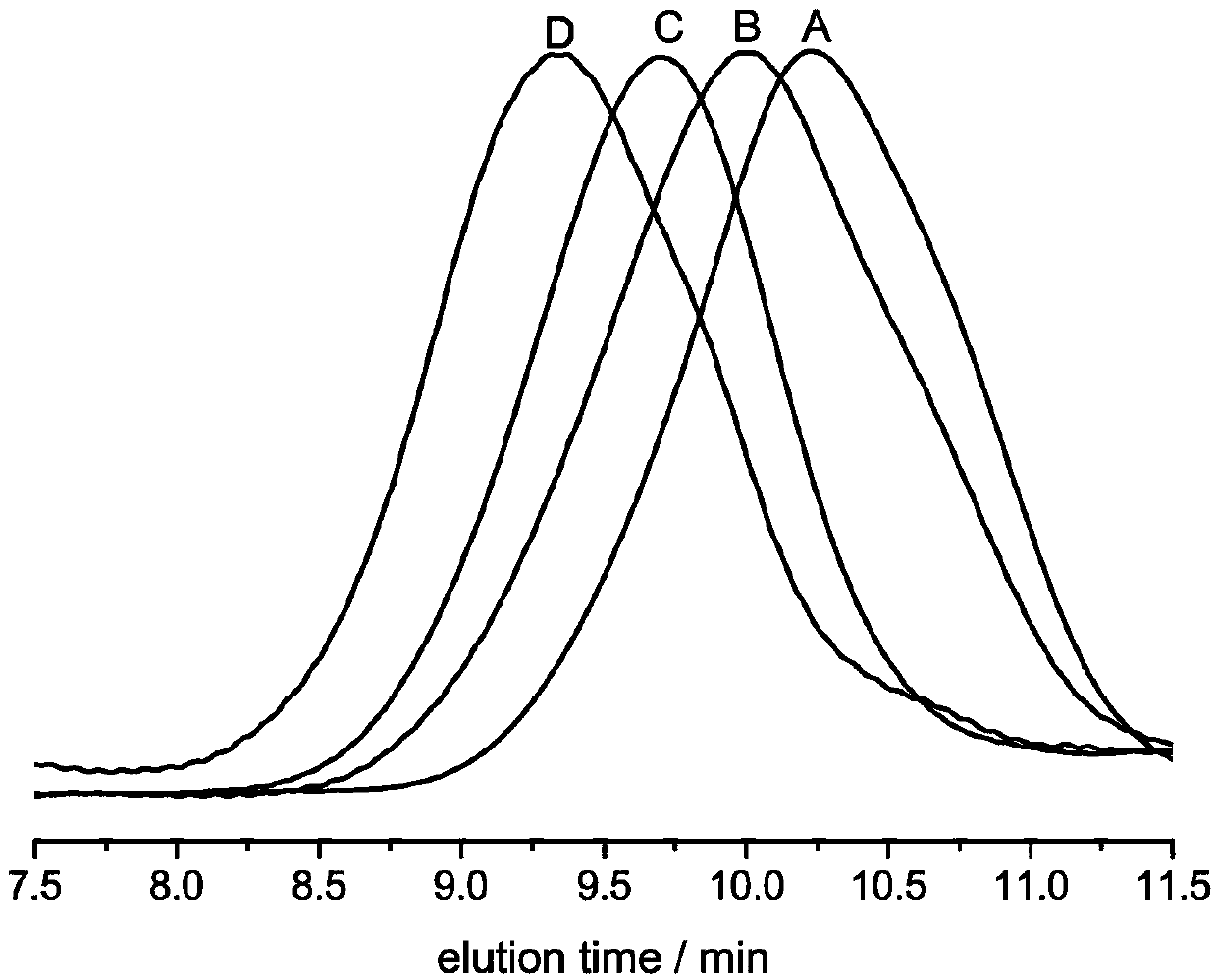
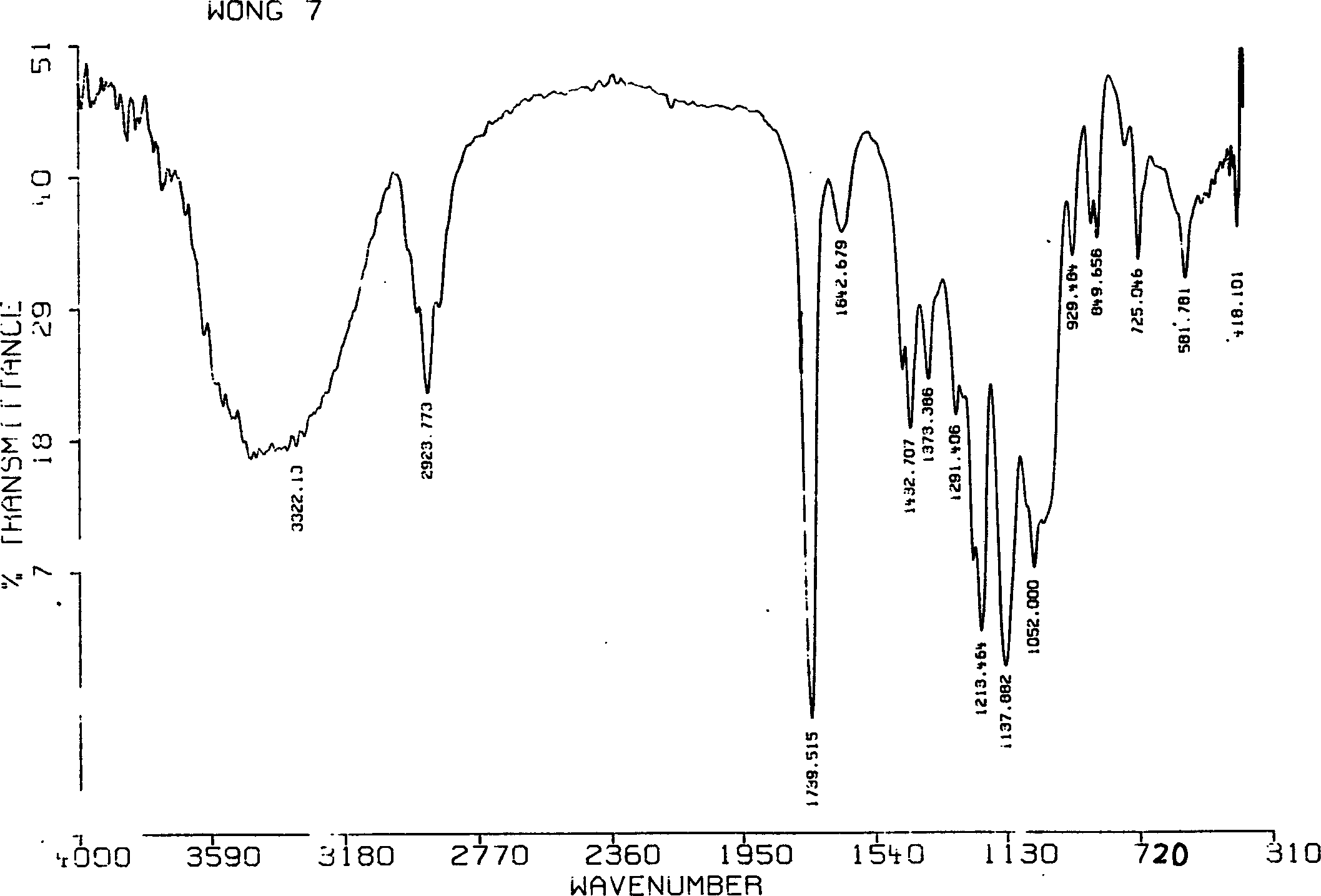
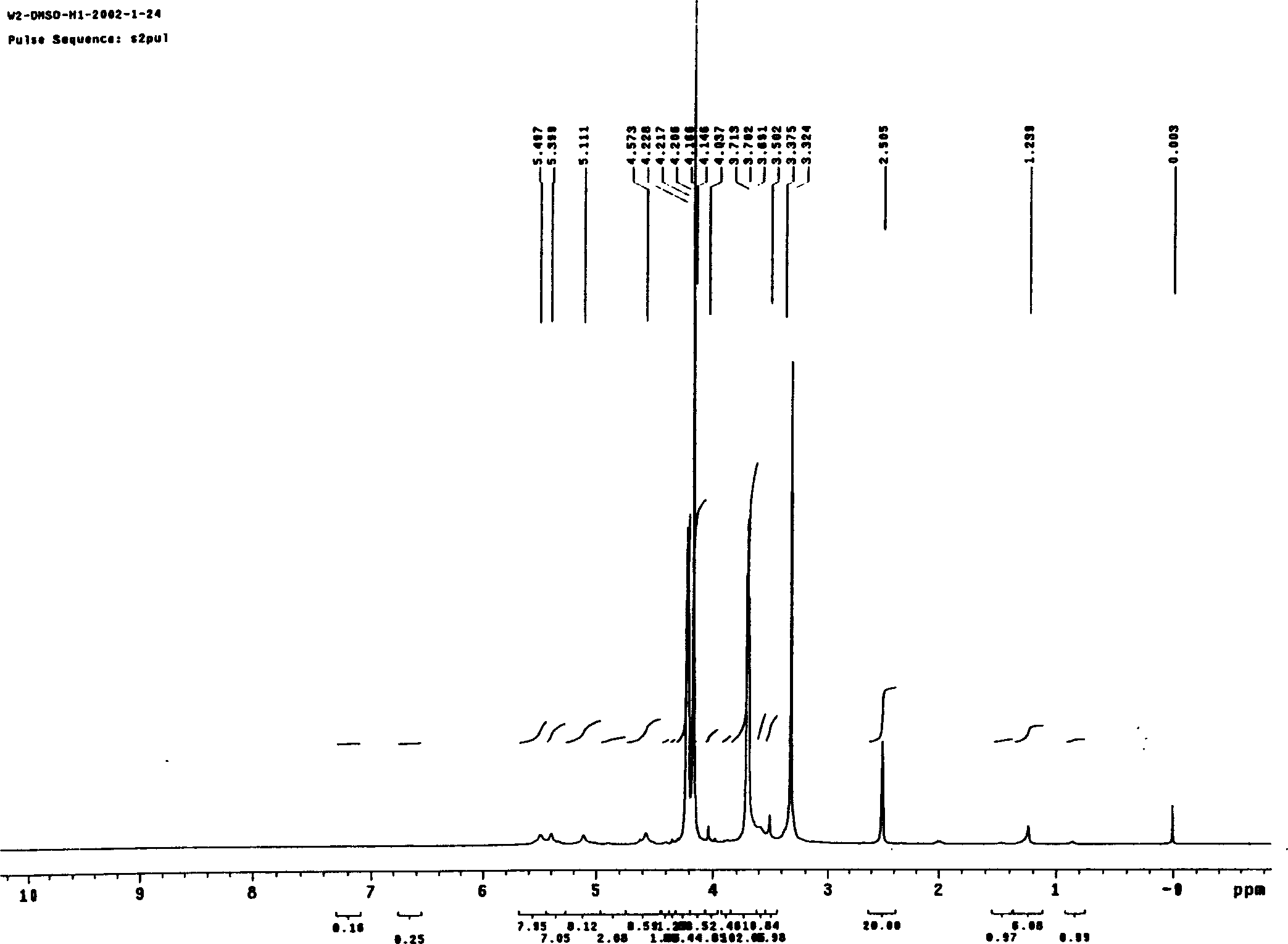
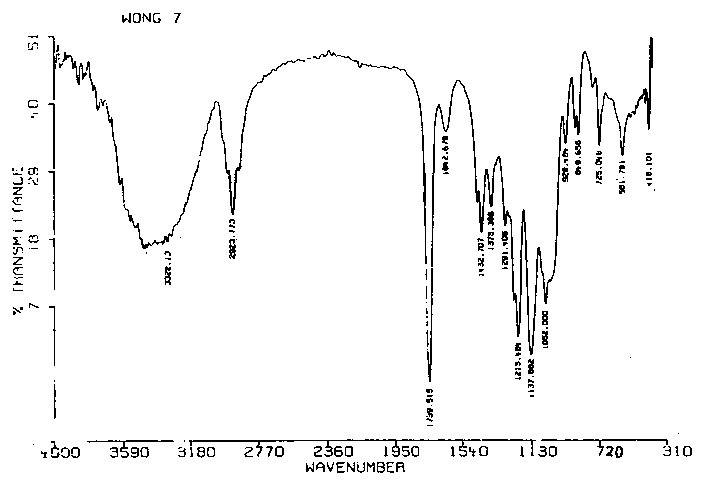
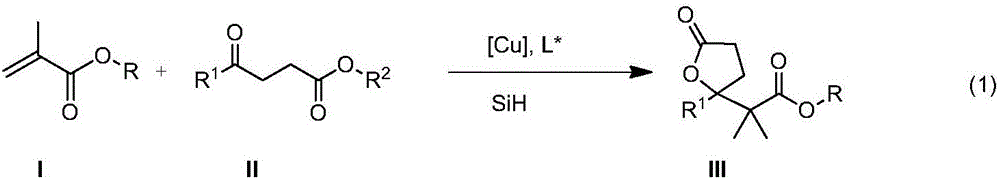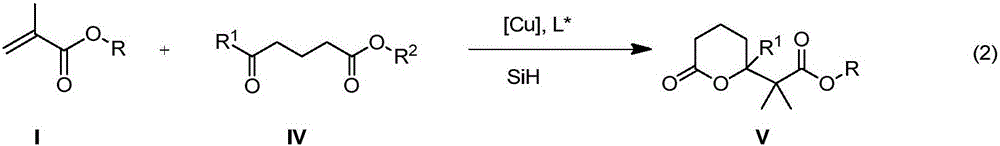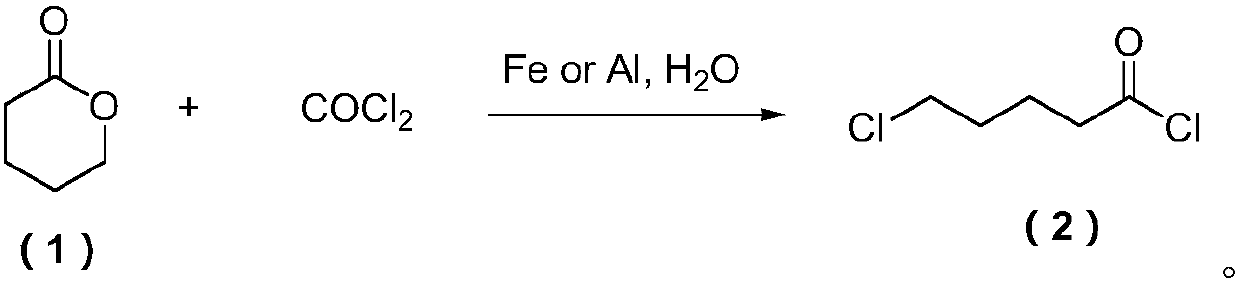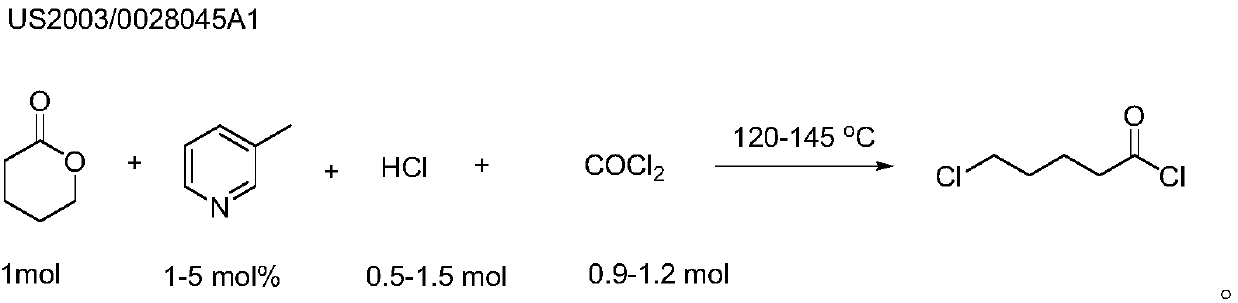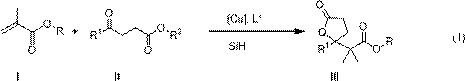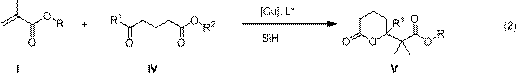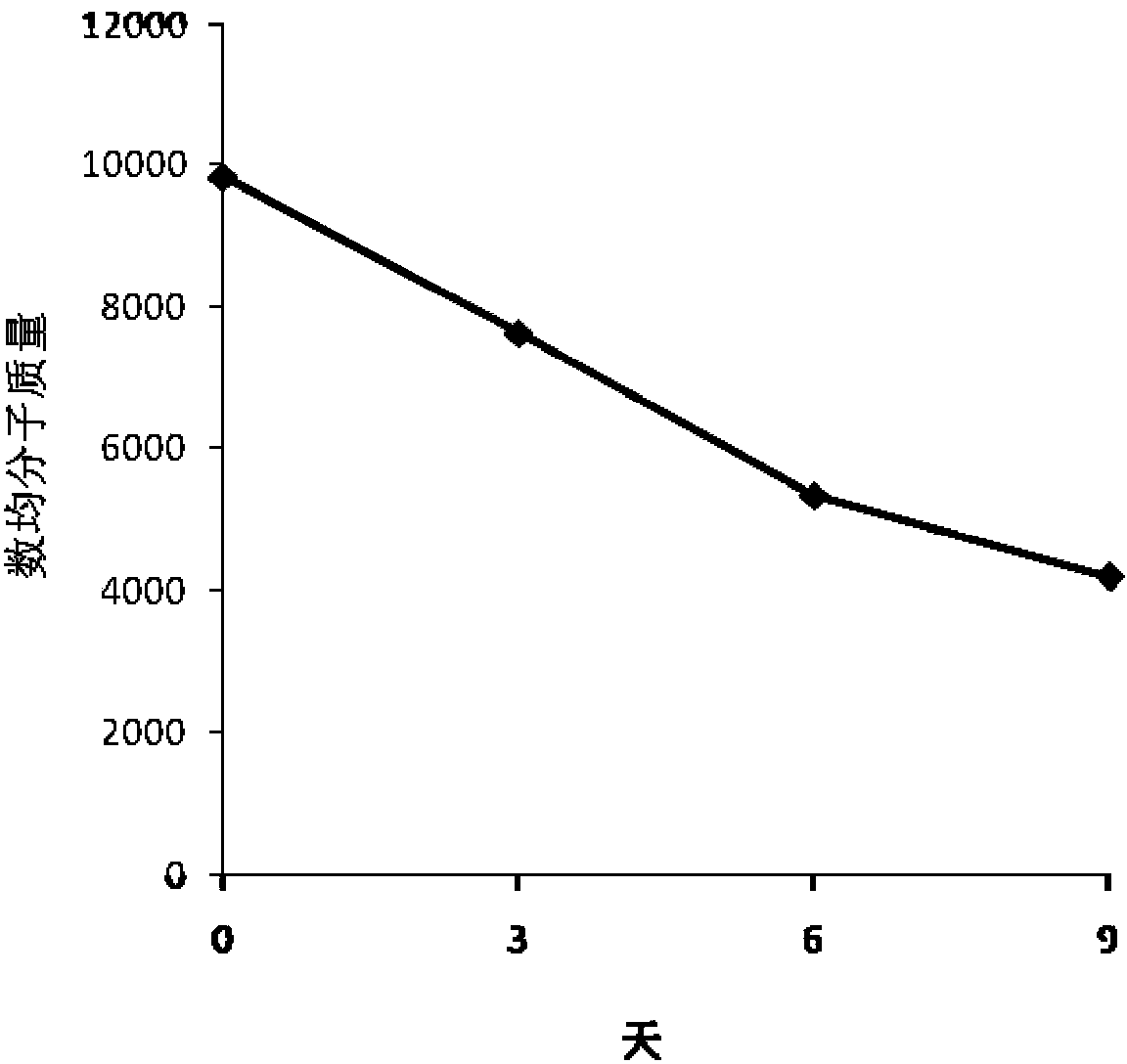Patents
Literature
35 results about "Delta-Valerolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
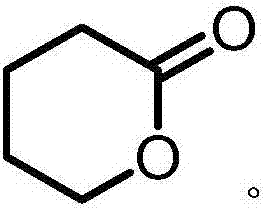
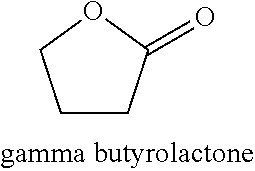
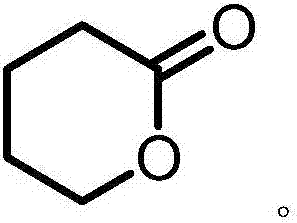
Δ-Valerolactone is a lactone used as a chemical intermediate in processes such as the production of polyesters.
Load type nano gold catalyst for preparing lactone by catalyzing air oxidation alpha, omega-diol and preparation method thereof
InactiveCN101683619AImprove conversion rateHigh selectivityOrganic chemistryChemical recyclingChemical industryGold particles
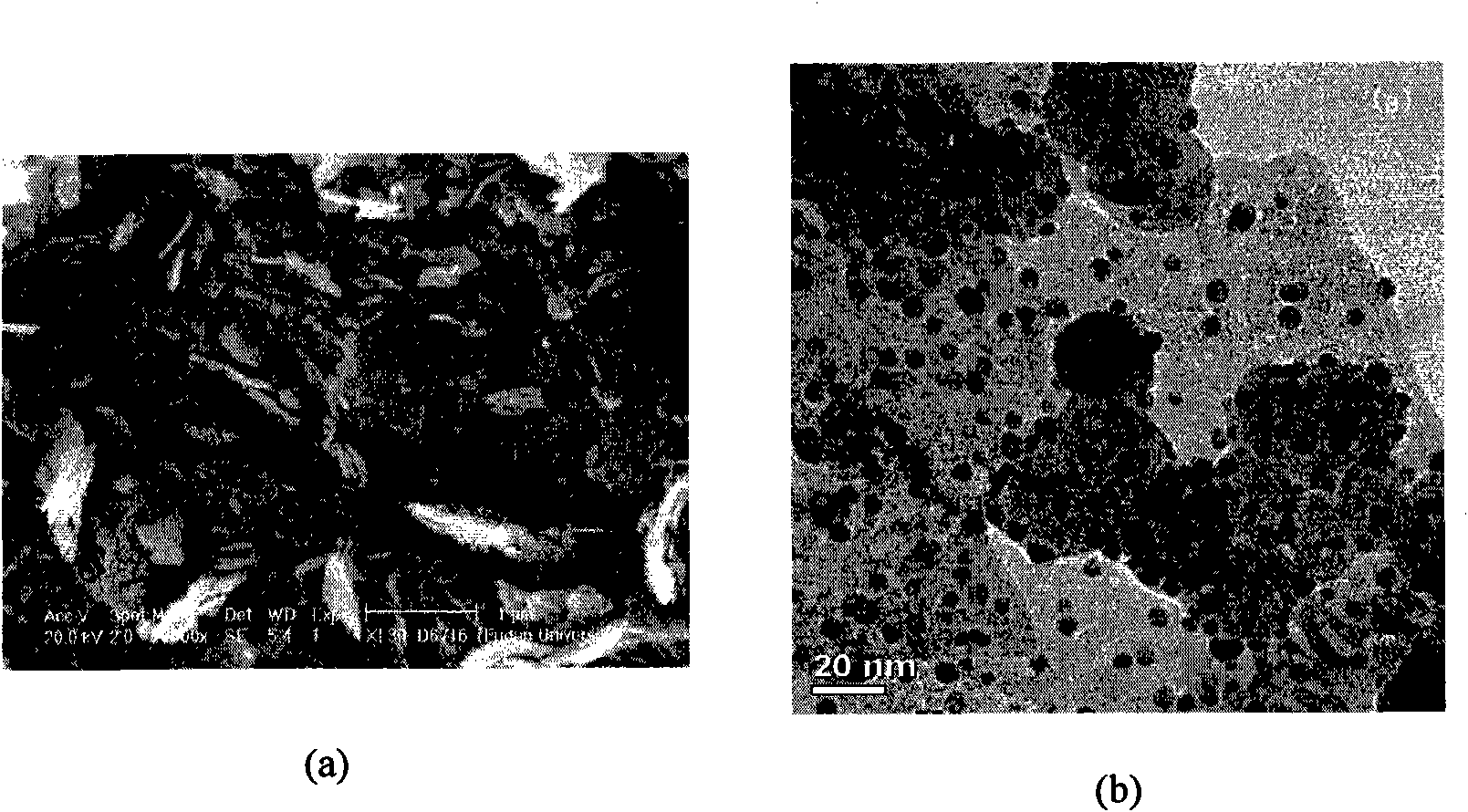
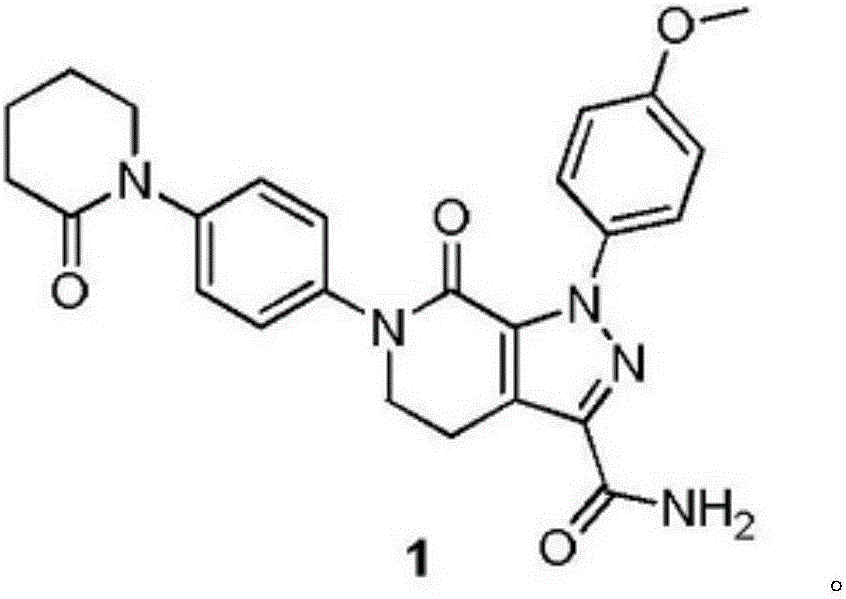
The invention belongs to the technical field of the chemical industry, which relates to a load type nano gold catalyst for preparing lactone by catalyzing air oxidation alpha, omega-diol. The invention adopts nano iron oxide as a carrier of a gold catalyst, the iron oxide is synthesized by a hydrothermal method, and nano iron oxide carriers with different crystal types and patterns can be preparedby calcination. The gold catalyst is prepared by a precipitation settling method, the prepared catalyst has small gold particles and good dispersivity, the interaction of gold and the carrier is strong, the gold catalyst represents an excellent activity in the preparation of gamma-butyrolactone by the direct oxidation of 1,4-butanediol through catalyzing air and the preparation of delta-valerolactone by oxidizing 1,5-pentanediol and conforms to the requirement of green chemistry, and the generation of lactone by oxidizing alpha, omega-diol in one step is realized. A magnetic iron oxide carrier can be obtained by selecting a proper preparation method, and the invention is convenient to separate and recover the catalyst and has better industrial application prospect.
Owner:FUDAN UNIV
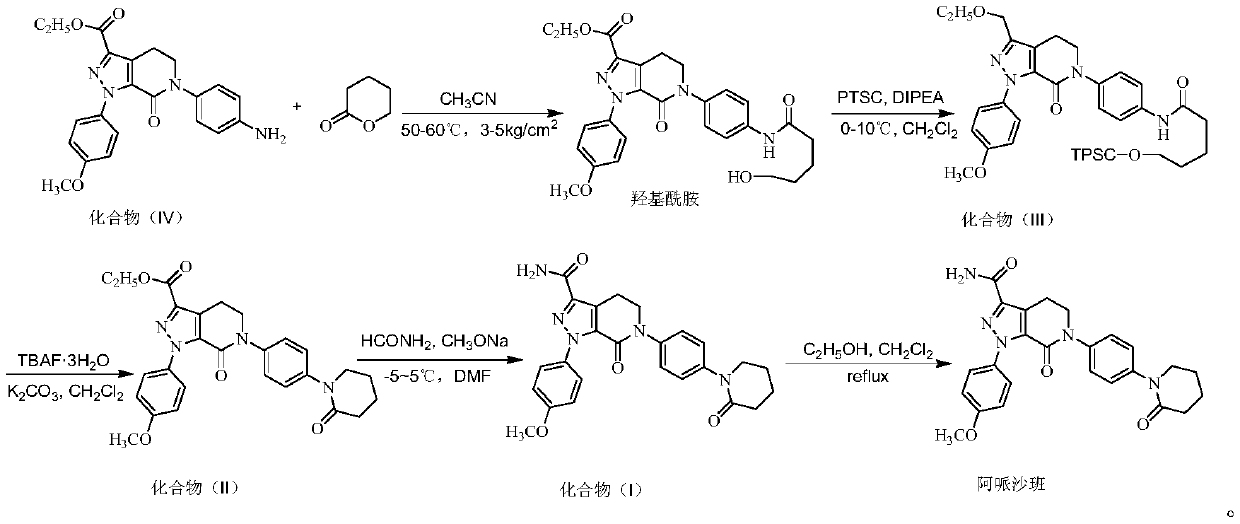
