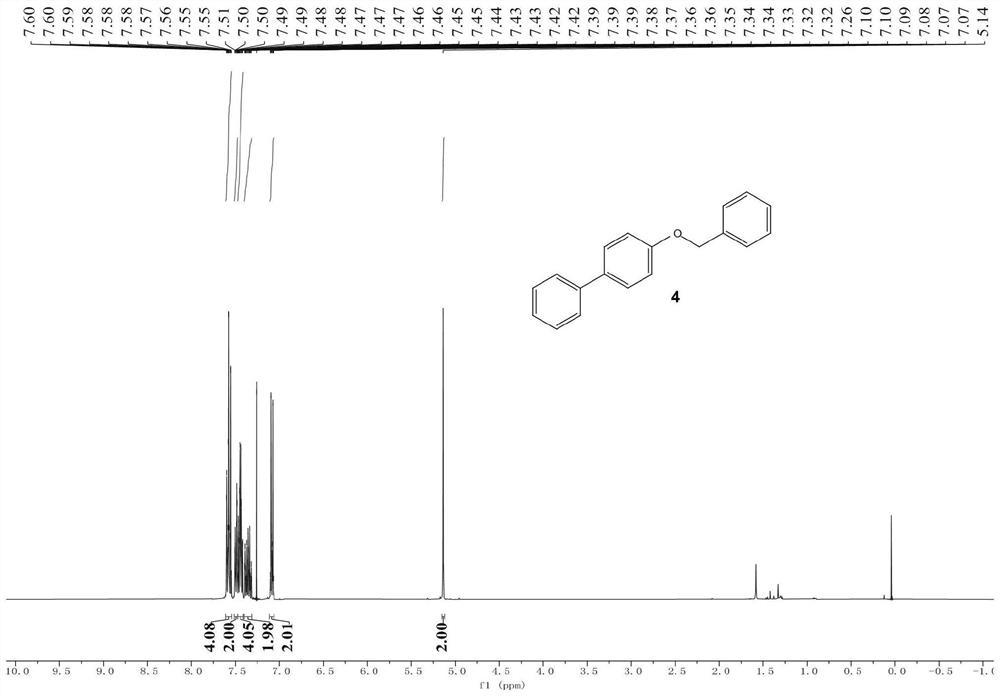
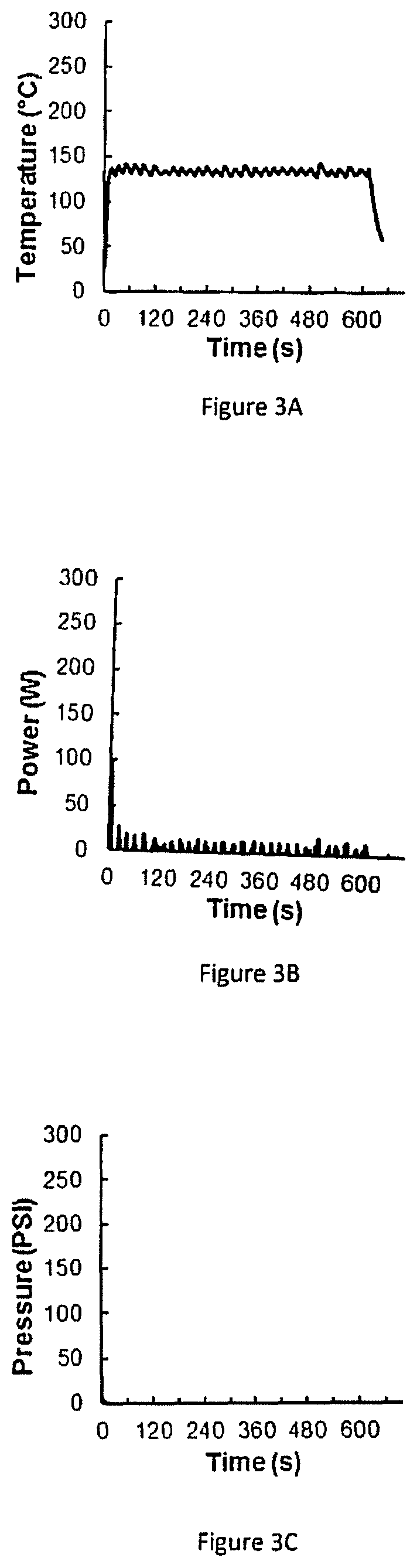
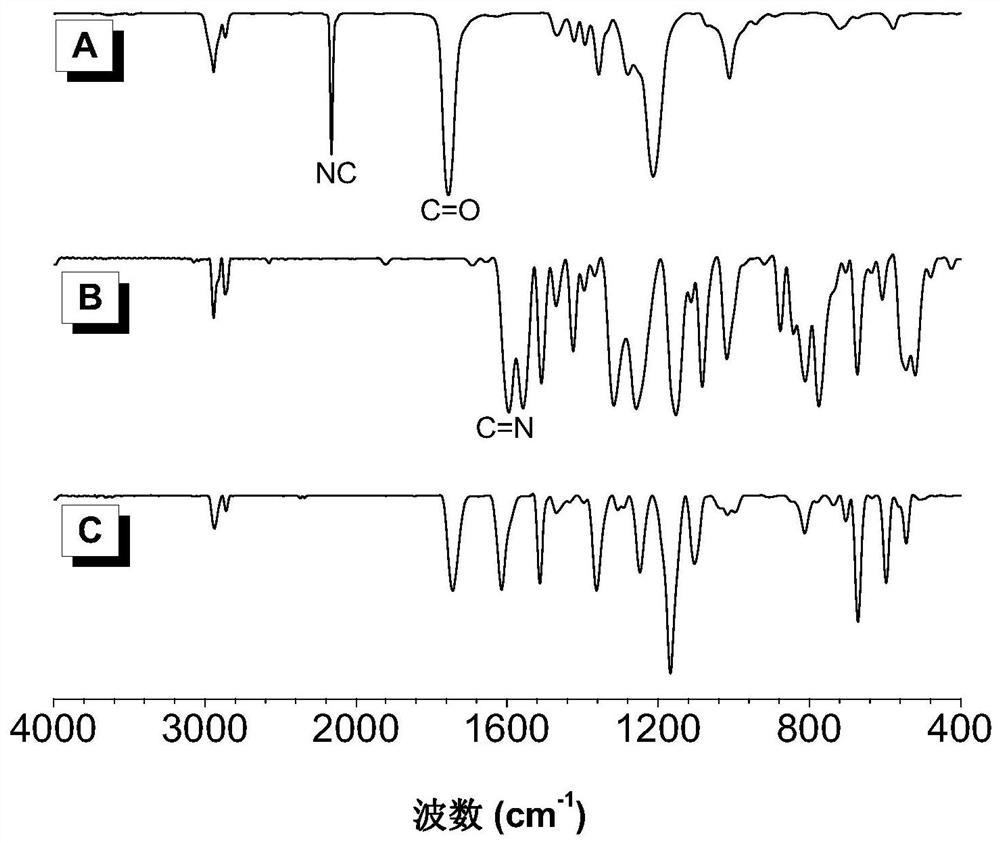
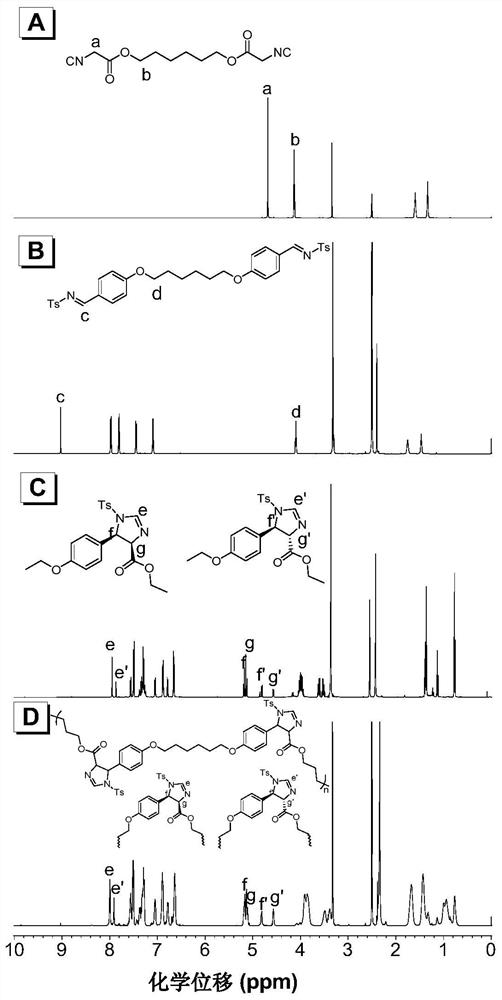
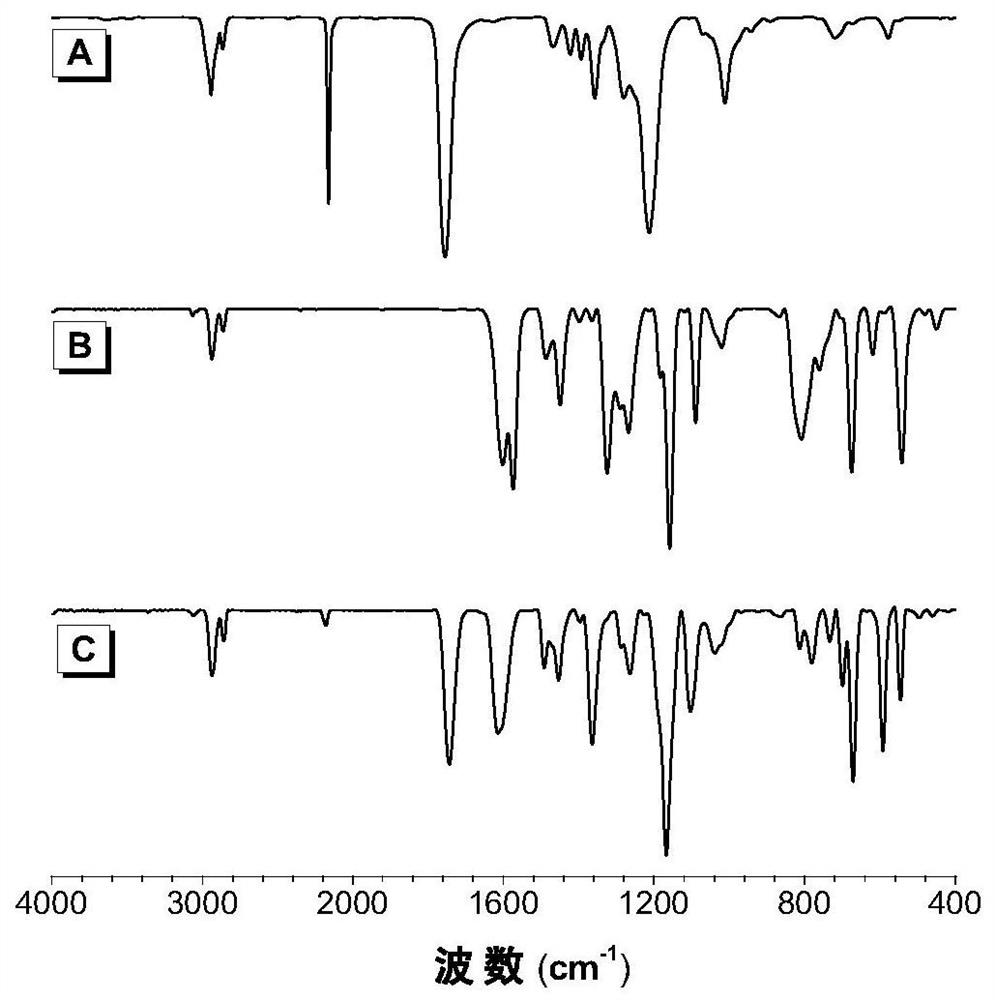
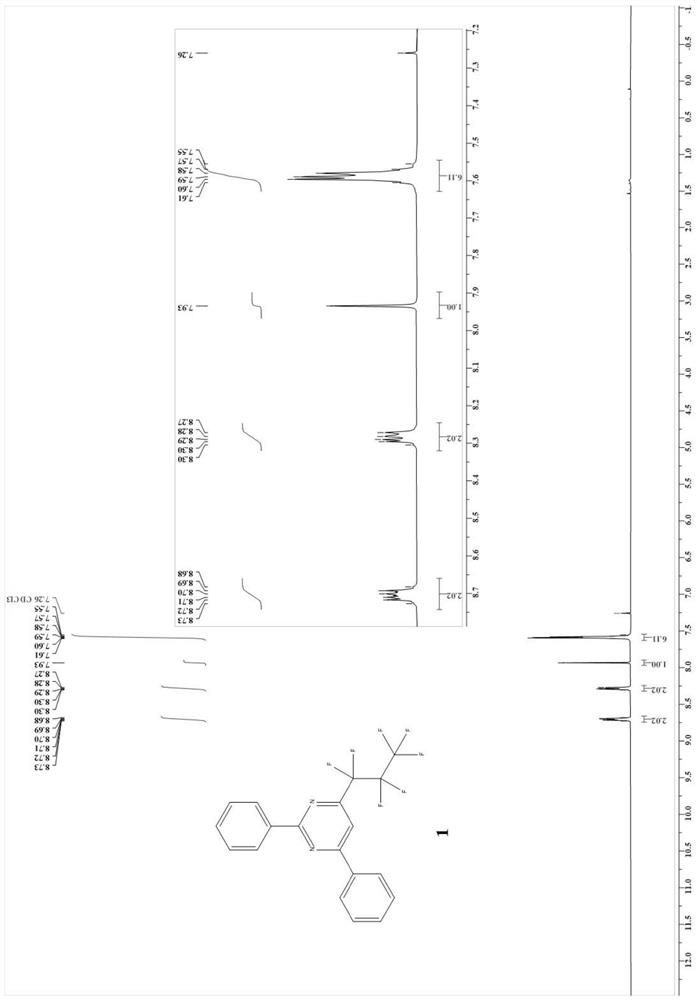
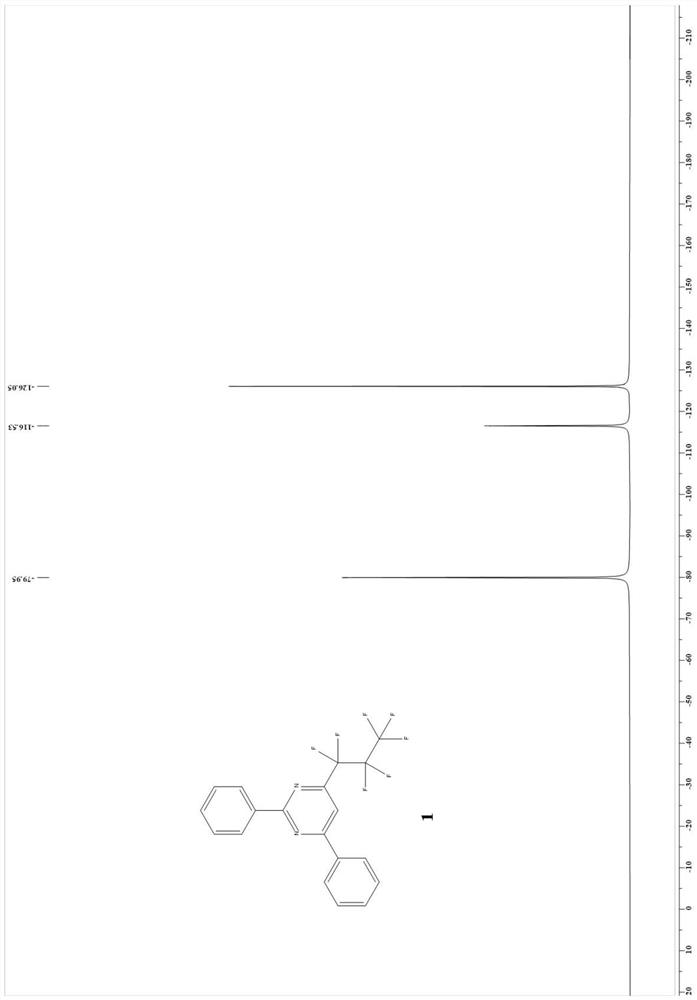
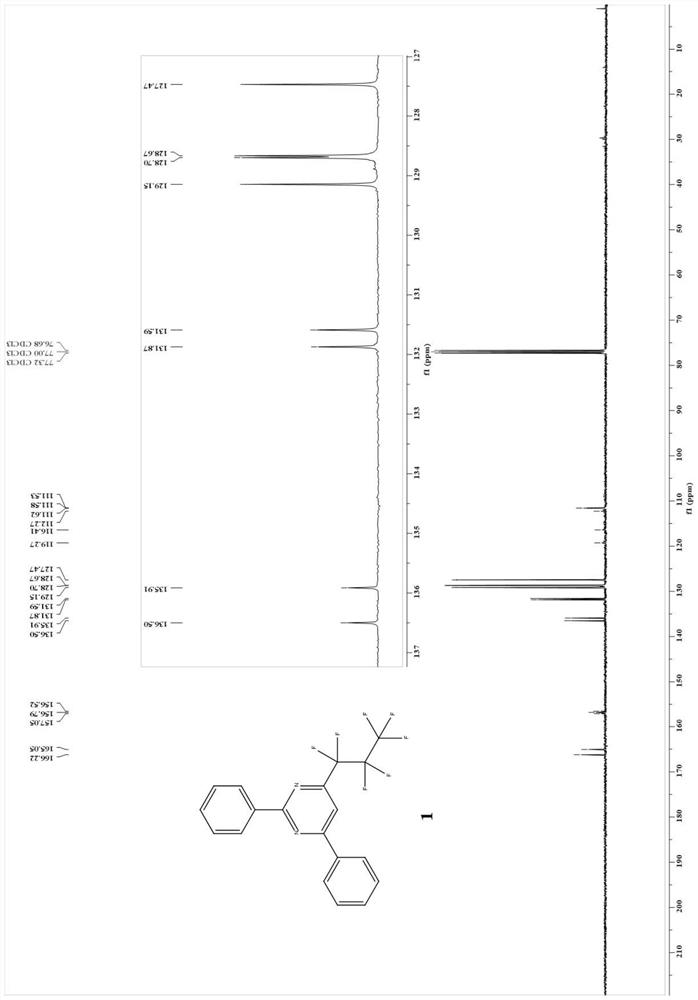
