Method of photo-induced catalytic selective synthesis of Z- and E-olefins
A light-induced and selective technology, applied in the field of pharmaceutical and chemical applications, can solve the problems of unfavorable large-scale industrial production, difficulty in obtaining trans-olefins, expensive ammonia borane, etc., achieve good catalytic effect, facilitate post-processing procedures, The effect of maintaining the catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of (E)-1,2-diphenylethylene:
[0043]
[0044] Add raw material 1a (0.20 mmol), [Ir(dtbbpy)(ppy)2][PF6] (10μmol, 8.4mg), PdCl 2 (0.04 mmol, 7.1 mg), DPPE (0.04 mmol, 15.9 mg), TEOA (0.4 mmol), HCOOH (0.4 mmol) and acetonitrile (1.5 mL), stirred at room temperature under white light for 16 hours in an air atmosphere, added acetic acid Diluted with ester (5 mL), washed with saturated brine (5 mL), dried the organic phase with anhydrous magnesium sulfate, spin-dried and then column chromatographed with (n-hexane) as the eluent to obtain 32 mg of product 2a as a white solid. The rate is 90%. 1 H NMR (400 MHz, CDCl 3 ): 7.49 (d, J =8.0 Hz, 4H), 7.34 (t, J = 8.0 Hz, 4H), 7.26 – 7.22 (m, 2H), 7.09 (s, 2H); 13 CNMR (100 MHz, CDCl 3 ) 137.4, 128.8, 127.7, 126.6.
Embodiment 2
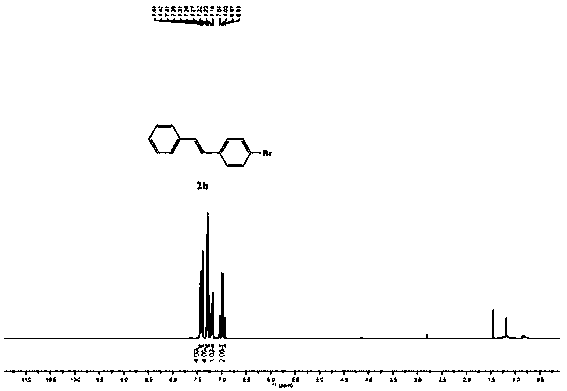
[0046] Synthesis of (E)-1-bromo-4-styrylbenzene:
[0047]
[0048] Add raw material 1b (0.20 mmol), [Ir(dtbbpy)(ppy)2][PF6] (10 μmol, 8.4 mg), PdCl2 (0.04 mmol, 7.1 mg), DPPE (0.04 mmol, 15.9 mg) in the reaction flask in sequence , TEOA (0.4mmol), HCOOH (0.4mmol) and acetonitrile (1.5 mL), in air atmosphere, under white light irradiation, stirred at room temperature for 16 hours, added ethyl acetate (5 mL) to dilute, washed with saturated brine (5 mL), The organic phase was dried with anhydrous magnesium sulfate, spin-dried and then column chromatographed with (n-hexane) as the eluent to obtain 42 mg of product 2b as a white solid with a yield of 81%. 1 H NMR (400 MHz, CDCl 3 ): 7.48 – 7.43(m, 4H), 7.35 – 7.31 (m, 4H), 7.23 (d, J =8.0 Hz, 1H), 7.07 (dd, J = 24.0,12.0 Hz, 2H); 13 C NMR (100 MHz, CDCl 3 ) 137.1, 136.4, 131.9, 129.6, 128.9, 128.1, 128.1, 127.6, 126.7, 121.5.
Embodiment 3
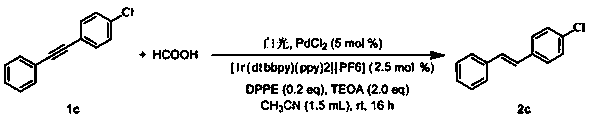
[0050] Synthesis of (E)-1-chloro-4-styrylbenzene:
[0051]
[0052] Add raw material 1c (0.20 mmol), [Ir(dtbbpy)(ppy)2][PF6] (10 μmol, 8.4 mg), PdCl2 (0.04 mmol, 7.1 mg), DPPE (0.04 mmol, 15.9 mg) in the reaction flask in sequence , TEOA (0.4mmol), HCOOH (0.4mmol) and acetonitrile (1.5 mL), in air atmosphere, under white light irradiation, stirred at room temperature for 16 hours, added ethyl acetate (5 mL) to dilute, washed with saturated brine (5 mL), The organic phase was dried with anhydrous magnesium sulfate, spin-dried and then column chromatographed with (n-hexane) as the eluent to obtain 36 mg of product 2c as a white solid with a yield of 84%. 1 H NMR (400 MHz, CDCl 3 ):7.50 (d, J =8.0 Hz, 2H), 7.44 (d, J = 8.0 Hz, 2H), 7.38-7.25 (m, 5H), 7.07 (dd, J = 20.0,16.0 Hz, 2H); 13 C NMR (100 MHz, CDCl 3 ) d 137.1, 136.0, 133.3, 129.5, 129.0, 128.9, 128.0, 127.8, 127.5, 126.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com