Preparation method of apixaban
ActiveCN105732622AAdaptable to conditionsFast aminolysisOrganic chemistryP-NitroanilineNitro reduction
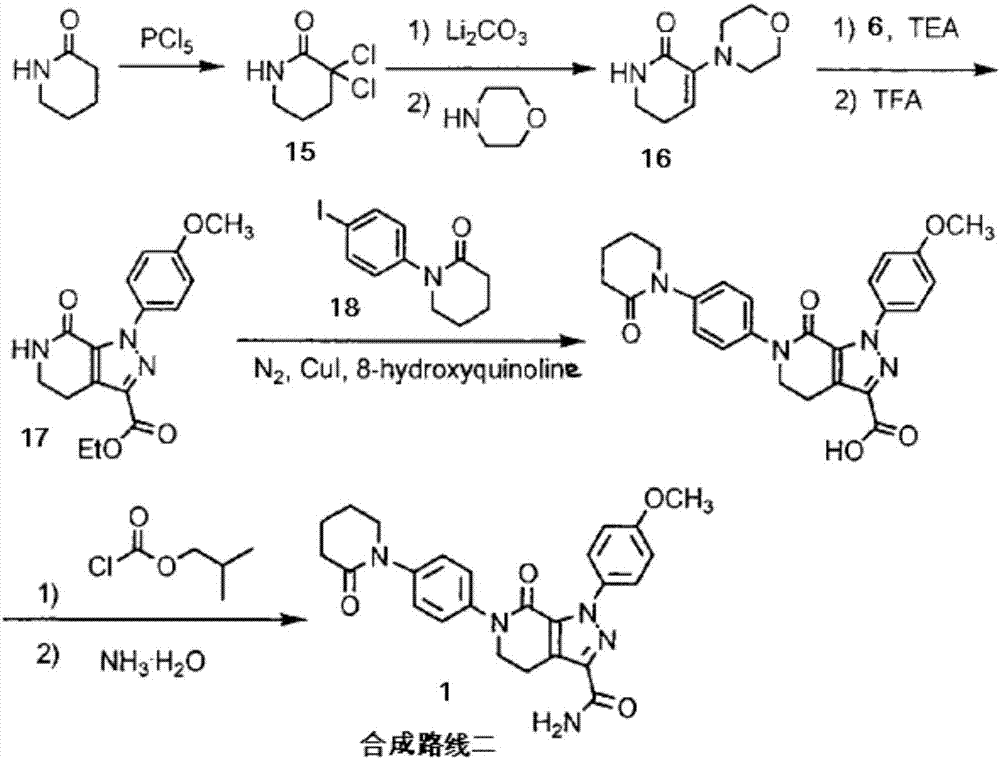
The invention discloses a preparation method of apixaban.The method comprises the steps that paranitroaniline serves as the raw material, paranitroaniline and delta-valerolactone are subjected to amidation ring-opening, substituting and ring-closing reactions under the action of AlMe3, and a compound 8 is obtained; the compound 8 is subjected to alpha-position dichloro substituting and condensation-elimination reactions, and a compound 7 is obtained; the compound 7 and a compound 6 are subjected to [3+2] cyclization-elimination and nitro reduction, and a compound 4 is obtained; the compound 4 sequentially reacts with delta-valerolactone and ammonium chloride under the action of AlMe3, and a compound 3 is obtained; the compound 3 is subjected to substituting and ring closing, and apixaban is obtained.According to the preparation method of apixaban, paranitroaniline and delta-valerolactone which are low in price are adopted to serve as the raw materials, operation of the whole route is simple, conditions of each reaction are mild, and the synthesizing method is easy to operate, high in yield and purity and suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for preparing bromopentoic acid
InactiveCN1757624ASimple processEasy to form large-scale productionPreparation from carboxylic acid esters/lactonesAlkaneOrganic solvent
This invention relates to a manufacturing technique of organic compounds, or a preparation method for 5-bromo-valeric acid. Based on the method dry hydrogen bromide gas is going through a delta-valerolactone containing organic solvent, the ring of delta-valerolactone is easy to have cleavage reaction, corresponding 5-bromo-valeric acid is produced with high yield. As 5-bromo-valeric acid is hard to dissolve in alkanes, cycloalkanes and aromatic compounds solvents, cooling it below 30 DEG C, lower than the melting point of 5-bromo-valeric acid, 5-bromo-valeric acid crystallized and deposited. Then, 5-bromo-valeric acid solid with high purity is separated through filtration technique. This method has simple technique, easy to form scaled production, with wide application prospect and good economic benefit.
