Manganese series lithium ion sieve adsorbent and preparation method of precursor thereof
A technology for lithium ions and adsorbents, which is applied in the field of preparation of manganese-based lithium ion sieve adsorbents and precursors, can solve the problems of difficulty in meeting industrialization requirements, long process flow, complicated process, etc., and achieves low raw material price and process flow. Short, high adsorption selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Take MnO 2 Baked at 680℃ for 6h in air atmosphere to obtain Mn 2 O 3 .
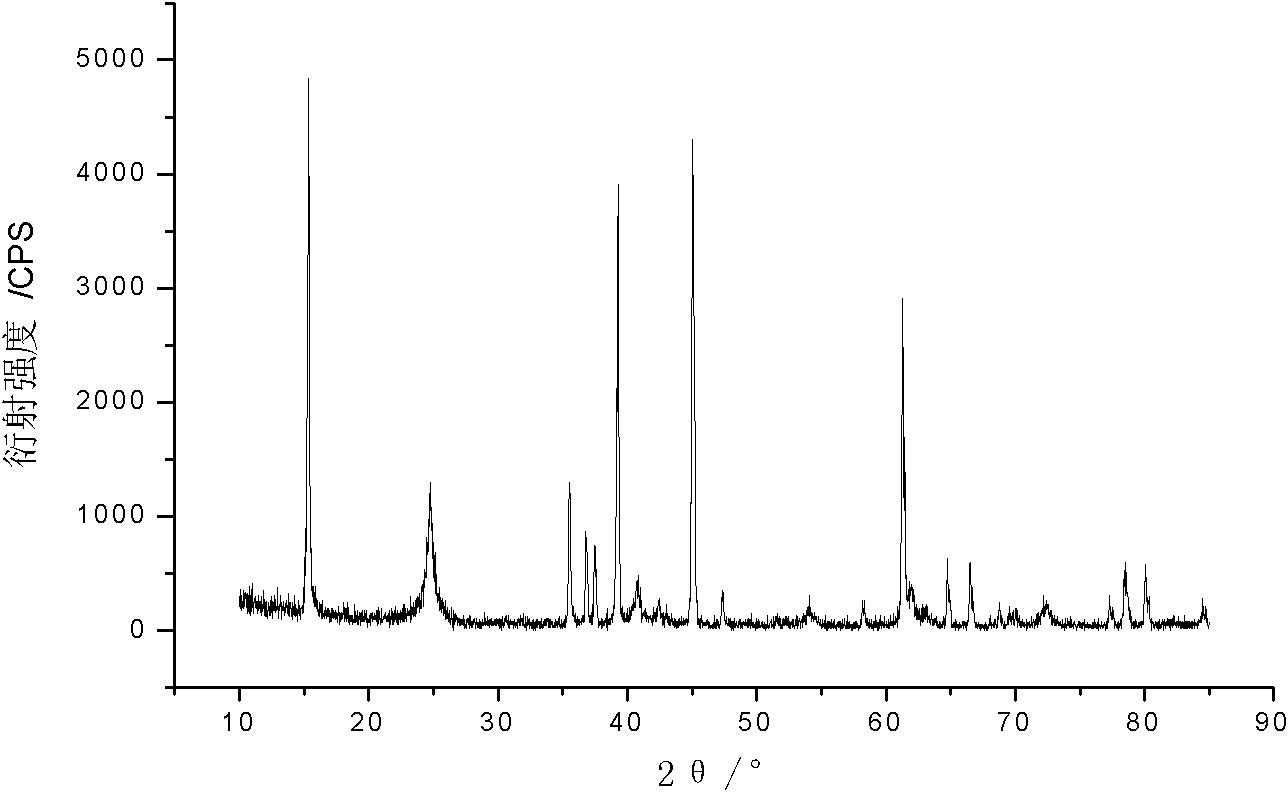
[0028] (2) Take Mn 2 O 3 15.78g, LiOH·H 2 O 8.8g (lithium-manganese molar ratio 1.05:1), using acetone as the dispersion medium, ground in a ball mill for 2 hours; after the ground mixture is dried, put it into a corundum crucible, and roast it under an inert atmosphere (nitrogen or argon). . After the temperature was raised to 800 °C, the temperature was kept constant for 12 hours. After the synthesis, the sample was cooled in the furnace to obtain LiMnO. 2 , and its XRD pattern is shown in the attached figure 2 shown.
