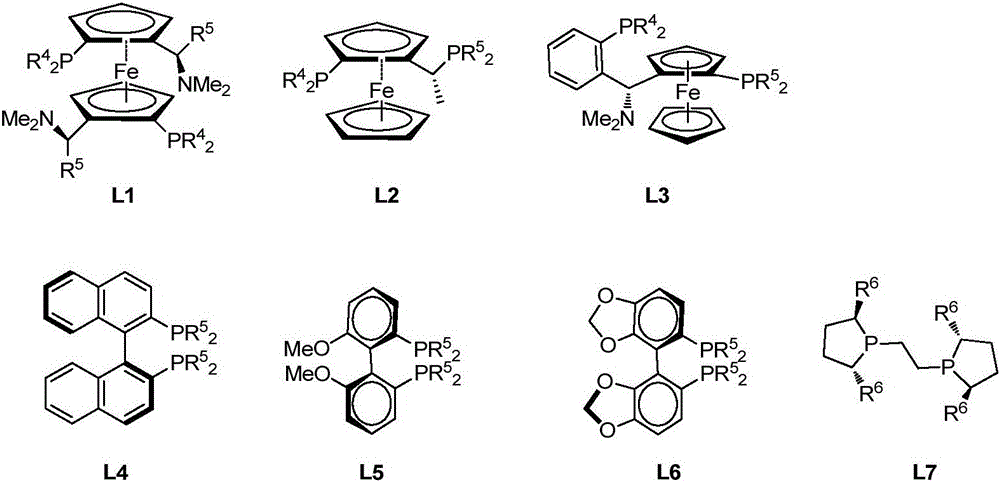
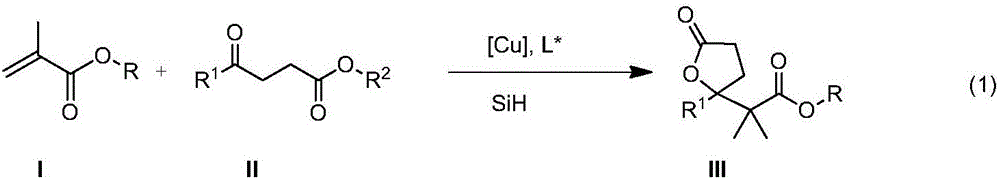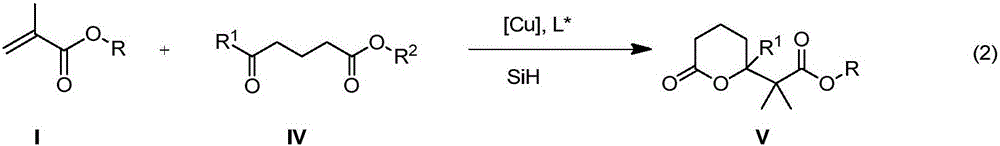Method for enantioselective synthesis of gamma-substituted-gamma-butyrolactone and delta-substituted-delta-valerolactone
A technology of enantioselectivity and synthesis method, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of unobtainable and no enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under nitrogen protection, add (R)-(+)-SEGPHOS(L6,R 5 =Ph)(7.4mg), Cu(OAc) 2 ·H 2 O (2.2 mg), toluene (1.0 mL), and polymethylhydrogensiloxane (48 μL) was added while stirring well. Add methyl methacrylate (85 μL) and methyl 3-benzoylpropionate (77 mg) in toluene (2.0 mL) dropwise under stirring, stir at room temperature for 4 h, then add saturated NH 4F in water (2 mL), and stirring was continued for 30 min. The phases were separated, and the aqueous phase was extracted with dichloromethane (3×5mL). The combined organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and then separated by column chromatography to obtain a colorless solid γ-phenyl - γ-(1-methyl-1-methoxyformylethyl)-γ-cyclobutyrolactone (102 mg, yield 97%). The product was analyzed by chiral HPLC, and the ee value was 84%.
Embodiment 2
[0027] Under nitrogen protection, add (R)-MeO-BIPHEP (L5,R 5 =Ph) (7.5mg, Cu(OAc) 2 ·H 2 O (2.2mgl), benzene (1.0mL), stir well, add polymethylhydrogensiloxane (48μL), add methyl methacrylate (85μL), 3-benzoylpropionic acid dropwise under stirring at room temperature A solution of the methyl ester (77 mg) in benzene (2.0 mL) was stirred for another 4 h. Add saturated NH to the reaction mixture 4 F in water (2 mL), and stirring was continued for 30 min. The phases were separated, and the aqueous phase was extracted with dichloromethane (3×5mL). The combined organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and then separated by column chromatography to obtain a colorless solid γ-phenyl - γ-(1-methyl-1-methoxyformylethyl)-γ-cyclobutyrolactone (94 mg, yield 90%, enantiomeric ratio ee 82%).
Embodiment 3
[0029] Under nitrogen protection, add (S,S)-Ph-BPE(L7,R 6 =Ph)(2.0mg,), Cu(OAc) 2 ·H 2 O (2.2 mg), toluene (1.0 mL), stirred well, and polymethylhydrogensiloxane (30 μL) was added. Cool to 0°C, add methyl methacrylate (53 μL), methyl 3-benzoylpropionate (77 mg) in toluene (2.0 mL), stir at 0°C for 24 h, then add saturated NH 4 F aqueous solution (2mL), and continue to stir for 30min, separate the phases, extract the aqueous phase (3×5mL) with dichloromethane, wash the combined organic phase with saturated brine, dry over anhydrous sodium sulfate, concentrate, and pass through the column Chromatographic separation gave γ-phenyl-γ-(1-methyl-1-methoxyformylethyl)-γ-cyclobutyrolactone (80 mg, yield 76%, ee 78%) as a colorless solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com