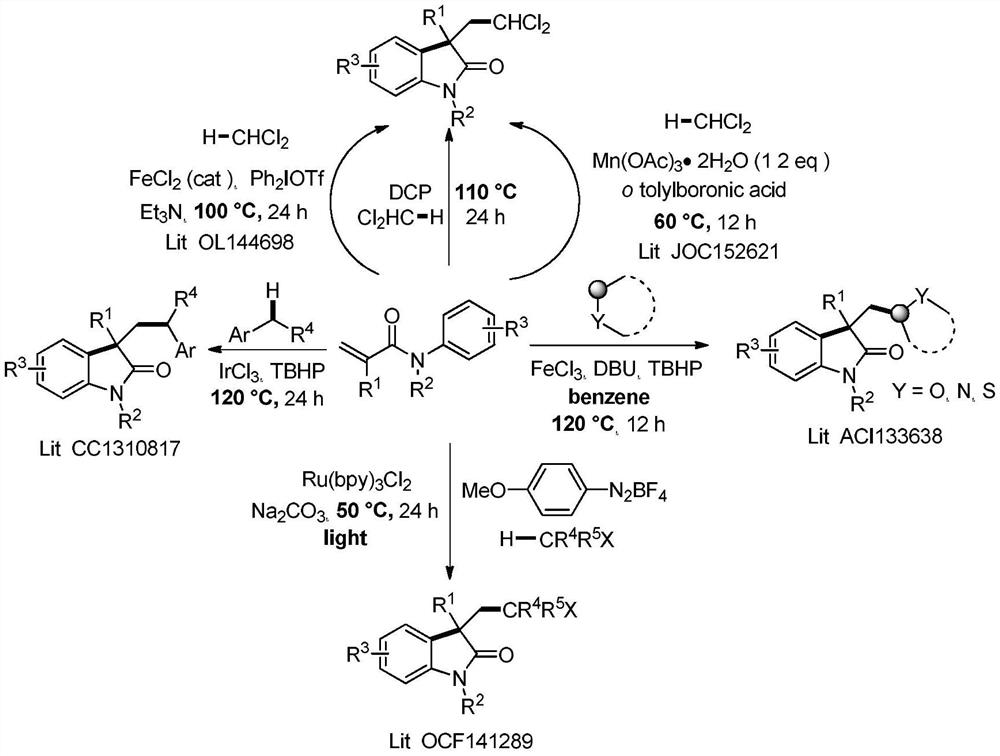
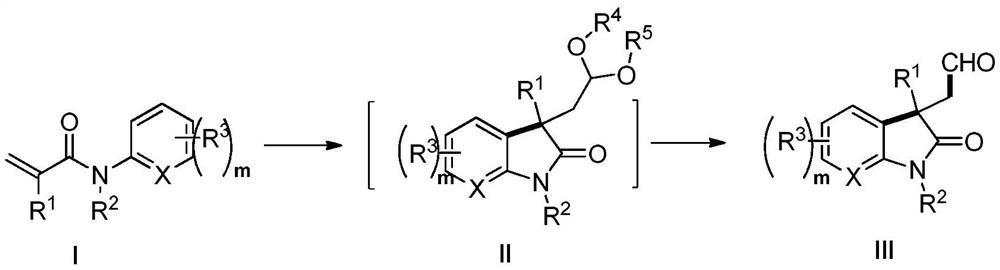
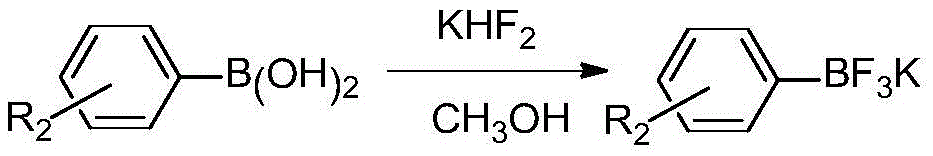
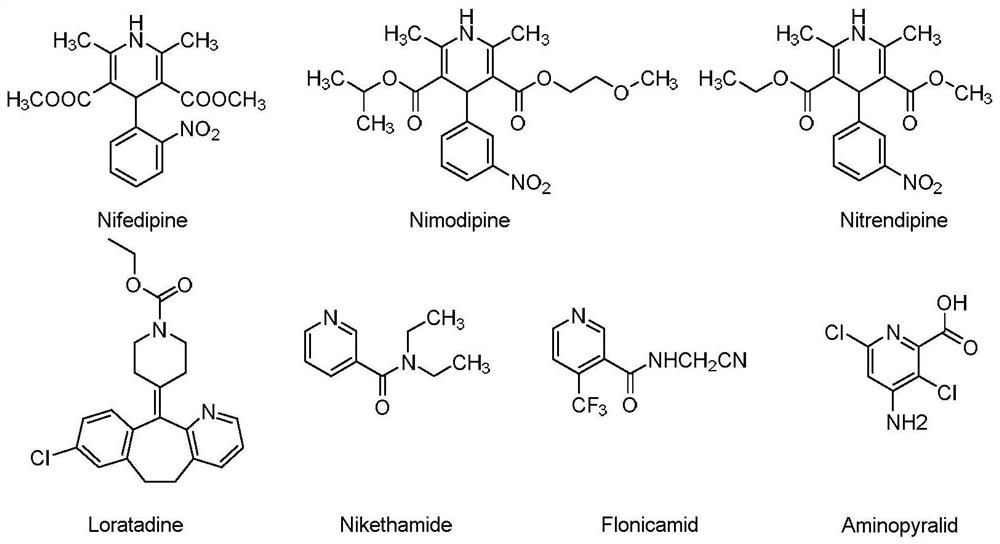
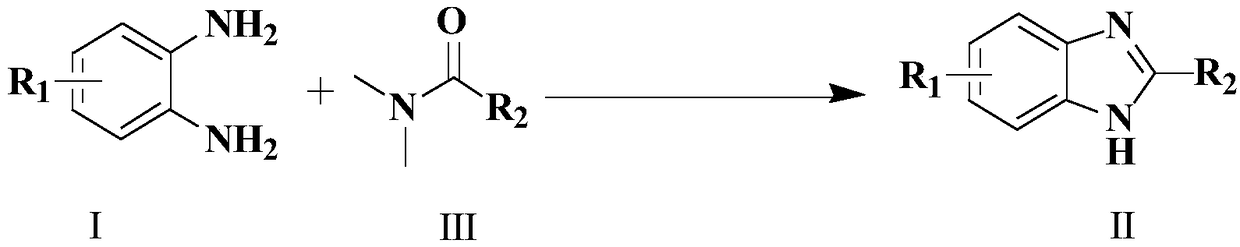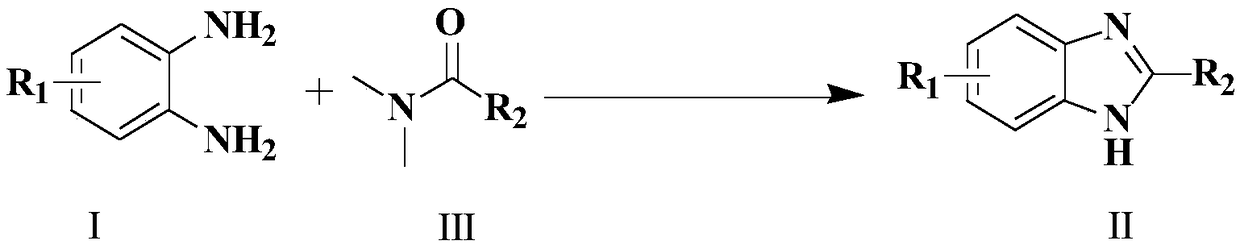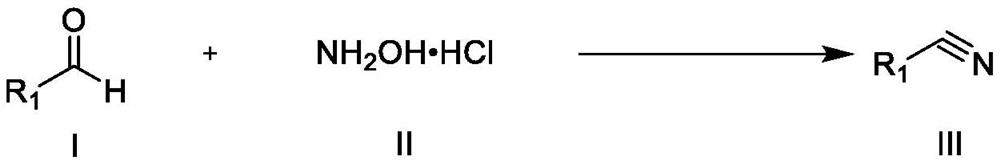