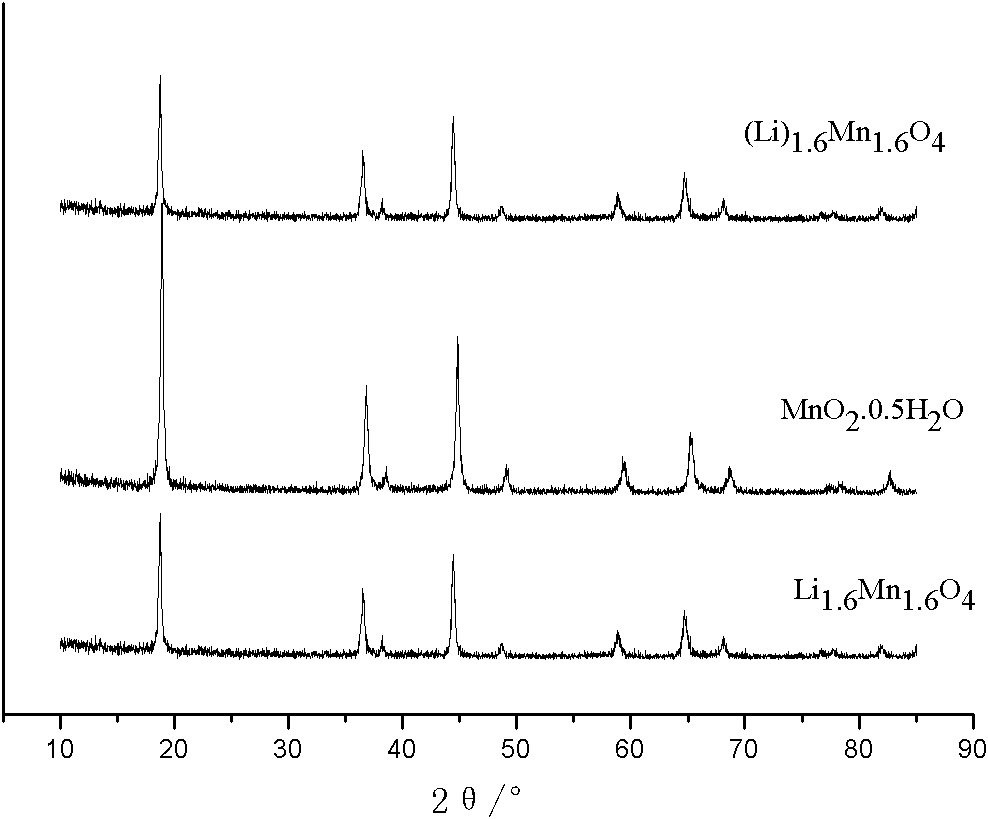
[0029] (3) Put LiMnO 2Put it into a corundum crucible, put it in a furnace, roast it at 450°C for 5 hours in an air atmosphere, and then cool it naturally to obtain Li, a precursor of a cubic crystal system lithium ion sieve. 1.6 Mn 1.6 O 4 . The lithium ion sieve precursor Li 1.6 Mn 1.6 O 4 Acid leaching with 0.5mol / L HCl for 2h, filtration and drying to obt...
Embodiment 2
[0032] (1) Take Mn 2 O 3 15.78g, Li 2 CO 3 7.97g (lithium-manganese molar ratio 1.08:1), with absolute ethanol as the dispersion medium, ground in a ball mill for 2 hours; after the ground mixture is dried, put it into a corundum crucible, under an inert atmosphere (nitrogen or argon) ) roasting. After the temperature was raised to 750 °C, the temperature was kept constant for 12 hours. After the synthesis, the sample was cooled in the furnace to obtain LiMnO. 2 .
[0033] (2) Put LiMnO 2 Put it into a corundum crucible, put it in a furnace, roast it at 500 ° C for 5 hours in an air atmosphere, and then cool it naturally to obtain the precursor Li of the cubic crystal system lithium ion sieve. 1.6 Mn 1.6 O 4 . The lithium ion sieve precursor Li 1.6 Mn 1.6 O 4 Acid leaching with 0.5mol / L sulfuric acid for 2h, filtration and drying to obtain lithium ion sieve MnO 2 ·0.5H 2 O. The adsorption analysis of the ion sieve is the same as (4) in Example 1.
Embodiment 3
[0035] (1) Take Mn 2 O 3 15.78g, Li 2 CO 3 7.97g (lithium-manganese molar ratio 1.08:1), with absolute ethanol as the dispersion medium, ground in a ball mill for 2 hours; after the ground mixture is dried, put it into a corundum crucible, under an inert atmosphere (nitrogen or argon) Roasting. After the temperature was raised to 500 °C, the temperature was kept constant for 24 hours. After the synthesis, the samples were cooled in the furnace. LiMnO is obtained after calcination 2 .
[0036] (2) Put LiMnO 2 Put it into a corundum crucible, place it in a furnace, roast it at 600°C for 5 hours in an air atmosphere, and then cool it naturally to obtain Li, the precursor of a cubic crystal system lithium ion sieve. 1.6 Mn 1.6 O 4 . The lithium ion sieve precursor Li 1.6 Mn 1.6 O 4 Acid leaching with 0.5mol / L HCl for 2h, filtration and drying to obtain lithium ion sieve MnO 2 ·0.5H 2 O. Lithium ion sieve MnO 2 ·0.5H 2 The adsorption and desorption process of O ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com