Owner:FUDAN UNIV
Method for preparing aliphatic polyesters
The invention discloses a method for preparing aliphatic polyesters and belongs to the field of macromolecular synthetic chemistry. According to the method, delta-valerolactone (delta-VL) and epsilon-caprolactone (epsilon-CL) monomers serve as reaction substrates, a combination of thiourea and carboxylic acid serves as a co-catalyst, organic alcohol serves as an initiator, a polymerization reaction is carried out for 25-195 hours in an organic solvent or under solvent-free conditions, and polyvalerolactone and polycaprolactone are obtained after the reaction ends. According to the method provided by the invention, the disadvantage that metal residual is caused due to the fact that polyvalerolactone and polycaprolactone are prepared by using a metal catalyst in the past is overcome, and the preparation rate is accelerated. The method, which is simple in process, low in cost and high in catalysis efficiency and is environment-friendly, is provided for preparing polyvalerolactone and polycaprolactone.
Owner:NANJING UNIV OF TECH
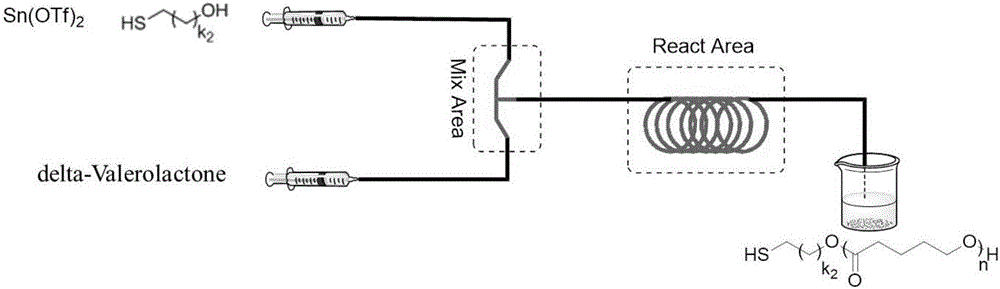
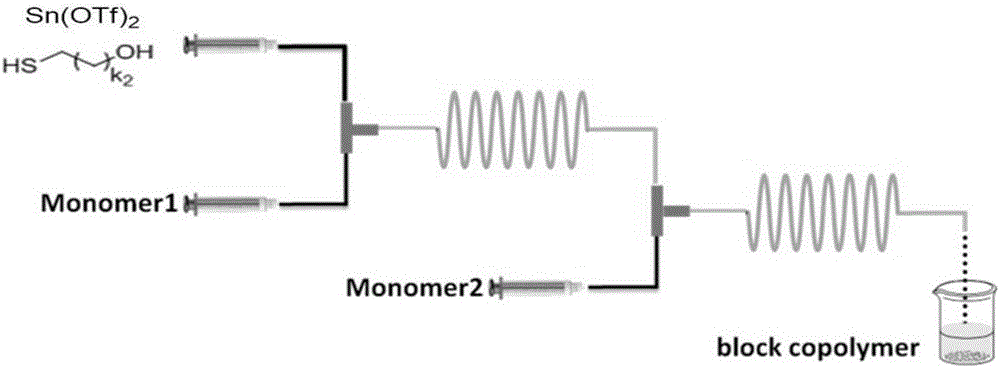
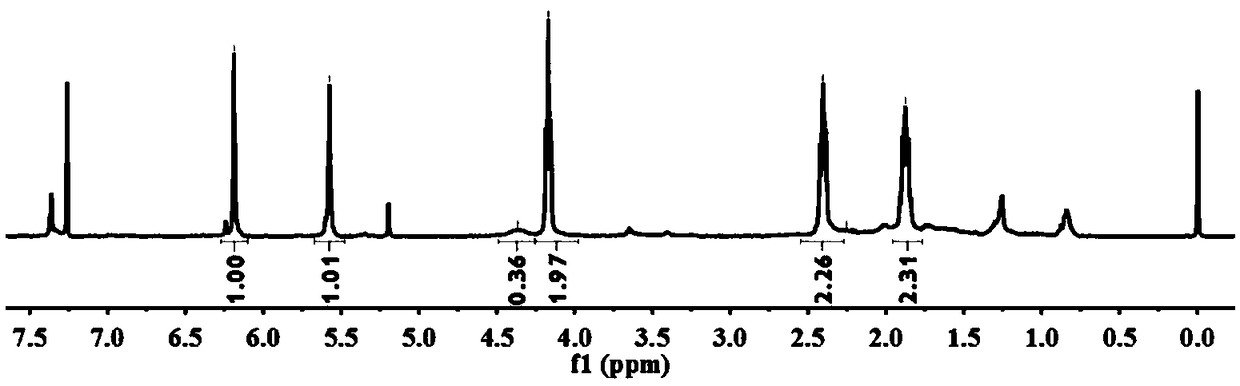
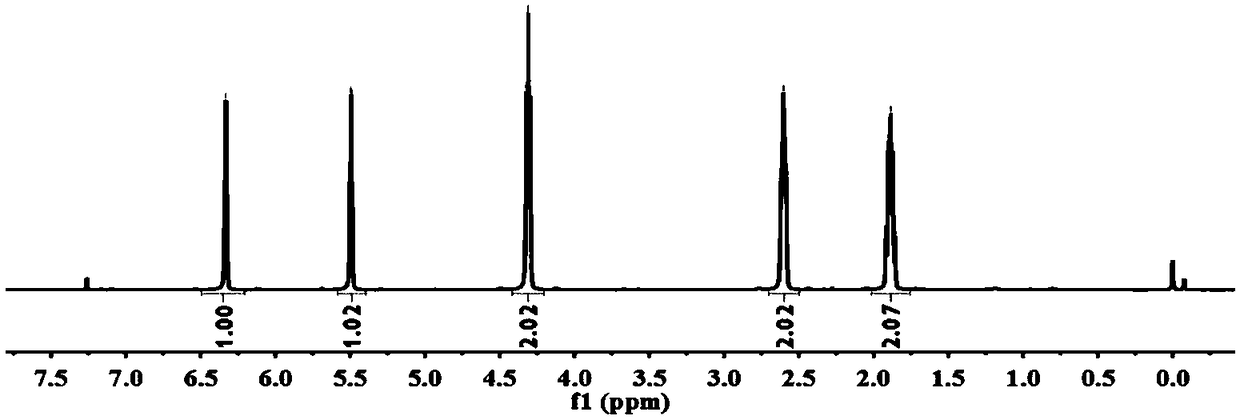
Method for preparing thiol-functionalized polyester with micro-reaction unit
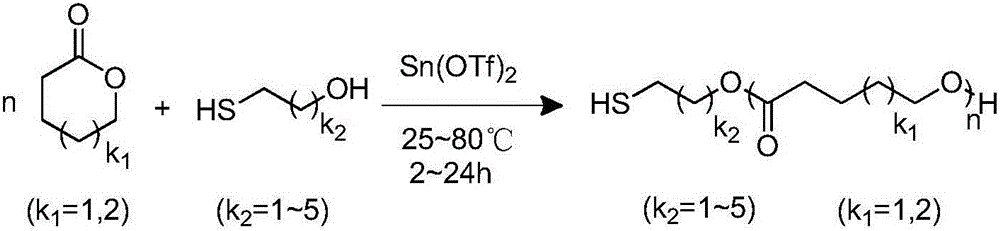
The invention discloses a method for preparing thiol-functionalized polyester with a micro-reaction unit. Delta-valerolactone (VL) or epsilon-caprolactone (CL) is used as a reaction monomer, tin trifluoromethanesulfonate (Sn(OTf)2) is used as a metal complex catalyst, fatty alcohol with an end thiol group is used as a functionalization initiator, and the thiol-functionalized polyester is prepared in the micro-reaction unit. Compared with the prior art, the adopted metal complex catalyst is simple and easy to obtain and high in catalytic activity, the technology is high in operability, and cost is low. Meanwhile, the whole polymerization process is short, reaction conversion rate is high, polymer thiol introduction rate is high, and high-precision control of polymerization reaction is achieved.
Owner:NANJING UNIV OF TECH
Recyclable polyester material with main chain containing active double bonds as well as preparation method and application thereof
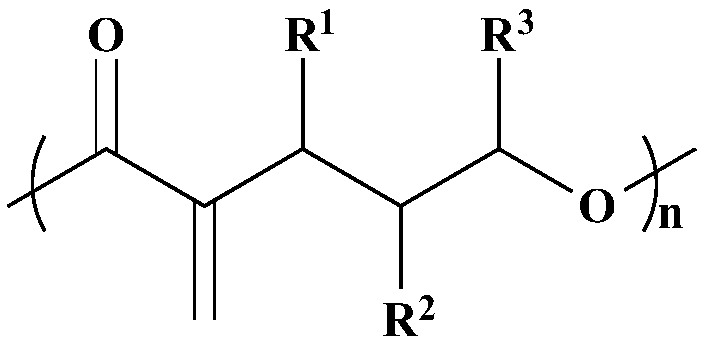
The invention belongs to the field of high polymer materials, and relates to a recyclable polyester material with a main chain containing active double bonds. According to the polyester material, 2-vinyl-delta-valerolactone is polymerized under the action of a catalyst so as to form the recyclable polyester material, and the polyester material can be degraded into the 2-vinyl-delta-valerolactone under the action of the catalyst. The polyester material has the advantages of being easy to obtain, capable of being quantitatively degraded into polymeric monomers and the like.
Owner:DALIAN UNIV OF TECH
Delta-valerolactone-based polymer and preparation method and application thereof
ActiveCN107118340AEffective cohesionLow toxicityOther foreign material introduction processesPolymer scienceGreen fluorescent protein
The invention provides a delta-valerolactone-based polymer. The structure of the delta-valerolactone-based polymer is subjected to infrared, nuclear magnetism and gel permeation chromatography characterization. The invention further discloses a preparation method and application of the delta-valerolactone-based polymer. The delta-valerolactone-based polymer is prepared through a Click reaction and a ring-opening polymerization, the reaction is simple and convenient, and the yield is high. The delta-valerolactone-based polymer is degradable under a certain condition, and acts with nucleic acid to form nano-particles easy to adsorb by cells; green fluorescent protein (GFP) and luciferase (Luciferase) expression experiments testify that the polymer can serve as a degradable non-viral gene vector and has high transfection efficiency; and the preparation method of the polymer and a preparation method of a transgenic vector are simple, mature and easy to learn.
Owner:BEIJING NORMAL UNIVERSITY
Starch / C4~C8 ring graft copolymer and prep. and use thereof
The present invention is starch / C4-C8 cyclic monomer grafted copolymer and its preparation process. Starch of corn, beans, potato and grains in grain size of 1-20 microns or modified starch in 1-60 portions and one or several C4-C8 cyclic monomer p-dioxy cyclohexanone, 1, 5-dioxy suberyl-2-one, gamma-butyrolactone, beta-butyrolactone, delta-valerolactone, epsilon-caprolactone, diglycolide and lactide in 40-99 portions react in a reaction bottle under the protection of inert gas and in the presence of catalyst in 0.01-5 portions at 60-150 deg.c for 10-48 hr to produce grafted copolymer. The unreacted monomer and homopolymer are extracted separately with solvent, the product is vacuum dried to constant weight, and the monomer converting rate, grafting rate and grafting efficiency are measured.
Owner:SICHUAN UNIV
Method for enantioselective synthesis of gamma-substituted-gamma-butyrolactone and delta-substituted-delta-valerolactone
The invention belongs to the field of chemical technology and relates to a method for enantioselective synthesis of gamma-substituted-gamma-butyrolactone and delta-substituted-delta-valerolactone. A cu-chiral phosphine catalyst is used to promote the reaction of silicon hydride, methacrylates, 3-acyl propionate or 4-acyl butyrate in an organic solvent to synthesize chiral gamma-alkoxyl formyl methyl-gamma-butyrolactone and delta-alkoxyl formyl methy-delta-valerolactone with important biological activity. Compared with the prior art, the method provided by the invention has enantioselectivity, the ee value of the product is up to 84%, and any one isomer of enantiomers can be preferentially obtained by adjusting the configuration of the ligand; the target compound obtained according to the invention contains a chiral ring-lactone structure and has application value.
Owner:DALIAN UNIV
Epsilon-caprolactone polymer
The present invention relates to one kind of epsilon-caprolactone polymer capable of being used as biomedicine material. The epsilon-caprolactone polymer is prepared with 4-carbonyl-epsilon-caprolactone as initial material, and through the first homopolymerization to obtain homopolymer or copolymerization together with epsilon-caprolactone, delta-valerolactone, lactide and / or glycolide to obtain copolymer, the subsequent selective reduction of the homopolymer or copolymer, and the final graft copolymerization of the reduced product and epsilon-caprolactone, delta-valerolactone, lactide and / or glycolide to obtain the target product. The epsilon-caprolactone polymer of the present invention has controllable molecular weight, and thus controllable crystaallinity, hydrophilicity, degradation rate, etc.