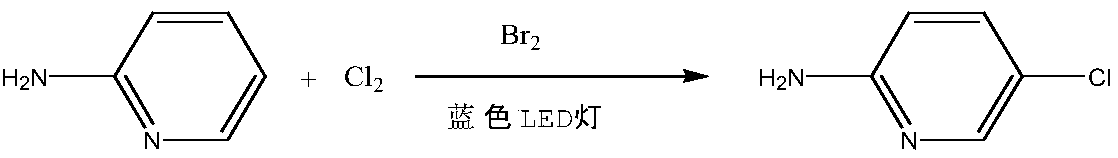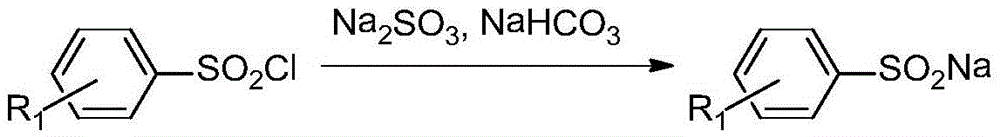Patents
Literature
30results about How to "Good functional group tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
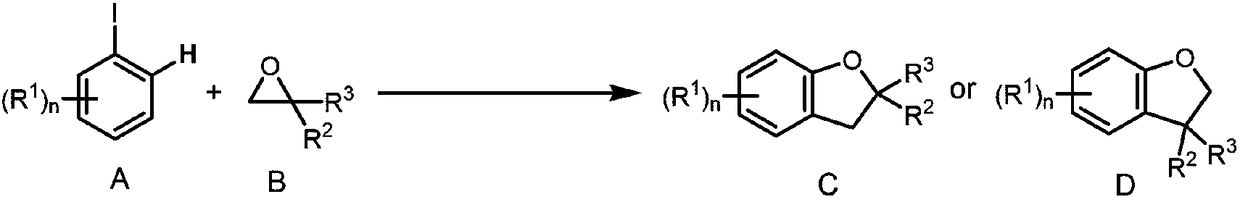
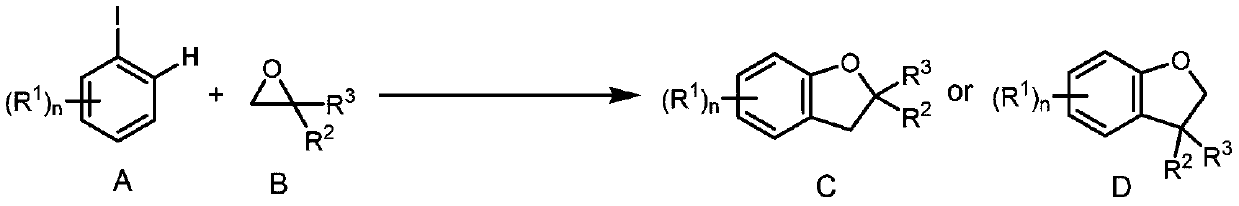
Method for synthesizing 2,3-dihydrobenzofurans compound
InactiveCN108329285ANo special handling requiredLow priceGroup 4/14 element organic compoundsSteroidsOrganic solventIodide
The invention provides a method for synthesizing a 2,3-dihydrobenzofurans compound. The method comprises the following steps: dissolving aromatic iodide, an epoxy compound, a palladium catalyst, a phosphine ligand and a norborene derivative in an organic solvent together; then carrying out stirring reaction at the temperature of 30-120 DEG C; and carrying out separation and purification after reaction to obtain the 2,3-dihydrobenzofurans compound. By the method, the 2,3-dihydrobenzofurans compound can be synthesized efficiently, economically and environmentally friendly. The method is gentle in condition, good in substrate universality and high in yield, and the prepared 2,3-dihydrobenzofurans compound is widely applied to the fields of medicinal chemistry and organic chemistry.
