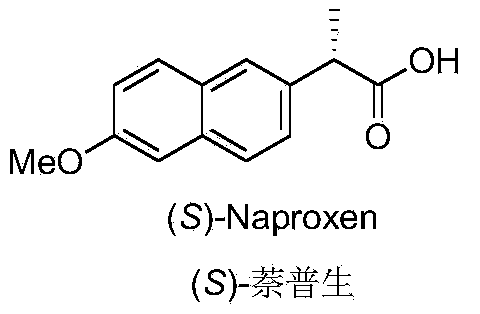
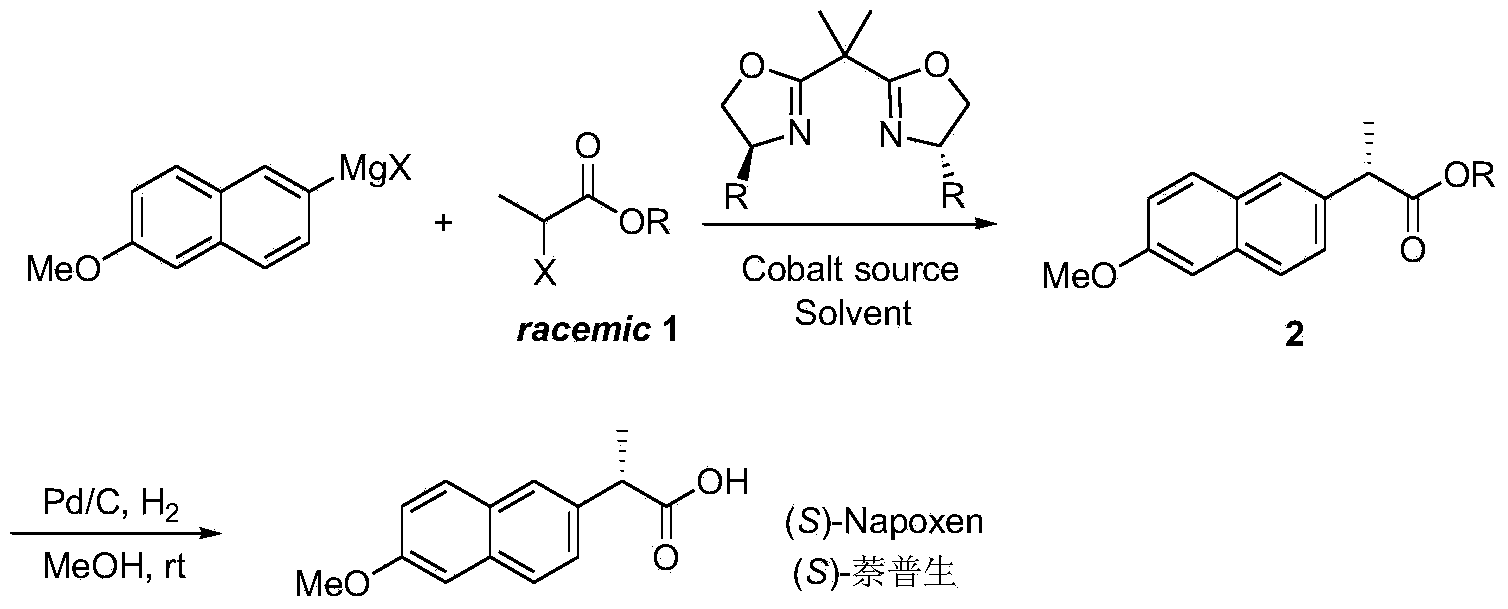
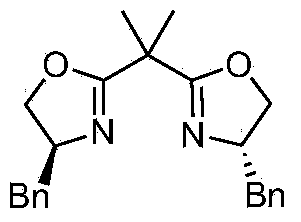
Novel asymmetric catalytic synthesis method of (S)-naproxen
A synthetic method and asymmetric technology, applied in the field of medicine, can solve the problems of cumbersome reaction steps and harsh reaction conditions, and achieve the effect of short synthetic route and high optical purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Under the protection of argon, add CoI in a dry Schlenk bottle 2 (62.4mg, 0.2mmol), after vacuum drying for 2h, add anhydrous tetrahydrofuran (6mL) and bisoxazoline chiral ligand L1 (88.0mg, 0.24mmol), and stir at room temperature for 1h. Add racemic benzyl 2-bromophenylpropionate (485.0mg, 2.0mmol) to the mixed solution, reduce the reaction temperature to -80°C, and add 6-methoxy-2-naphthylmagnesium bromide dropwise ( 8.0 mL, 0.5M solution in THF, 4.0 mmol). Stirring was continued at -80°C for 12 h, and the reaction was quenched by adding saturated ammonium chloride aqueous solution. The reaction solution was extracted with ether (15mL×4), the organic layers were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure and purified by silica gel column chromatography (n-hexane / ethyl acetate 40:1) to obtain (S)-naphthalene as a white solid Proxanthate 2 (364.8 mg, yield 57%, optical purity 90%). The ee value of (S)-naproxen ester 2 was incre...
Embodiment 2
[0027] Under the protection of argon, add Pd / C (21mg, 10%, 0.02mmol) in a dry Schlenk tube, vacuum dry for 10min, add (S)-naproxen ester 2 (64mg, 0.2mmol) in methanol (1mL) solution. Continue to stir the reaction for 6h and filter. The filtrate was concentrated under reduced pressure and purified by silica gel column chromatography (n-hexane / ethyl acetate 3:2) to obtain (S)-naproxen (34.9 mg, yield 76%, optical purity > 99%) as a white solid. [α] D 20 =+70.0(c1.0,CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ:7.70–7.68(m,3H),7.42–7.39(m,1H),7.15–7.10(m,2H),3.90(s,3H),3.85(q,J=7.2Hz,1H),1.58 (d,J=7.2Hz,3H); 13C NMR (75MHz, CDCl 3 )δ: 180.7, 157.7, 134.9, 133.8, 129.3, 128.9, 127.2, 126.2, 126.1, 119.0, 105.7, 55.3, 45.3, 18.1; HRMS (ESI-TOF): calcd for C 14 h 15 o 3 [M+H] + :231.1021, found 231.1016.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com