Owner:EAST CHINA UNIV OF SCI & TECH
Purification method of crude lactide and application
The invention provides a purification method of crude lactide. The purification method includes the steps that A, the crude lactide and a solvent are mixed at 60-150 DEG C to obtain a mixed solution,wherein the solvent is selected from one or more of epsilon-caprolactone, delta-valerolactone and gamma-valerolactone; B, the mixed solution is subjected to cooling crystallization, and after centrifugal separation, purified lactide is obtained. The purification method can effectively remove meso-lactide and acidic impurities in the crude lactide and also can effectively remove acidic impurities in crude glycolide, the finally obtained lactide is high in purity and is polymeric lactide, and meanwhile the yield is high. Besides, the purified lactide obtained by the method can be directly polymerized without removing a trace amount of residual solvent, and the residual solvent can be effectively utilized.
Owner:普立思生物科技有限公司
Method for manufacturing delta-valerolactone
ActiveCN107987044APrice comparisonEasy to buyOrganic chemistryChemical industryDelta-ValerolactonePentalenolactone
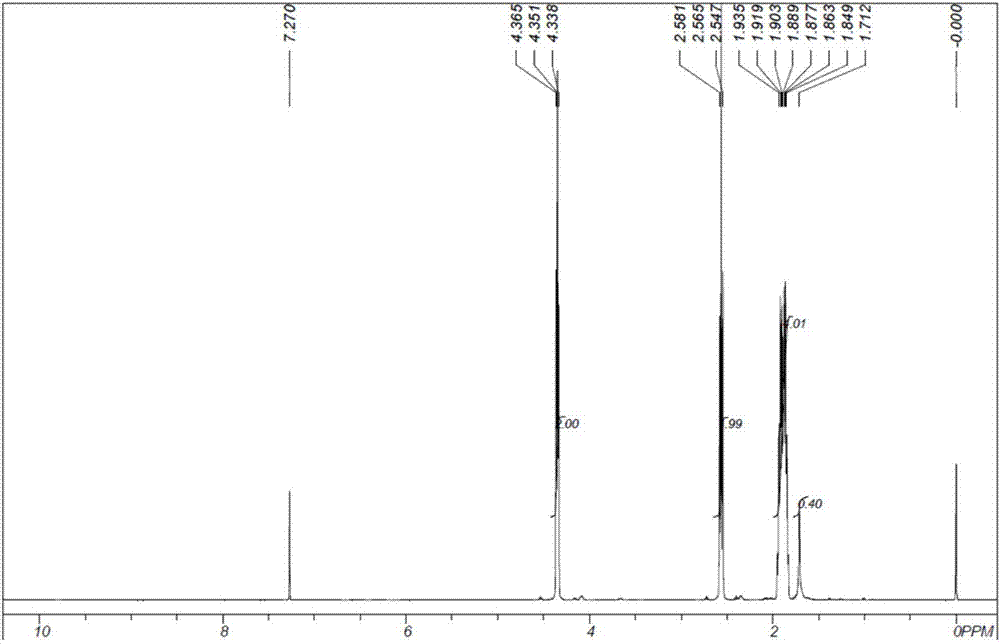
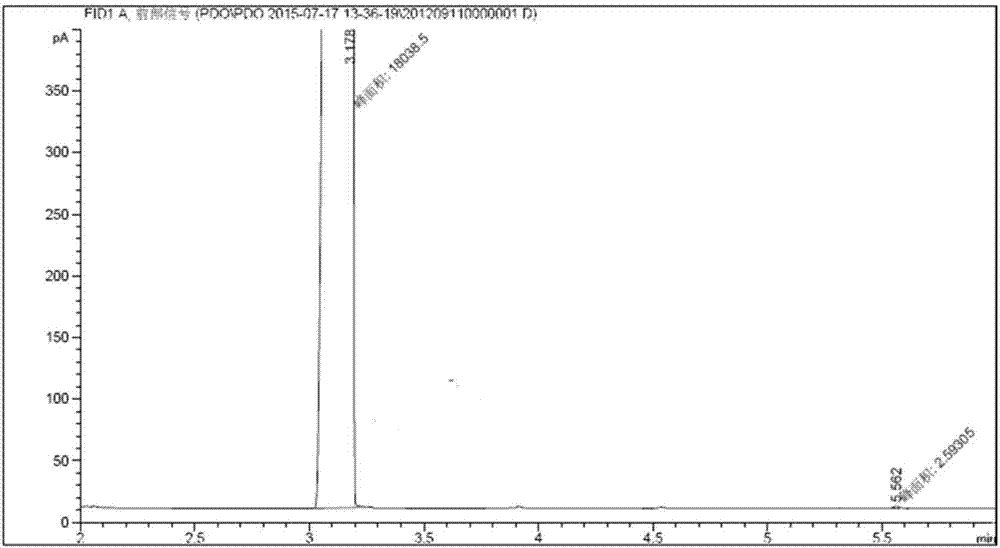
The invention provides a method for manufacturing delta-valerolactone. The method comprises the following steps: (1) carrying out nucleophilic substitution reactions between alkyl acetate representedby the formula (I) and epoxy chloropropane to generate 4,5-epoxy alkyl valerate represented by the formula (III); (2) subjecting 4,5-epoxy alkyl valerate obtained in the step (1) to a hydrogenation treatment to generate 5-alkyl hydroxyl valerate represented by the formula (IV); and (3) making 5-alkyl hydroxyl valerate obtained in the step (2) carry out cyclization reactions to generate delta-valerolactone. The total yield of the method can reach 95% or more. The method has the advantages of cheap and easily available raw materials, little environmental pollution, mild reaction conditions, lowcost, high yield, easy purification, and is suitable for industrial production.
Owner:WANHUA CHEM GRP CO LTD
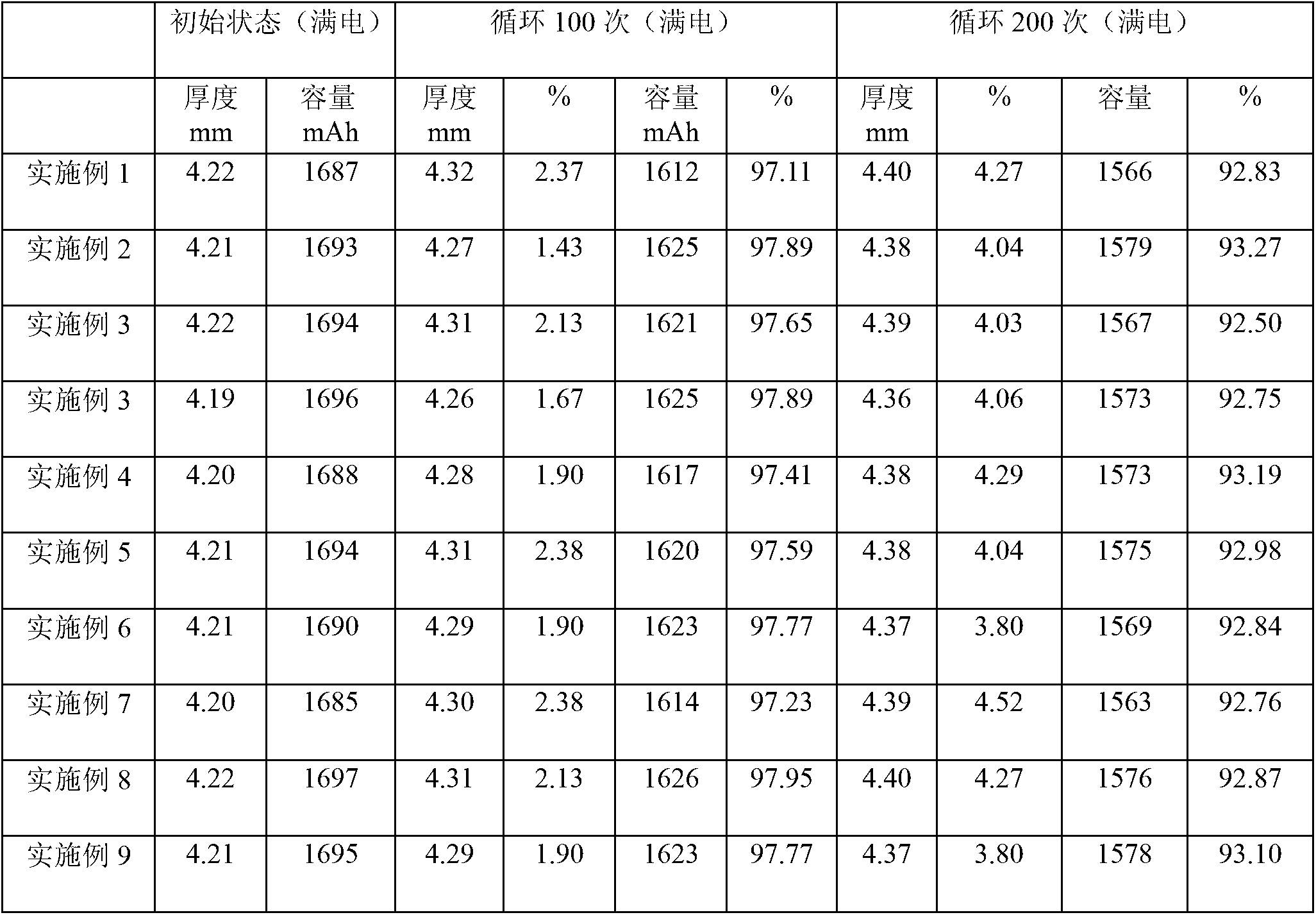
Electrolyte solution capable of improving high temperature cycling and storing performances of lithium secondary battery
InactiveCN103022561AImprove high temperature circulationImprove featuresSecondary cells servicing/maintenanceGamma-ValerolactoneLithium-ion battery
The invention discloses an electrolyte solution capable of improving high temperature cycling and storing performances of a lithium secondary battery. The electrolyte solution is prepared by lithium salt, an organic solvent and an addition agent, wherein the electrolyte solution also comprises a high temperature film-forming agent; the high temperature film-forming agent is one or any combinations of delta-valerolactone, gamma-valerolactone, gamma-caprolactone and epsilon-caprolactone; and the mass percentage of the high temperature film-forming agent accounts for 0.5%-15% of the total amount of the electrolyte solution. The high temperature film-forming agent is added into the electrolyte solution of the lithium secondary battery, a passivating film with excellent stability can be formed on the surface of a lithium secondary battery positive pole, a contact interface of the positive pole and the electrolyte solution can be improved, the decomposition reaction of the electrolyte solution on the positive pole material in the high temperature is inhibited, and the disadvantages that the existing lithium secondary battery is quick in storage capacity loss, low in recovery rate and quick in battery thickness swelling when being used under high temperature environments are overcome, so that the high temperature cycling and storing performances are improved.
Owner:TIANJIN LISHEN BATTERY
Preparation process of high-purity apixaban
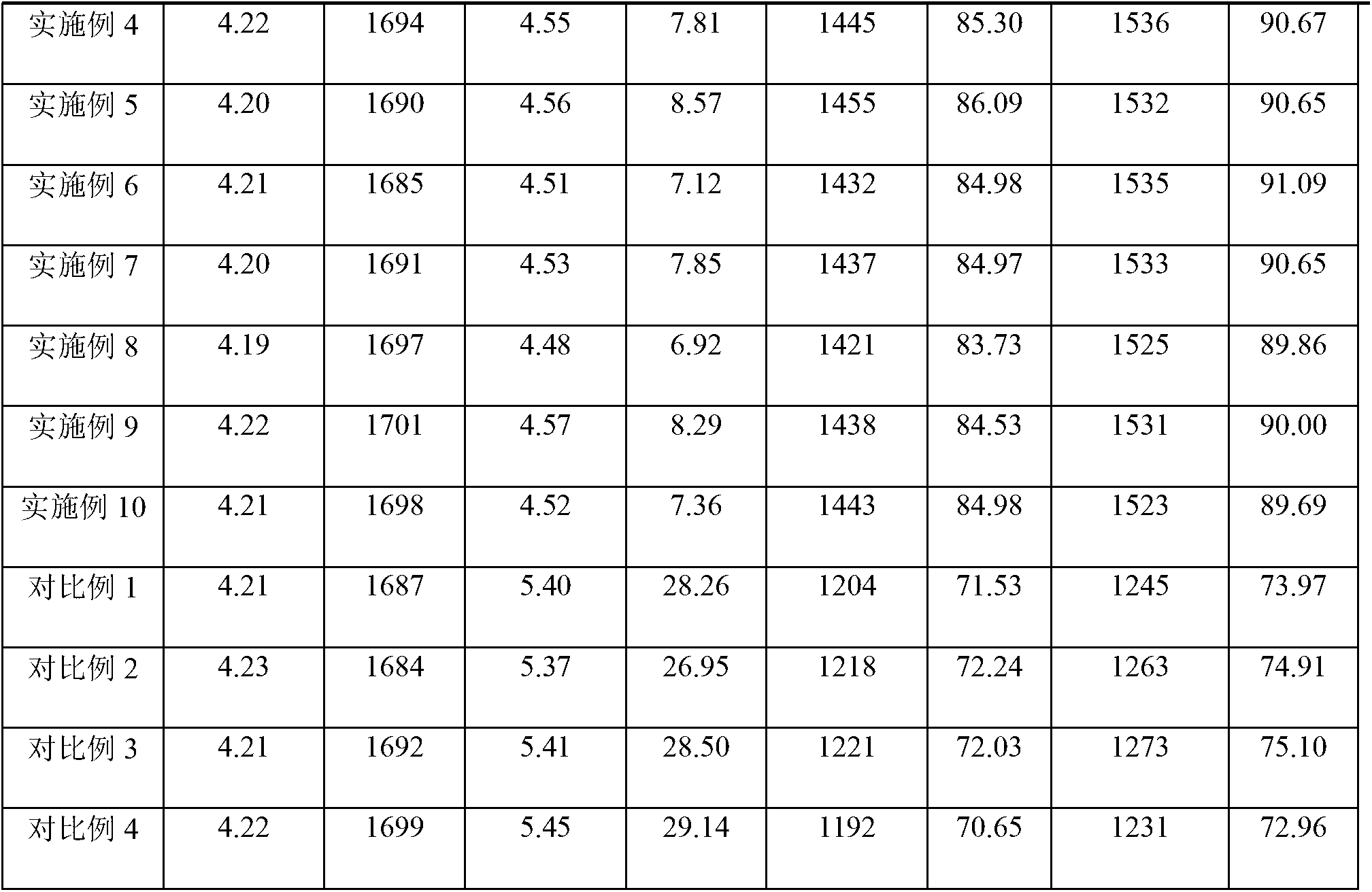
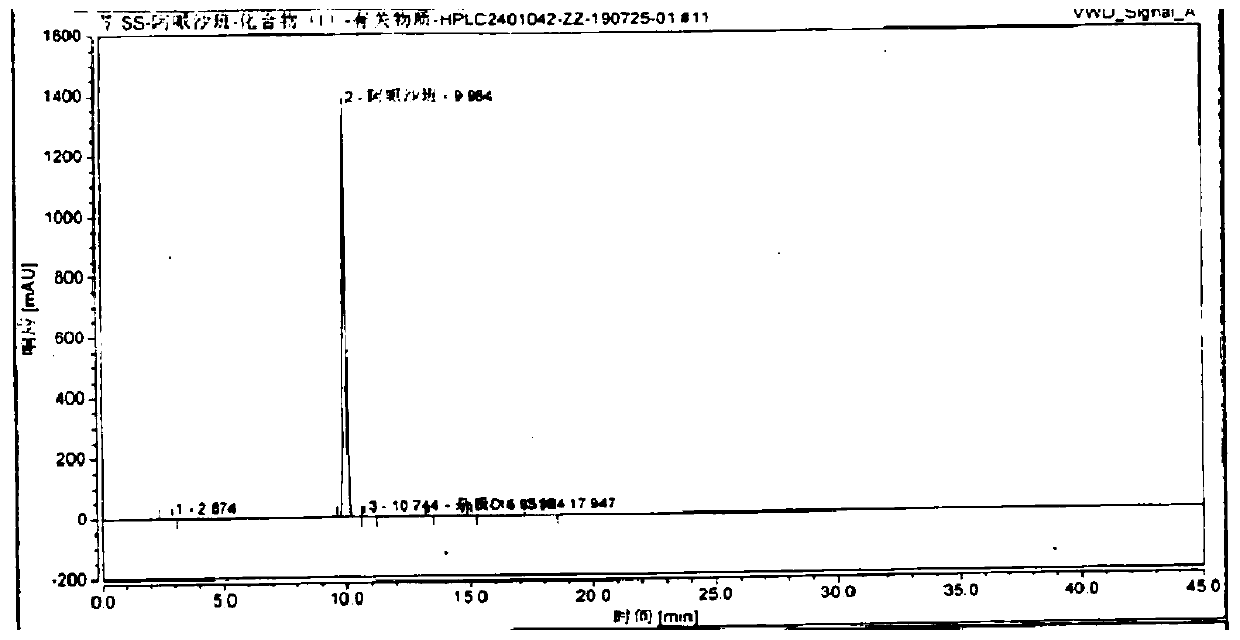
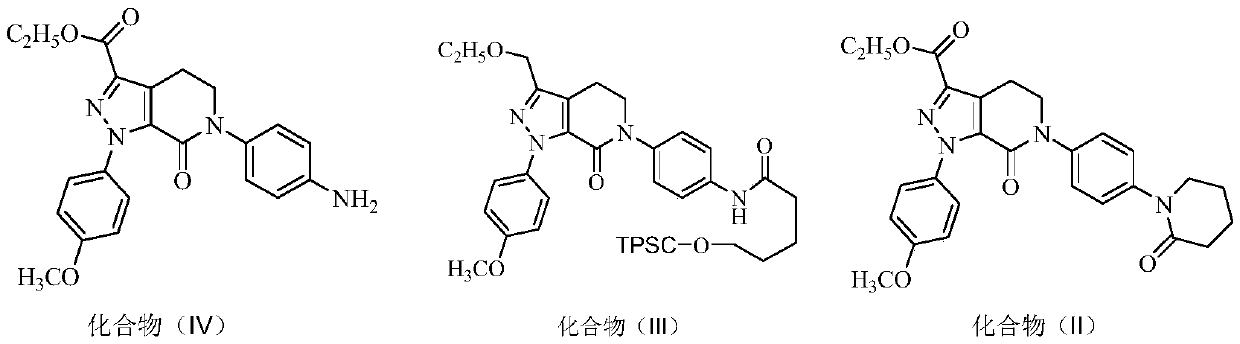
The invention discloses a preparation process of apixaban. The method comprises the following steps of: performing amidation on an initial raw material compound (IV) and delta-valerolactone under pressure; performing a one-pot reaction on the obtained hydroxyl amide and p-toluenesulfonyl chloride (PTSC)to obtain a compound (III); carrying out a reaction with tetrabutylammonium fluoride trihydrateto obtain a cyclization product compound (II); preparing a compound (I) from 2,4-dichlorobenzaldehyde and formamide under the action of sodium methoxide and a molecular sieve; refining the crude product by adopting an optimized ethanol / dichloromethane mixed solvent, so that impurity A is removed well; by means of the method, the apixaban product with high yield and high purity is prepared, whereinthe purity of the apixaban product is not lower than 99.5%, the impurity A does not exceed 0.05%, and any single impurity does not exceed 0.1%.
