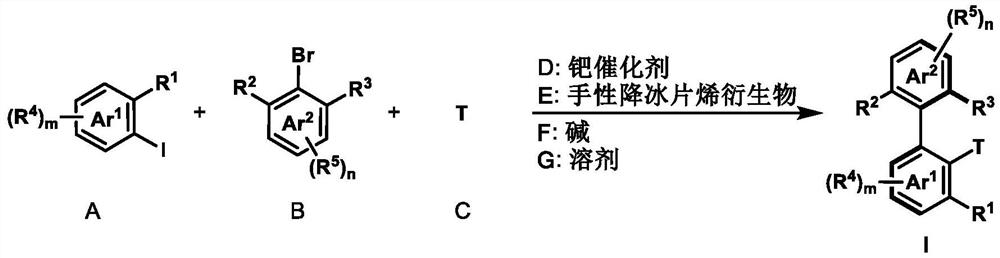
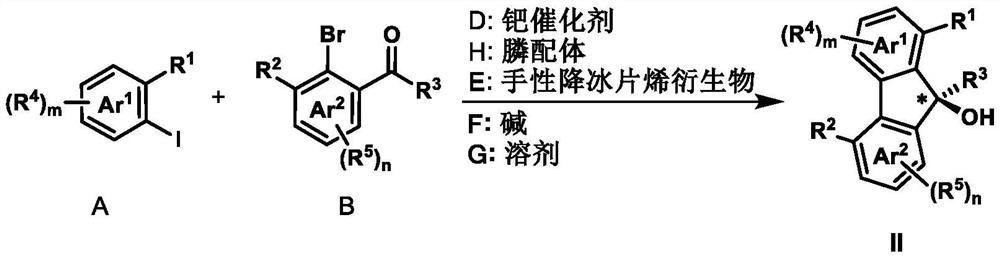
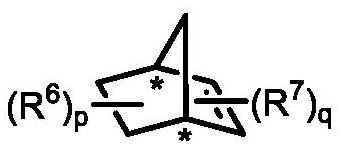
Preparation method of axially chiral biaryl compound and chiral fluorenol compound
A compound and biaryl technology, which is applied in the field of preparation of axial chiral biaryl compounds and chiral fluorenol compounds, can solve problems such as complex catalysts and limited application range, and achieve good tolerance, universality and low price Inexpensive, the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of Compound I-1
[0050]
[0051] Under argon protection, palladium acetate (2.3 mg, 0.01 mmol), potassium carbonate (34.6 mg, 0.25 mmol) and dry acetonitrile (1.0 mL) were added to a dry reaction tube equipped with a magnetic stirrer, and then ( 1S,4R)-2-norbornene-2-carboxylic acid ethyl ester (8.3mg, 0.05mmol), 1-iodonaphthalene (38.1mg, 0.15mmol), 2-bromo-3-methylbenzoate (22.9 mg, 0.1 mmol) and tert-butyl acrylate (19.2 mg, 0.15 mmol). The resulting mixture was reacted at 105° C. for 24 hours under an argon atmosphere. After the reaction was completed, it was cooled to room temperature, the mixture was filtered with diatomaceous earth, washed with ethyl acetate, the solvent was distilled off under reduced pressure, and purified by column chromatography to obtain compound I-1 (colorless oily liquid, 78% yield, 96% ee) . 1 H NMR (400MHz, CDCl 3 ):δ8.23–8.20(m,1H),7.89–7.82(m,3H),7.71(d,J=16.3Hz,1H),7.57–7.50(m,2H), 7.45–7.43(m,1H ),7.38–7.34(m,1H)...
Embodiment 2~19
[0053] Preparation of Compounds I-2~I-19
[0054] The operation steps are the same as in Example 1, except that the olefins used are: ethyl acrylate, benzyl acrylate, N,N-dimethylacrylamide, N,-methyl-N-methoxyacrylamide, acrolein , vinyl sulfone, vinyl phosphate, vinyl trimethylsilane, vinyl ethyl ether, 2-methyl-3-buten-2-ol, allyl alcohol, homoallyl alcohol, 5-hexyl- 1-alcohol, styrene, 4-fluorostyrene, 4-methoxystyrene, 4-nitrostyrene, 3-vinylbenzothiophene. Axial chiral biaryl products with different substituent terminations were obtained, and the results are shown in Table 1.
[0055] Table 1 Example 2~19 obtained axial chiral biaryl compound result
[0056]
Embodiment 20
[0058] Preparation of Compound I-20
[0059]
[0060] The operation steps were the same as in Example 1, except that the structure of the alkene (52.9 mg) used was as follows to obtain compound I-20 (yellow oily liquid, 96% yield, 97% ee). 1 H NMR (400MHz, CDCl 3 ):δ8.31–8.27(m,1H),7.93–7.89(m,1H),7.84(d,J=8.4Hz,1H),7.80(dd,J=8.1,1.2Hz,1H),7.76– 7.72(m,2H),7.69–7.67(m,2H),7.56–7.50(m,2H),7.41(d,J=7.2Hz,1H),7.36–7.32(m,3H),7.30–7.24( m,2H), 6.88–6.84(m,2H),6.67(d,J=16.5Hz,1H),5.14–5.05(m,1H),3.53(s,3H),2.00(s,3H),1.66 (s,6H),1.21(s,3H),1.20(s,3H); 13 C NMR (100MHz, CDCl 3 ):δ195.01,173.31,168.25,159.55, 141.86,141.27,137.35,137.07,136.95,134.30,133.75,133.25,132.45,132.06,131.73,131.11, 130.80,130.41,128.69,127.67,127.62,127.42,127.37,126.42, 126.11, 125.80, 125.61, 117.23, 79.45, 69.45, 52.02, 25.49, 21.66, 20.67; HRMS (ESI-TOF): calc'd for C 41 h 38 NaO 6 [M+Na] + 649.2561, found 649.2559; HPLC: Daicel chiralpak AD-H column, 15% i PrOH in n Hexane, 1 mL / min, λ=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com