Owner:WUHAN UNIV
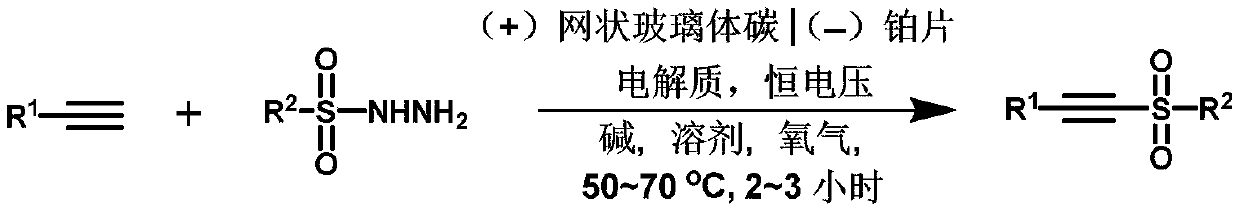
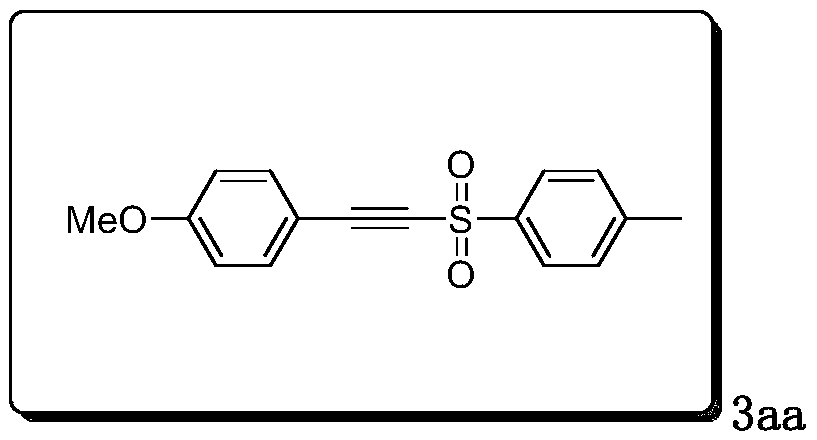
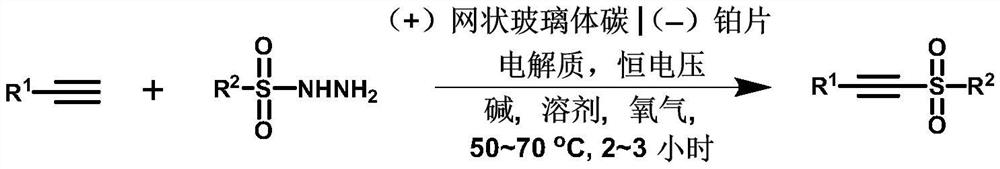
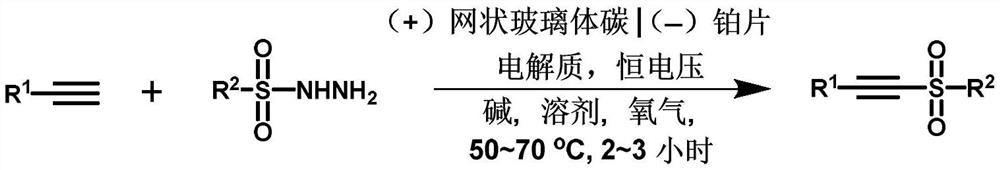
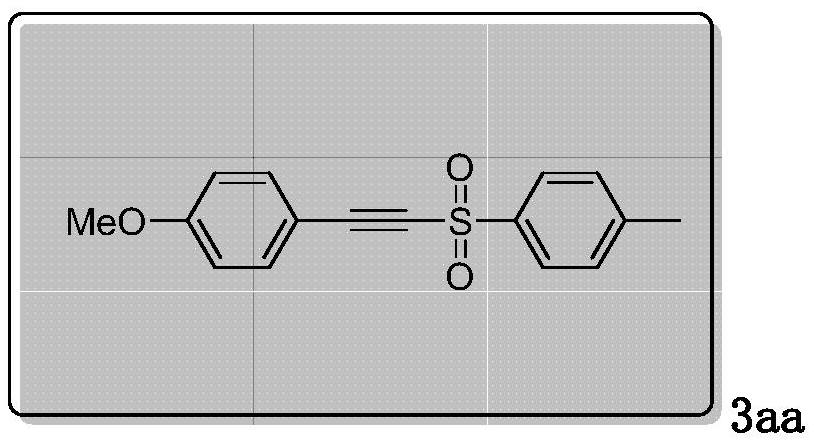
Method for synthesizing alkyne sulfone compounds from terminal alkyne and sulfonyl hydrazine
ActiveCN111139494AAtom economy is highGood functional group toleranceElectrolysis componentsElectrolytic organic productionSulfohydrazideAlkyne
The invention discloses a method for synthesizing alkyne sulfone compounds from terminal alkyne and sulfonyl hydrazine. According to the method, a series of the alkynyl sulfone compounds are synthesized through an oxidative cross-coupling reaction of terminal alkyne and sulfonyl hydrazine, and the method does not need pre-functionalization of alkyne, and has the advantages of high atom economy, nometal or oxidant, good functional group tolerance and the like. The compounds synthesized by the method are subjected to in-vitro antitumor activity screening, and experimental results show that mostof the compounds have relatively strong inhibitory activity on tumor cells.
Owner:GUANGXI NORMAL UNIV
Method for catalyzing alcohol dehydrogenation silicon alkylation by using azacyclo-cabbeen
InactiveCN102924502AHigh selectivityGood choiceGroup 4/14 element organic compoundsSilanesDistillation
Provided is a method for catalyzing alcohol dehydrogenation silicon alkylation by using azacyclo-cabbeen. Catalyst azacyclo-cabbeen is dissolved in an organic solvent or directly placed in a schlenk tube (Schlenk tube) and a nuclear magnetism tube, silane and alcohol are sequentially added, a molar ratio of the silane to the alcohol is 1:1-2, a molar ratio of the catalyst azacyclo-cabbeen to the silane is 0.002-0.05:1, reaction is carried out at the normal temperature for 0.5-24h, then a solvent is extracted under a vacuum condition or distillation or column chromatography purification is directly carried out, and the alcohol dehydrogenation silicon alkylation can be carried out to prepare siloxane. The method has the advantages of using small organic molecule azacyclo-cabbeen to serve as a catalytic agent to replace a metal catalyst, is cheap in raw material, easy to prepare, soft in catalysis condition, high in catalytic activity, good in selectivity, easy in aftertreatment and wide in applicable scope of substrate silane, has good functional group tolerance, is single in reaction product, and can efficiently and selectively obtain the siloxane.