Owner:JIANGXI GUOYAO PHARMA LLC
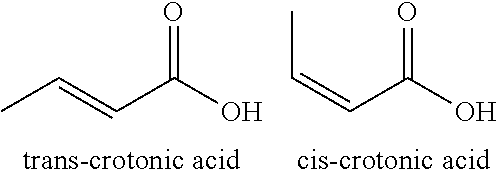
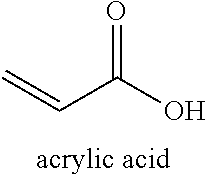
Process for Ultra Pure Chemical Production from Biobased Raw Starting Materials
InactiveUS20150376152A1Rapid productionLow detectable odorBiocidePreparation from carboxylic acid esters/lactonesGamma-ButyrolactoneLactone formation
Processes and methods for making ultra-pure (>99.50% by weight), biobased crotonic acid, gamma-butyro lactone, acrylic acid and delta-valerolactone from renewable carbon resources are described herein.
Owner:CJ CHEILJEDANG CORP
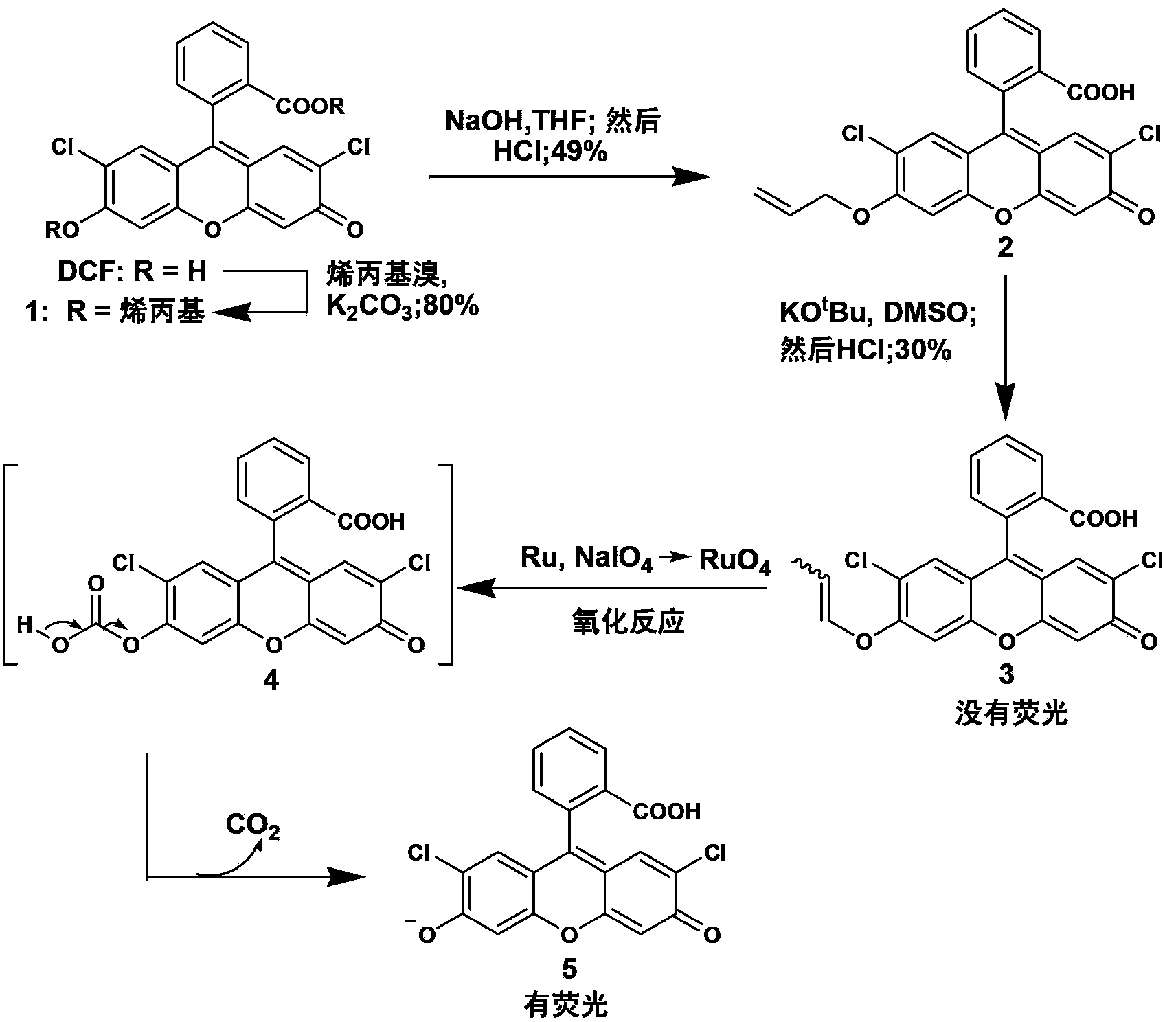
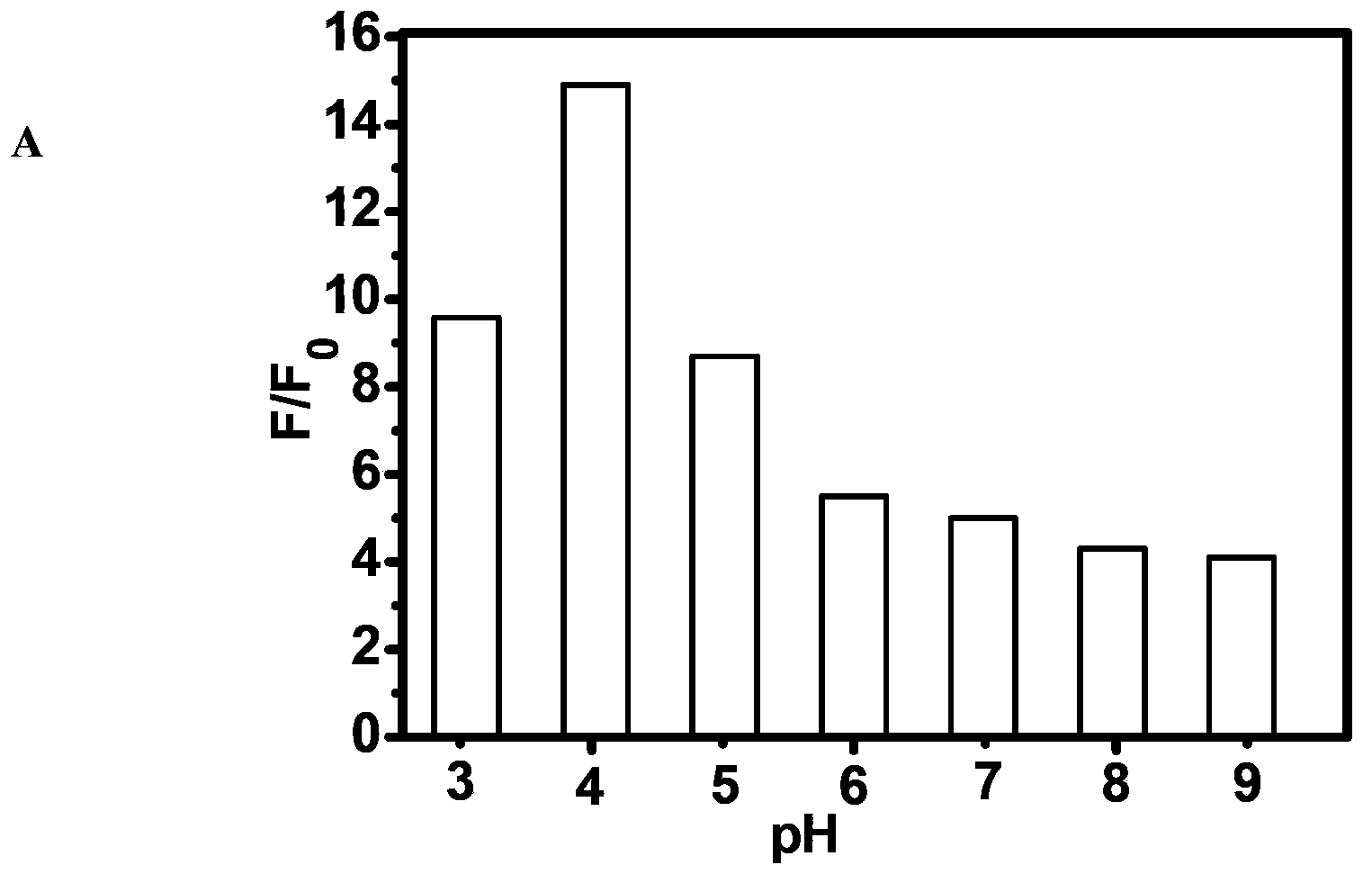
Fluorescent compound and application in ruthenium detection
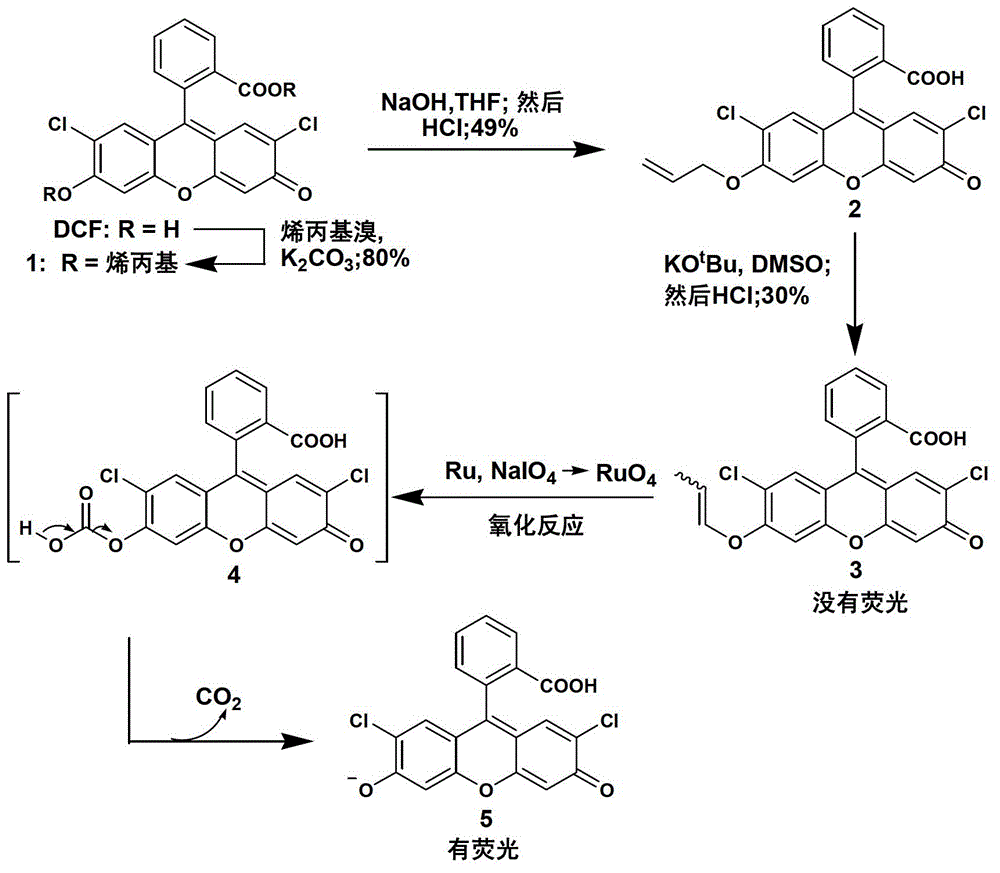
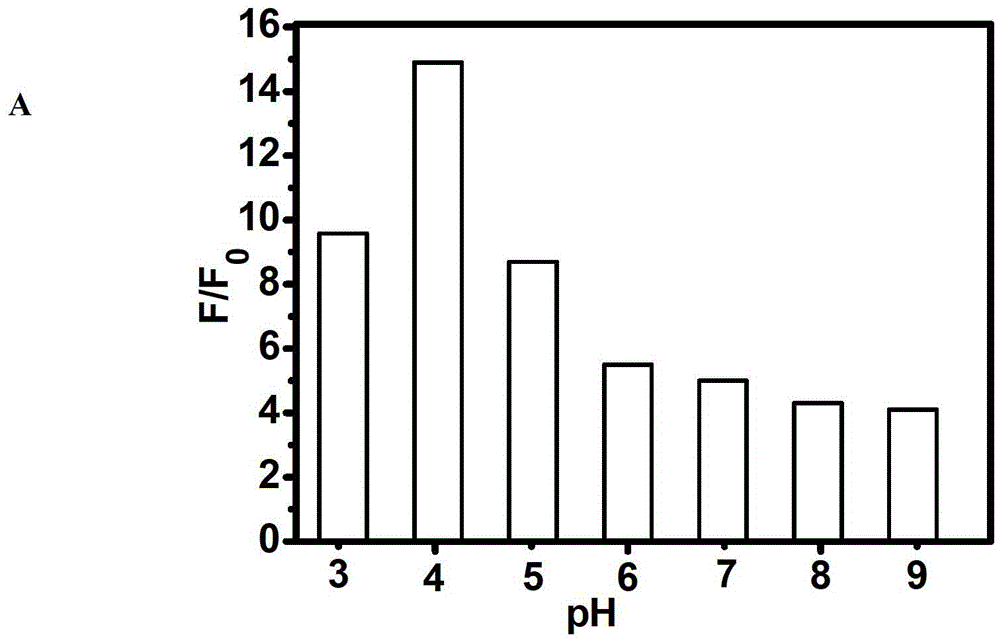
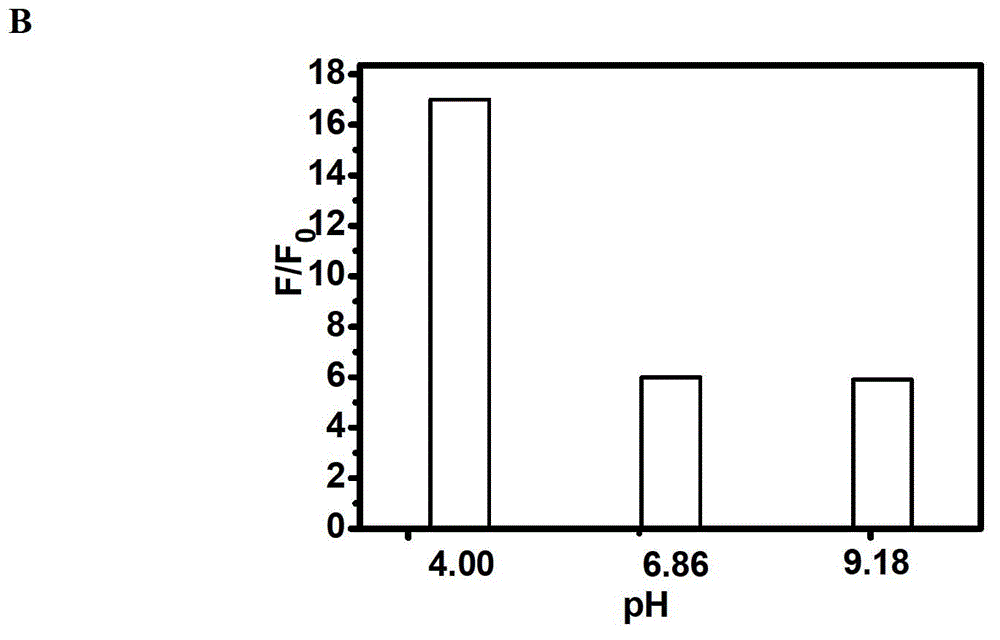
The invention discloses a fluorescent compound with a general formula I structure, and additionally relates to a method for detecting ruthenium metal in a sample by utilizing the fluorescent compound described in the general formula I. The method comprises the following steps of: (1) providing an appropriate pH system for the sample; (2) mixing the following detection solution and the sample, wherein the detection solution is composed of the components of a fluorescent compound I, an oxidizing agent and a cosolvent acetonitrile; and (3) detecting the fluorescence intensity of the sample after mixing so as to obtain the ruthenium metal content in the sample. The principle is that Ru in the sample is oxidized into RuO4 through an oxidizing material, then olefin in a fluorogen is oxidized into carboxylic acid by the RuO4, and finally a carboxylic acid structure is removed in a mode of CO2, gama-butyrolactone or delta-valerolactone, so that the fluorescence is enhanced. The method can detect the low ruthenium content in the sample, and the method has a wide ruthenium application prospect.
