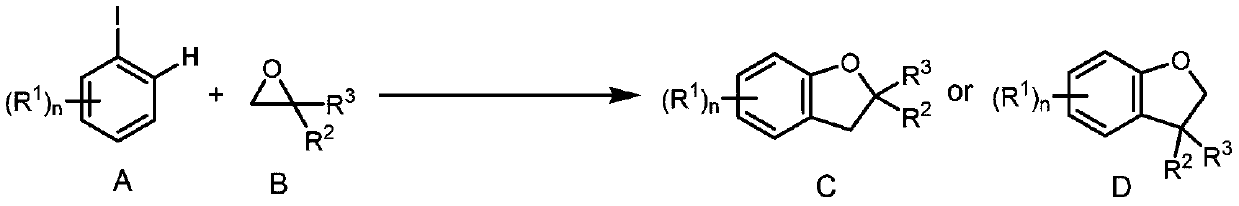
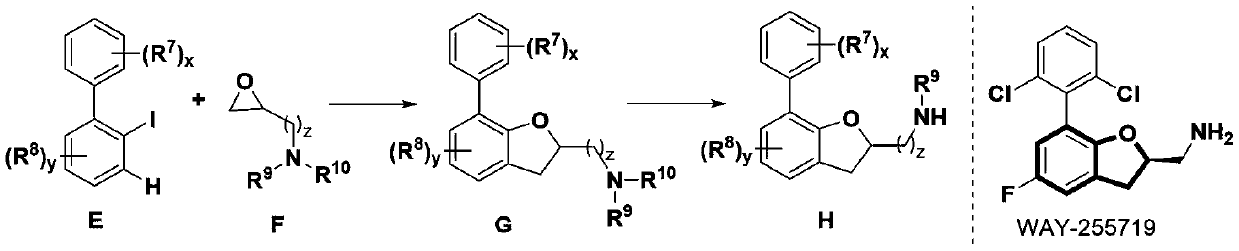
A method for synthesizing 2,3-dihydrobenzofuran compounds
A technology for dihydrobenzene and compounds, which is applied in steroids, chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, etc., can solve the problem of poor universality of epoxy substrates, poor reaction selectivity, and raw material synthesis. difficult problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of 7-methyl-2-(phenoxymethyl)-2,3-dihydrobenzofuran-5-carboxylic acid methyl ester
[0052]
[0053] Under the protection of an inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2 (2.2mg, 10mol%), XPhos (19.1mg, 20mol%), NBE-CO 2 K (3.5 mg, 10 mol%), methyl 3-methyl-4-iodobenzoate (0.2 mmol, 1.0 equiv.), phenyl glycidyl ether (0.6 mmol, 3.0 equiv.) and dry N-methylpyrrolidone (1.0 mL). The vial was capped and stirred at room temperature for about 5 minutes, after which the mixture was heated to 80°C and stirred for 24 hours. After the reaction vessel was cooled to room temperature, it was quenched with water (10 mL), extracted with methyl tert-butyl ether (3×10 mL), Na 2 SO 4 Dry, filter and concentrate in vacuo. Purified by column chromatography, the eluent is petroleum ether:ethyl acetate=10:1 (v / v), to obtain 7-methyl-2-(phenoxymethyl)-2,3-dihydrobenzofuran - 49 mg of methyl 5-carboxylate (white so...
Embodiment 2
[0054] Example 2: Preparation and gram-level preparation of (R)-7-methyl-2-(benzyloxymethyl)-2,3-dihydrobenzofuran-5-carboxylic acid methyl ester
[0055]
[0056] Under the protection of an inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2 (2.2mg, 10mol%), XPhos (19.1mg, 20mol%), NBE-CO 2 K (3.5mg, 10mol%), methyl 3-methyl-4 iodobenzoate (0.2mmol, 1.0equiv.), (R)-benzyl glycidyl ether (0.6mmol, 3.0equiv.) and dry N - Methylpyrrolidone (1.0 mL). The vial was capped and stirred at room temperature for about 5 minutes, after which the mixture was heated to 80°C and stirred for 24 hours. After the reaction vessel was cooled to room temperature, it was quenched with water (10 mL), extracted with methyl tert-butyl ether (3×10 mL), Na 2 SO 4 Dry, filter and concentrate in vacuo. Purified by column chromatography, the eluent is petroleum ether:ethyl acetate=10:1 (v / v), to obtain 7-methyl-2-(benzyloxymethyl)-2,3-dihydrobenzofuran - 58 m...
Embodiment 3
[0058] Example 3: Preparation of 7-methyl-2-(butyryloxymethyl)-2,3-dihydrobenzofuran-5-carboxylic acid methyl ester
[0059]
[0060] Under the protection of an inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2 (2.2mg, 10mol%), XPhos (19.1mg, 20mol%), NBE-CO 2 K (3.5 mg, 10 mol%), methyl 3-methyl-4 iodobenzoate (0.2 mmol, 1.0 equiv.), glycidyl n-butyrate (0.6 mmol, 3.0 equiv.) and dry N-methyl Pyrrolidone (1.0 mL). The vial was capped and stirred at room temperature for about 5 minutes, after which the mixture was heated to 80°C and stirred for 24 hours. After the reaction vessel was cooled to room temperature, it was quenched with water (10 mL), extracted with methyl tert-butyl ether (3×10 mL), Na 2 SO 4 Dry, filter and concentrate in vacuo. Purified by column chromatography, the eluent was petroleum ether:ethyl acetate=10:1 (v / v), to obtain 7-methyl-2-(butyryloxymethyl)-2,3-dihydrobenzo 53 mg of methyl furan-5-carboxylate (lig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com