Owner:DALIAN UNIV OF TECH
Biodegradable triblock miktoarm star-shaped amphiphilic high molecular material and preparation method thereof
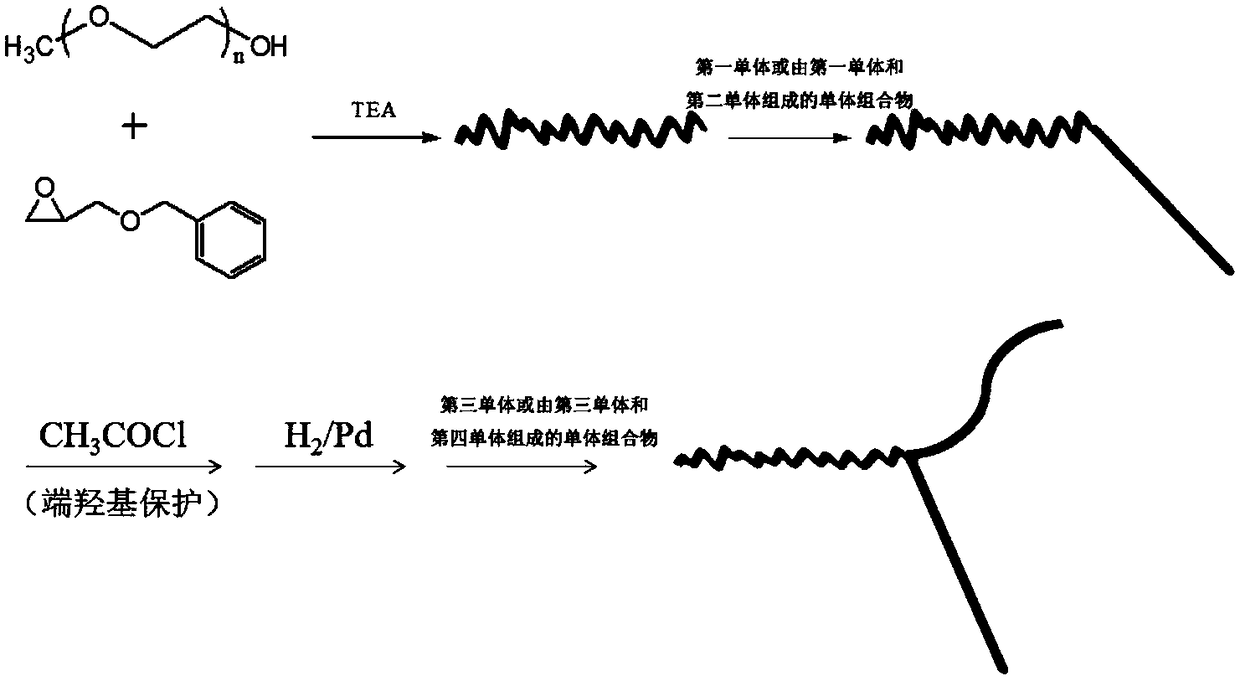
The invention discloses a biodegradable triblock miktoarm star-shaped amphiphilic high molecular material and a preparation method thereof. The biodegradable triblock miktoarm star-shaped amphiphilichigh molecular material takes cyclic esters including methoxypolyethylene glycol, L-lactide, glycolide, epsilon-caprolactone, trimethylene carbonate and delta-valerolactone and the like as raw materials and a first-core and second-arm synthetic strategy is adopted; firstly, a terminal hydroxyl group of methoxypolyethylene glycol is modified by utilizing benzyl glycidyl ether and benzyl glycidyl ether is used as an initiator to initiate different monomers to be subjected to ring-opening polymerization in sequence to finally obtain the triblock miktoarm star-shaped high molecular material composed of three different high molecular chains; a biodegradable triblock miktoarm star-shaped amphiphilic high molecular drug-loading material with relatively high research value and application value isprovided for a nano drug transmission system.
Owner:四川迈可隆生物科技有限公司
Method for producing DVL (delta-valerolactone)
The invention provides a method for producing DVL (delta-valerolactone). The method comprises the steps as follows: 1) acetonitrile and acraldehyde are subjected to an addition reaction, and a reaction solution containing an intermediate product 5-formyl butylcyanide is obtained; 2) 5-formyl butylcyanide is obtained is obtained after the reaction solution in the step 1) is subjected to reduced pressure distillation and separation purification, 5-formyl butylcyanide is subjected to a hydrogenation reaction, and 5-hydroxyl butylcyanide is produced; 3) 5-hydroxyl butylcyanide obtained in step 2)is subjected to a self-alcoholysis cyclization reaction and DVL is obtained. The total reaction yield is up to 95% or above. Raw materials are cheap and easy to obtain, the method causes small environmental pollution, adopts mild reaction conditions and is high in yield, low in cost and suitable for industrial production, and the product is easy to purify.
Owner:WANHUA CHEM GRP CO LTD
Preparation method of delta-valerolactone
ActiveCN109651318AImprove adsorption capacityConducive to solid loadingOrganic chemistryChemical industryActive componentLITHIUM PHOSPHATE
The invention relates to a preparation method of delta-valerolactone. The preparation method comprises the following steps: alkyl acetate and propylene oxide are subjected to an isomeric addition cyclization reaction in the presence of a catalyst, and the delta-valerolactone is obtained by one step, wherein the catalyst is a supported catalyst; a carrier is aluminium oxide; and the active component is lithium phosphate. The process has the following advantages: the raw materials are easily available; the reaction steps are simple; environmental pollution is less; yield is high; and the productis easy to purify. The preparation method is suitable for industrial production.
Owner:WANHUA CHEM GRP CO LTD
First-class rare earth macrolide/valerolactone/caprolactone terpolymer and preparation method thereof
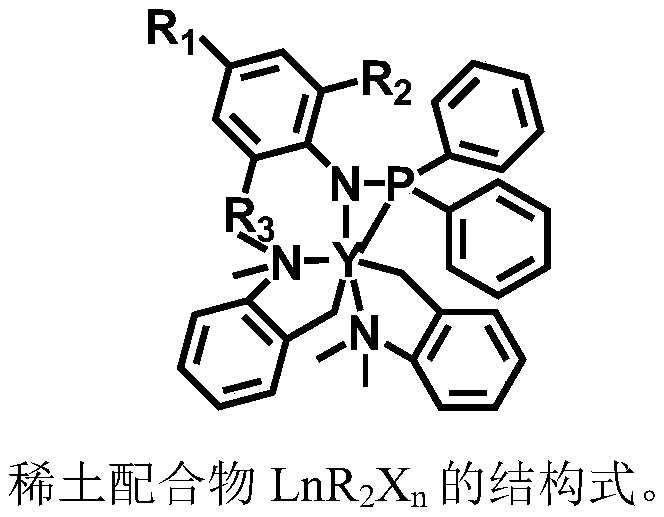
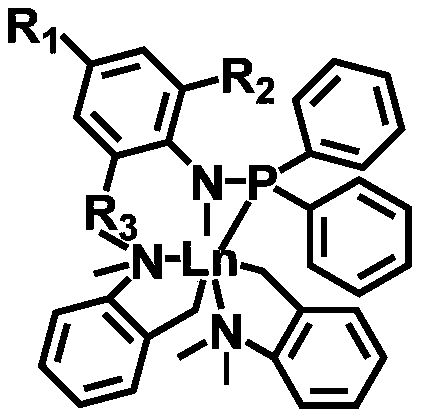
The invention provides a first-class rare earth macrolide / valerolactone / caprolactone terpolymer and a preparation method thereof, and belongs to the technical filed of high polymer materials. The first-class rare earth macrolide / valerolactone / caprolactone terpolymer is prepared by catalyzing macrolide, delta-valerolactone and e-caprolactone to terpolymerize by using a rare earth catalyst under room temperature; the number-average molecular weight is 1*10<4> to 50*10<4> g / mol, and the molecular weight distribution is narrow; on the basis of mole percent, the sum of macrolide, delta-valerolactone and e-caprolactone is 100%; macrolide accounts for 10-70%, delta-valerolactone accounts for 10-80%, and e-caprolactone accounts for 10-80%; macrolide is selected from 14-16 macrolides; the rare earth catalyst is LnR2Xn; Ln is rare earth metal; R is alkyl connected to the rare earth metal; X is radical which is complexed with the rare earth; n is the number of Lewis acid. According to the first-class rare earth macrolide / valerolactone / caprolactone terpolymer, phosphine amine type non-cyclopentadienyl rare earth complex rare earth catalyst is treated as a main catalyst; the catalyzing activityis high; the reaction conditions are mild; the product structure is controllable; the terpolymer in which macrolide is introduced is capable of greatly improving the mechanical performance and the heat stability of polyester materials; a high-performance polyester material with outstanding heat resistance can be obtained.
Owner:DALIAN UNIV OF TECH
Preparation method for 1-benzosuberone
InactiveCN106554265AWide variety of sourcesShort stepsPreparation from heterocyclic compoundsBenzeneReaction temperature
The invention provides a preparation method for 1-benzosuberone. The preparation method for the 1-benzosuberone includes the following steps that (A) a catalytic reaction is performed through a Lewis acid catalyst by taking delta-valerolactone and benzene as raw materials, the reaction temperature is controlled to be 80-90 DEG C, and the reaction time is controlled to be 12-16 h; and (B) after a quenching reaction, an organic phase is obtained through extraction, the organic phase is subjected to impurity removing and concentration treatment, and then the 1-benzosuberone is obtained. The preparation method for the 1-benzosuberone has the effects that the reaction route is short, operation is easy, the raw materials are easy to obtain, and the product is high in yield and purity.
Owner:SICHUAN TONGSHENG BIOTECH
Preparation method of 5-chlorovaleryl chloride
ActiveCN107628943AFew synthetic stepsHigh synthesis efficiencyCarboxylic acid halides preparationDistillationHydrogen chloride
The invention relates to a preparation method of 5-chlorovaleryl chloride and belongs to the field of synthesis of pesticide intermediates. The preparation method comprises the following steps: in delta-valerolactone, adding iron or aluminum with the mass being 0.05-1% of that of the delta-valerolactone and water with the weight of 1-5% of that of the delta-valerolactone as a cocatalyst, then introducing a certain amount of phosgene into a reaction system, and after the reaction mixed liquid reacts for 2-10 hours at the temperature of 60-100 DEG C, obtaining a crude 5-chlorovaleryl chloride product, and carrying out decompressed distillation on the crude 5-chlorovaleryl chloride product to obtain high-purity 5-chlorovaleryl chloride. The preparation method adopts the iron (or aluminum) andthe water as the cocatalyst, does not need introduction of hydrogen chloride gas, has the advantages of simple and easy obtaining of the materials and the catalyst, mild reaction condition and simpleoperation and the like, and has a good industrial application prospect.
Owner:宁波科诺华化工有限公司
A kind of preparation method of Apixaban
ActiveCN105732622BAdaptable to conditionsFast aminolysisOrganic chemistryP-NitroanilineNitro reduction
The invention discloses a preparation method of apixaban.The method comprises the steps that paranitroaniline serves as the raw material, paranitroaniline and delta-valerolactone are subjected to amidation ring-opening, substituting and ring-closing reactions under the action of AlMe3, and a compound 8 is obtained; the compound 8 is subjected to alpha-position dichloro substituting and condensation-elimination reactions, and a compound 7 is obtained; the compound 7 and a compound 6 are subjected to [3+2] cyclization-elimination and nitro reduction, and a compound 4 is obtained; the compound 4 sequentially reacts with delta-valerolactone and ammonium chloride under the action of AlMe3, and a compound 3 is obtained; the compound 3 is subjected to substituting and ring closing, and apixaban is obtained.According to the preparation method of apixaban, paranitroaniline and delta-valerolactone which are low in price are adopted to serve as the raw materials, operation of the whole route is simple, conditions of each reaction are mild, and the synthesizing method is easy to operate, high in yield and purity and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Synthetic method of γ-alkoxyacylmethyl-γ-butyrolactone and δ-alkoxyacylmethyl-δ-valerolactone
The invention belongs to the field of chemical technology, and in particular to a synthetic method of gamma-alkyl oxyacyl methyl-gamma-butyrolactone and delta- alkyl oxyacyl methyl-delta-valerolactone. CuH compound as a reducing agent reacts with keto ester, alpha, beta-unsaturated carboxylic ester; keto ester, alpha,beta-unsaturated carboxylic ester, a silicon hydride or boron hydride react with each other with CuH compound as the catalyst; and other copper compound, phosphine ligand, silicon hydride or boron hydride react with each other to generate a catalyst, which directly catalyses silicon hydride or boron hydrogen to react with keto ester, alpha,beta-unsaturated carboxylic ester. The synthesis reaction is carried out through the above three methods. reaction of this three method of The method of the invention uses cascade reaction for synthesis, three steps of reaction are carried out continuously in the same reaction vessel; separation of intermediates is not required, so as to avoid the separation process and the resulting loss; and the method has simple operation, improved reaction efficiency, and good application value.
Owner:DALIAN UNIVERSITY
Method for preparing delta-valerolactone by catalyzing oxidative chemical degradation and lactonization of 1,6-hexanediol
The invention relates to a method for preparing delta-valerolactone by catalyzing oxidative chemical degradation and lactonization of 1,6-hexanediol. The method uses air and / or oxygen as oxygen source, 1,6-hexanediol is subjected to oxidative chemical degradation and lactonization under catalyst. The method provided by the invention has high oxidation efficiency and high product yield; air is usedas oxygen source, so that the method is economical and environmentally-friendly. The product and catalyst are easy to separate, and the post-treatment is simple, so it has a good application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Enantioselective synthesis of γ-substituted-γ-butyrolactone and δ-substituted-δ-valerolactone
The invention belongs to the field of chemical technology and relates to a method for enantioselective synthesis of gamma-substituted-gamma-butyrolactone and delta-substituted-delta-valerolactone. A cu-chiral phosphine catalyst is used to promote the reaction of silicon hydride, methacrylates, 3-acyl propionate or 4-acyl butyrate in an organic solvent to synthesize chiral gamma-alkoxyl formyl methyl-gamma-butyrolactone and delta-alkoxyl formyl methy-delta-valerolactone with important biological activity. Compared with the prior art, the method provided by the invention has enantioselectivity, the ee value of the product is up to 84%, and any one isomer of enantiomers can be preferentially obtained by adjusting the configuration of the ligand; the target compound obtained according to the invention contains a chiral ring-lactone structure and has application value.
Owner:DALIAN UNIV
A Class of Fluorescent Compounds and Its Application in Detection of Ruthenium
The invention discloses a fluorescent compound with a general formula I structure, and additionally relates to a method for detecting ruthenium metal in a sample by utilizing the fluorescent compound described in the general formula I. The method comprises the following steps of: (1) providing an appropriate pH system for the sample; (2) mixing the following detection solution and the sample, wherein the detection solution is composed of the components of a fluorescent compound I, an oxidizing agent and a cosolvent acetonitrile; and (3) detecting the fluorescence intensity of the sample after mixing so as to obtain the ruthenium metal content in the sample. The principle is that Ru in the sample is oxidized into RuO4 through an oxidizing material, then olefin in a fluorogen is oxidized into carboxylic acid by the RuO4, and finally a carboxylic acid structure is removed in a mode of CO2, gama-butyrolactone or delta-valerolactone, so that the fluorescence is enhanced. The method can detect the low ruthenium content in the sample, and the method has a wide ruthenium application prospect.
Owner:DALIAN UNIV OF TECH
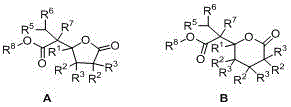
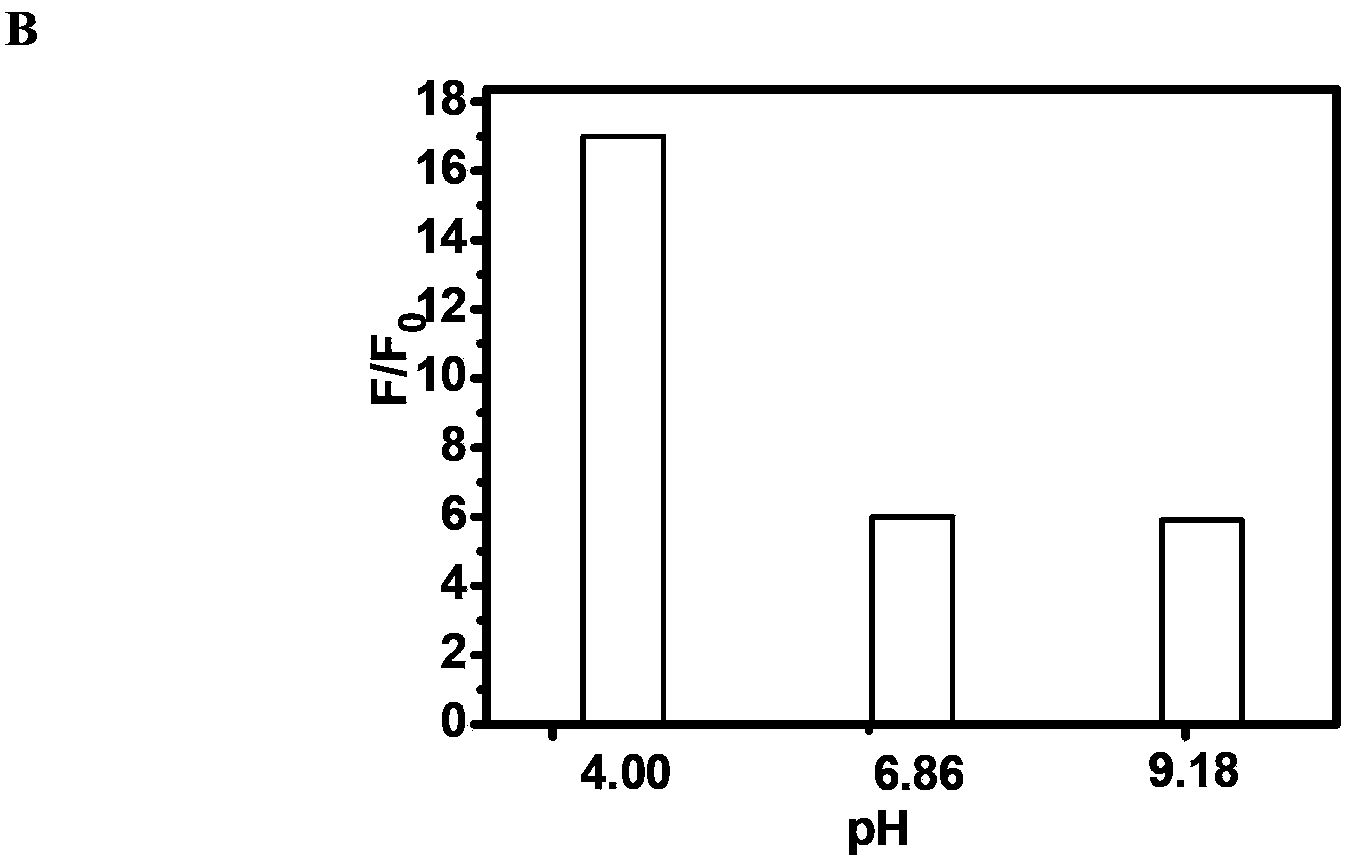
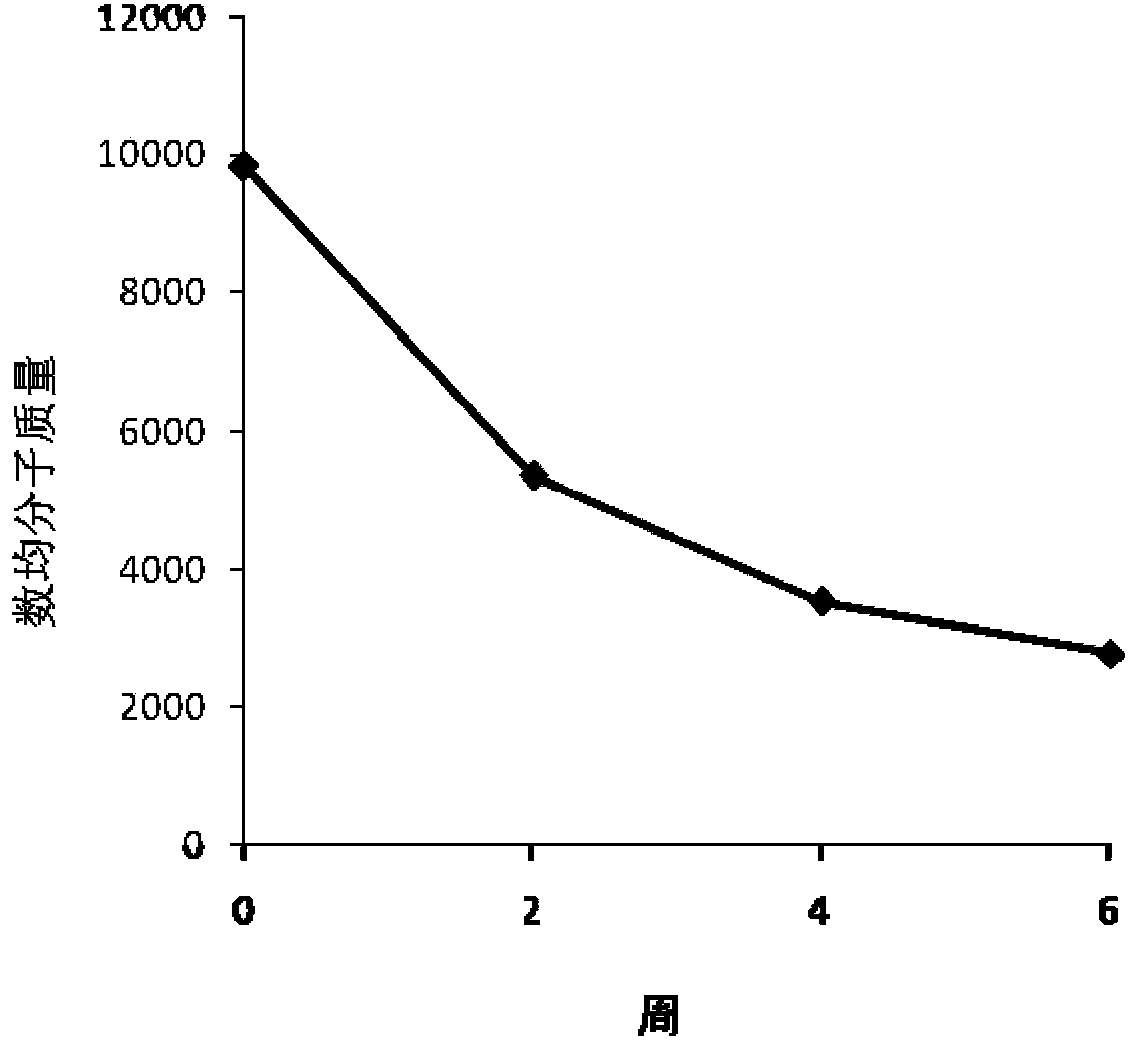
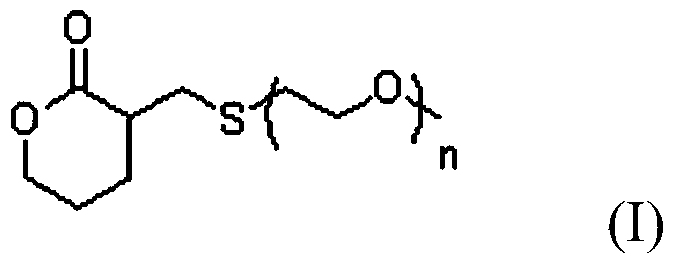
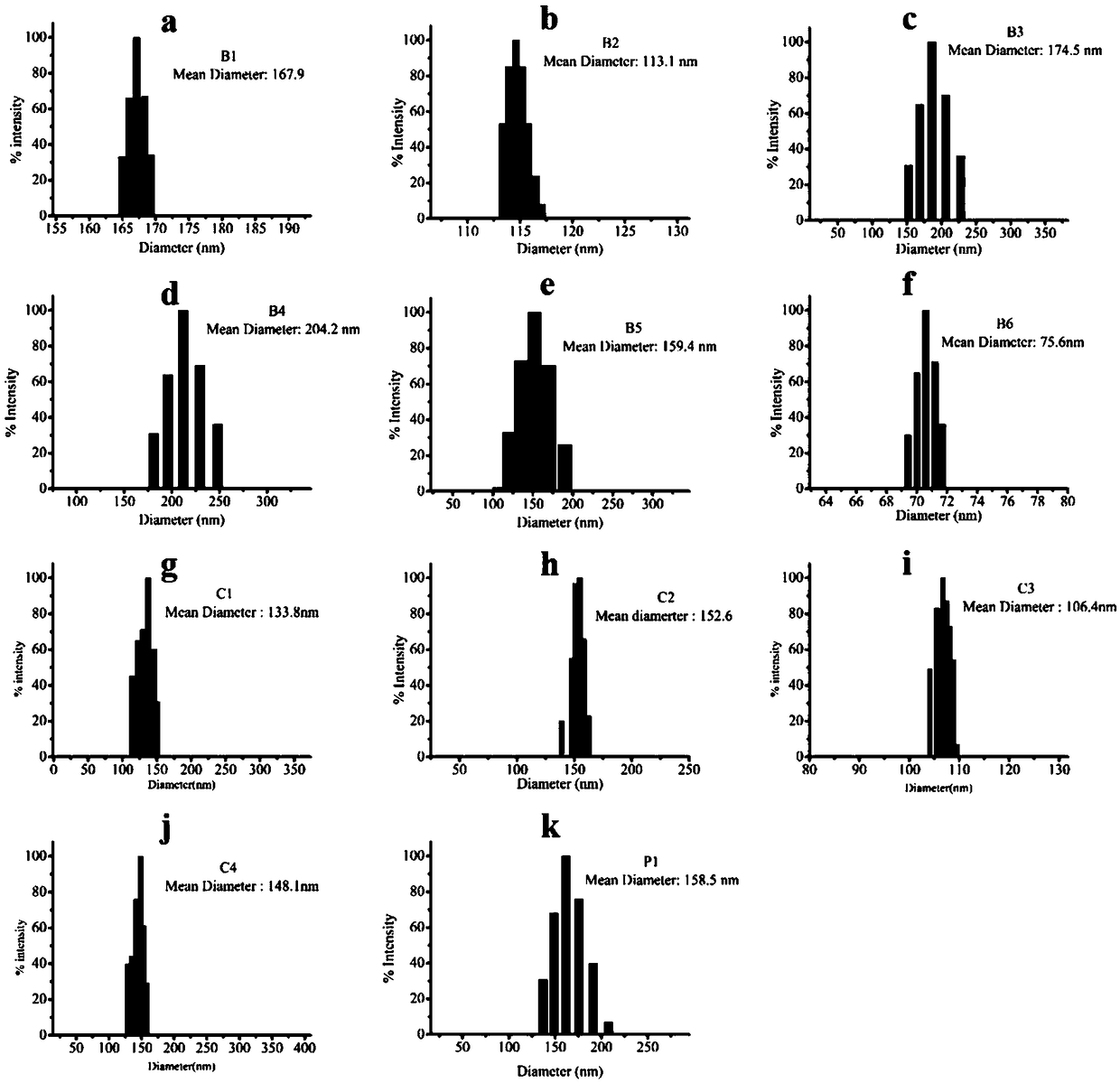
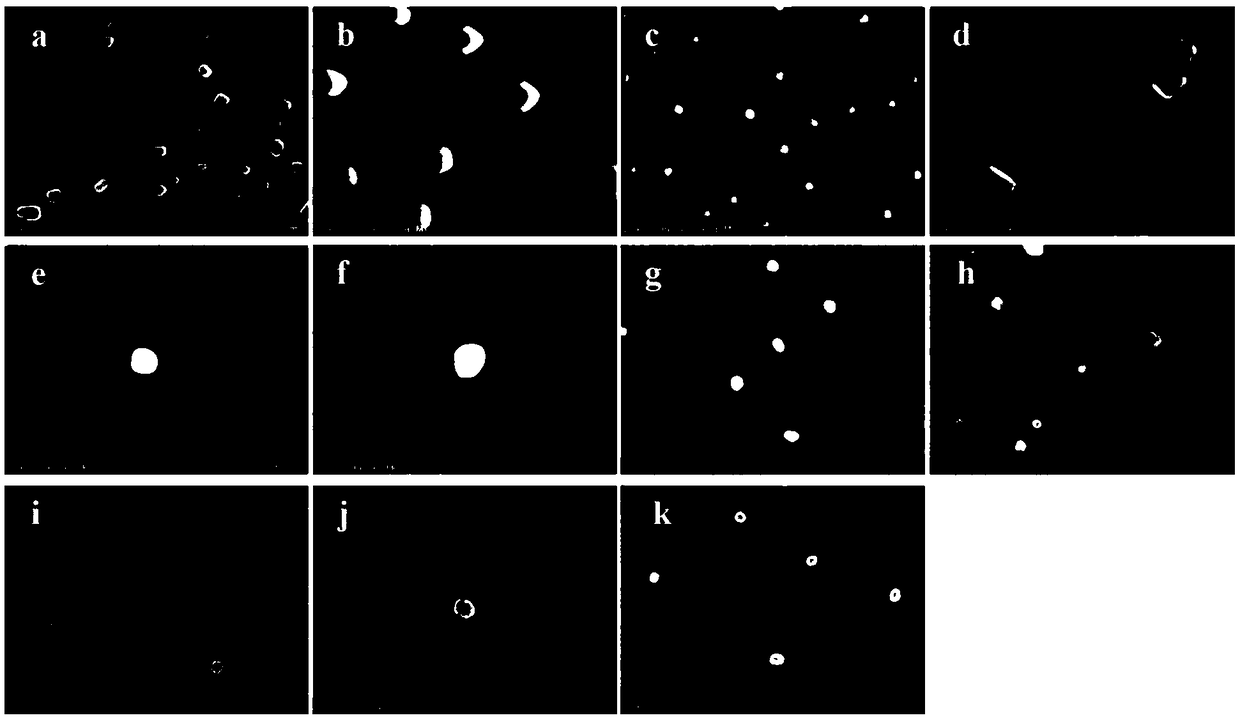
Delta-valerolactone compounds, preparation method and application
ActiveCN103288788BWide variety of sourcesBiocompatibleOrganic chemistryPharmaceutical non-active ingredientsPolyesterBiocompatibility Testing
The invention discloses delta-valerolactone compounds having structures expressed by a general formula (I); the invention further discloses a preparation method of the compounds, polyester prepared from the compounds and a preparation method thereof; the raw materials of the delta-valerolactone compounds prepared by the invention are extensive and renewable; and the polyester prepared from the delta-valerolactone compounds has biocompatibility and degradable hydrophily and can be utilized in a plurality of purposes, for example, medicine conveyance, protein protection, and the like.
Owner:YINGU PHARMA
Polymer based on delta-valerolactone and its preparation method and application
ActiveCN107118340BEffective cohesionLow toxicityOther foreign material introduction processesPolymer scienceGreen fluorescent protein
The invention provides a delta-valerolactone-based polymer. The structure of the delta-valerolactone-based polymer is subjected to infrared, nuclear magnetism and gel permeation chromatography characterization. The invention further discloses a preparation method and application of the delta-valerolactone-based polymer. The delta-valerolactone-based polymer is prepared through a Click reaction and a ring-opening polymerization, the reaction is simple and convenient, and the yield is high. The delta-valerolactone-based polymer is degradable under a certain condition, and acts with nucleic acid to form nano-particles easy to adsorb by cells; green fluorescent protein (GFP) and luciferase (Luciferase) expression experiments testify that the polymer can serve as a degradable non-viral gene vector and has high transfection efficiency; and the preparation method of the polymer and a preparation method of a transgenic vector are simple, mature and easy to learn.
Owner:BEIJING NORMAL UNIVERSITY
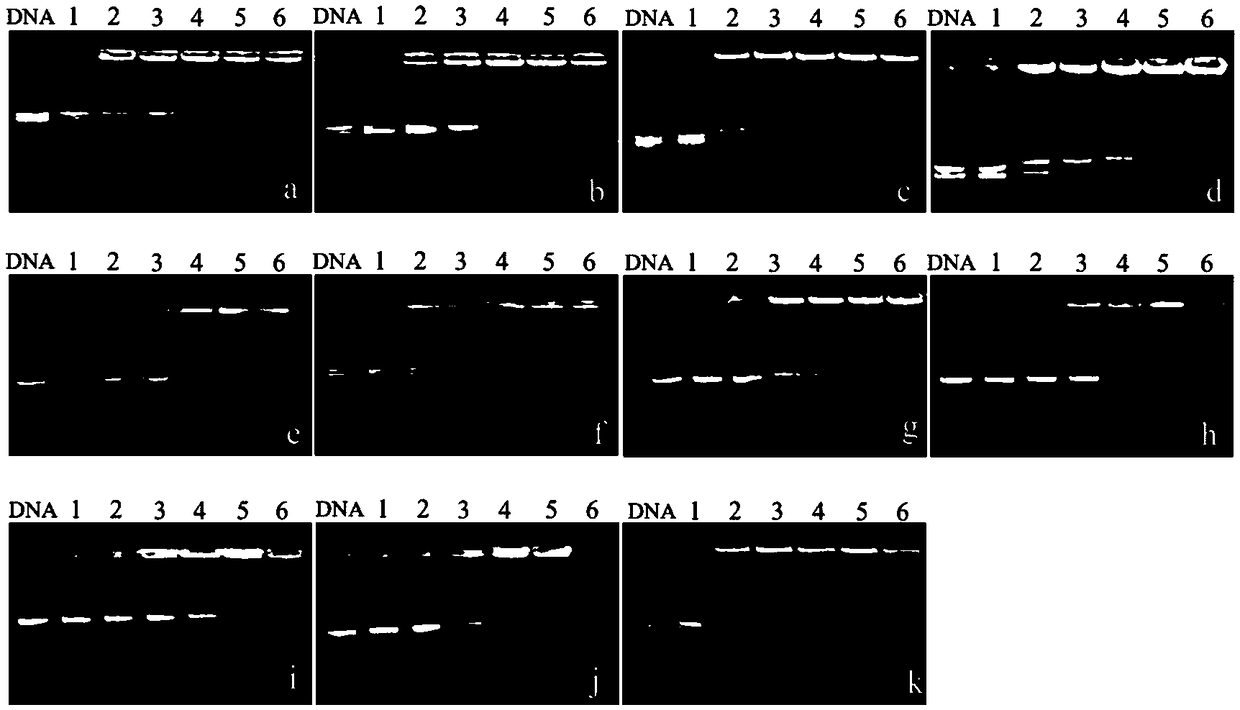
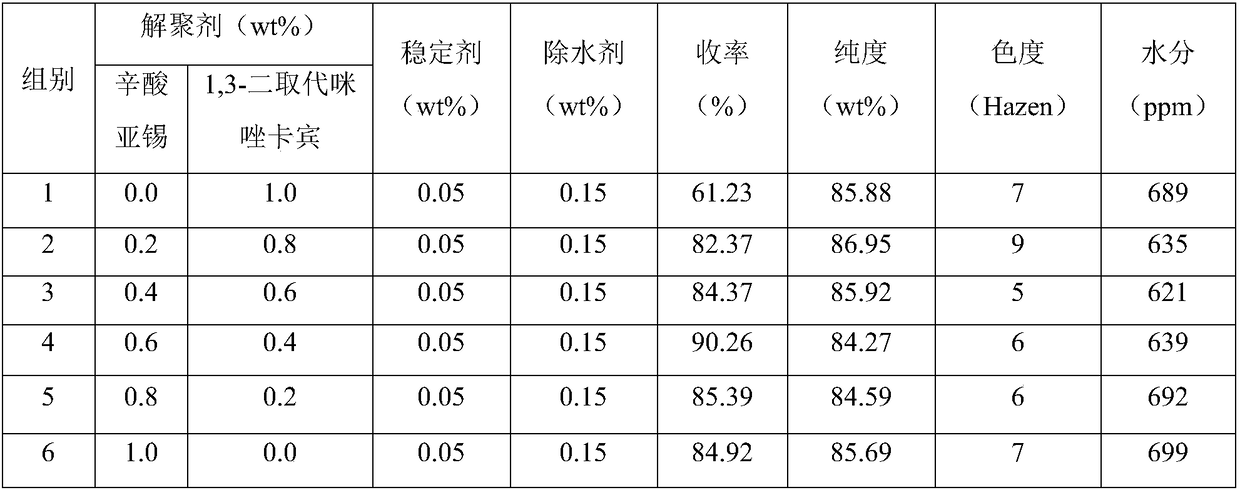
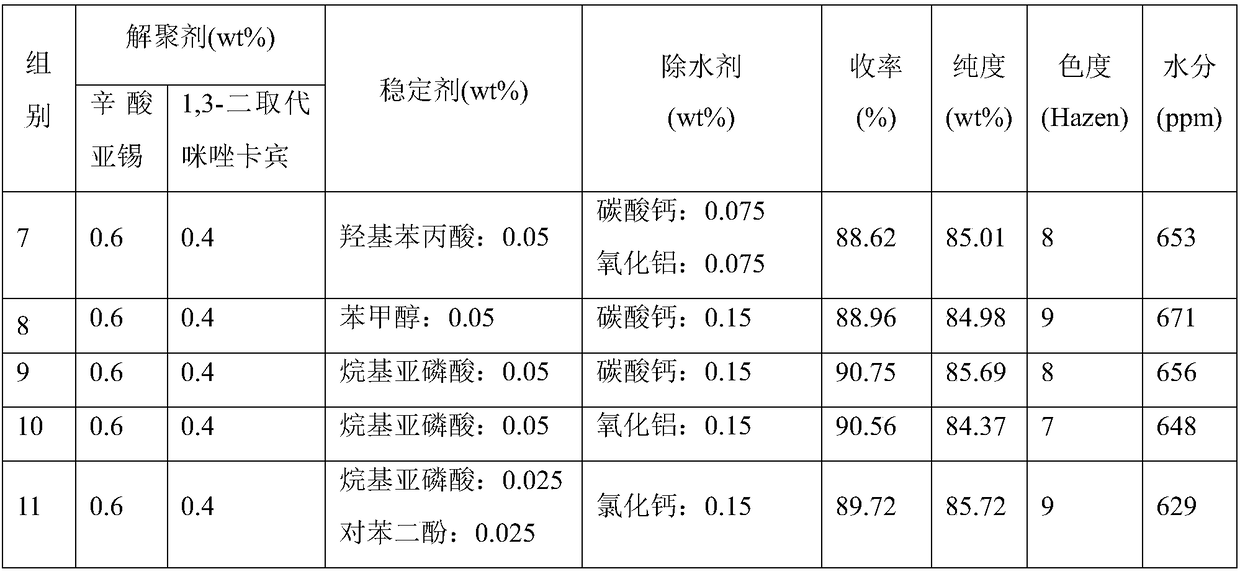
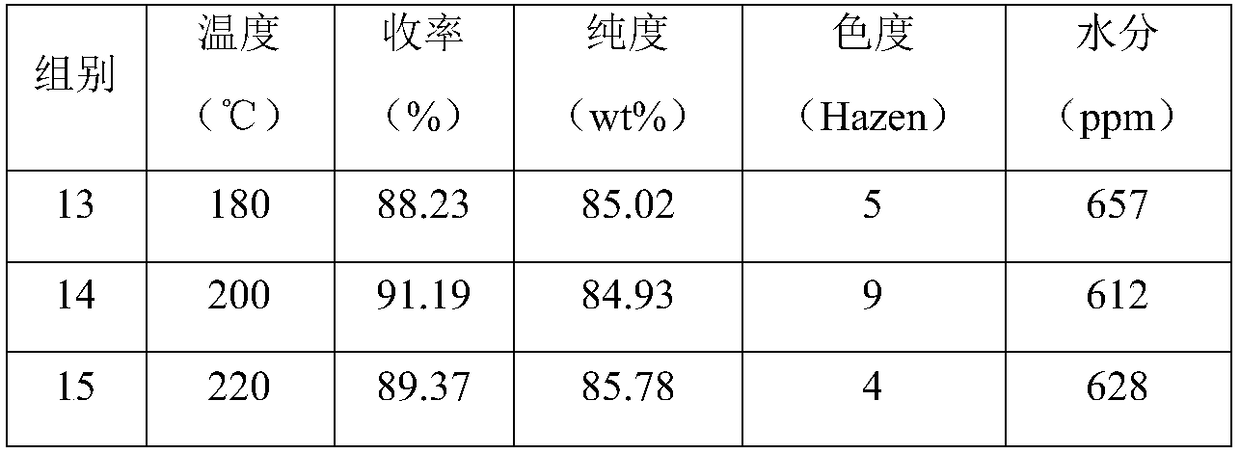
Composite depolymerizing agent for delta-valerolactone polymer, and using method of composite depolymerizing agent
InactiveCN108689977AGood depolymerization effectRich sourcesOrganic chemistryPhosphorous acidWater baths
The invention in particular relates to a composite depolymerizing agent for a delta-valerolactone polymer, and an using method of the composite depolymerizing agent. The composite depolymerizing agentis prepared from a depolymerizing agent, a stabilizing agent and a water removal agent; the depolymerizing agent is prepared from stannous octoate and / or 1, 3-disubstituted imidazole carbene; the stabilizing agent is prepared from one or two or more of alkyl phosphorous acid, aromatic phosphorous acid, hydroxyphenylpropionic acid, benzyl alcohol or hydroquinone; the water removal agent is prepared from one or two or more of clay, alumina, silica gel, zeolite, calcium chloride, calcium carbonate or potassium hydrogen carbonate. The using method of the composite depolymerizing agent comprises the following steps: sealing the delta-valerolactone polymer in a container, and heating for melting under the water bath condition at 85-95 DEG C; transferring the melted delta-valerolactone polymer into a four-neck bottle, enabling the four-neck bottle to be connected with a condenser pipe, adding the composite depolymerizing agent into the four-neck bottle, heating, standing for reacting, then stirring, and carrying out a reaction to obtain a delta-valerolactone crude product.
Owner:MAIQI CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com