Owner:NANKAI UNIV
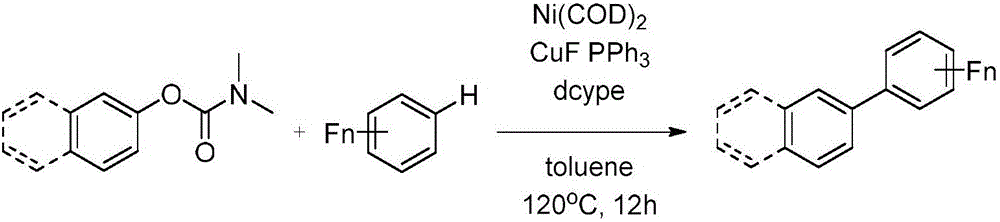
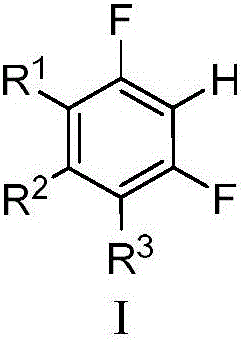
Magnesium assisted nickel catalyzed multi-fluoro aromatic hydrocarbon monoarylation method
InactiveCN106187656AGood functional group toleranceWide substrate applicabilitySilicon organic compoundsCarboxylic acid nitrile preparationChemistryMagnesium
The invention discloses a magnesium assisted nickel catalyzed multi-fluoro aromatic hydrocarbon monoarylation method. The method realizes cross coupling of multi-fluoro aromatic hydrocarbon and aryl halide or aryl sulfonate by adopting a compound formed by a nickel source and a diphosphine ligand as a catalyst. The coupling method has the advantages of high controllability, high monoarylation product proportion reaching 99% or above, good function group tolerance, wide substrate applicability, and realization of the yield of 80-96%. Compared with methods adopting previous catalysis catalysts (palladium system and copper system), the method adopting a brand new nickel catalysis system has the advantages of realization of cross coupling under mild conditions, excellent selectivity, low price, simplicity in operation, easiness in post-treatment, small pollution, and high social values and industrial promotion prospect.
Owner:SHAANXI NORMAL UNIV
Biaryl compound as well as preparation method and application thereof
ActiveCN113402350AExpand the range of prepared substratesEasy to operateGroup 4/14 element organic compoundsAmino preparation from aminesLithium chlorideMeth-
The invention discloses a biaryl compound as well as a preparation method and application thereof. The preparation method comprises the following steps: reacting magnesium chips and lithium chloride in a schlenk sealed tube to obtain a reactant 1, adding ultra-dry tetrahydrofuran into the sealed tube, extracting nitrogen from the sealed tube, then adding aryl quaternary ammonium salt, a 5mol% catalyst, aryl bromide and N, N, N' N'-tetramethyldisiloxane, stirring and reacting at 25-60 DEG C for 6-12 hours to obtain a reactant 2, and finally, sequentially carrying out extracting, washing, drying, extract liquor removing and purifying on the reactant 2 to obtain the biaryl compound which can be used for preparing a compound containing a biaryl structure. The preparation method is wide in substrate range, convenient to operate, mild in reaction condition, low in pollution and high in economic benefit, and the obtained biaryl compound has good functional group tolerance and substrate universality.
Owner:NANJING UNIV OF TECH
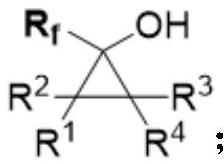
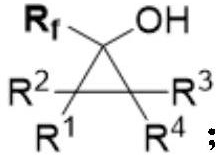
Alpha-fluoroalkyl substituted cyclopropyl alcohol compound as well as preparation method and application thereof
PendingCN114456134AGood diastereoselectivityAchieve cyclopropanationCarboxylic acid nitrile preparationOrganic compound preparationArylCarboxyl radical
The invention relates to the technical field of organic synthesis, in particular to an alpha-fluoroalkyl substituted cyclopropyl alcohol compound as well as a preparation method and application thereof, the molecular structural formula of the alpha-fluoroalkyl substituted cyclopropyl alcohol compound is shown in the specification, R1, R2, R3 and R4 are respectively any one of aryl, heteroaryl, alkyl, alkenyl, alkynyl, ester group, cyano, nitryl, sulfonyl, heteroatom and hydrogen atom, Rf is CF3, CF2R5 or CFR6R7, Rf is CF3, CF2R5 or CFR6R7, and R1, R2, R3 and R4 are respectively any one of aryl, heteroaryl, alkyl, alkenyl, alkynyl, ester group, cyano, nitryl, sulfonyl, heteroatom and hydrogen atom. R5 is any one of halogen, aryl, heteroaryl, alkyl, alkenyl, alkynyl, ester group, cyano, nitryl, sulfonyl, carboxyl, heteroatom and hydrogen atom; r6 and R7 are respectively any one of aryl, heteroaryl, alkyl, alkenyl, alkynyl, ester group, cyano, nitryl, sulfonyl, heteroatom and hydrogen atom, and can be the same or different. The alpha-fluoroalkyl substituted cyclopropyl alcohol compound disclosed by the invention is a novel compound structure and has high diastereoselectivity.
Owner:WUHAN UNIV
Tetrahydrobenzofuran Mannich alkali compounds, and preparation method and application thereof
ActiveCN110804035AGood functional group tolerancePost-processing is simpleOrganic chemistryAntineoplastic agentsIodideStomach cancer
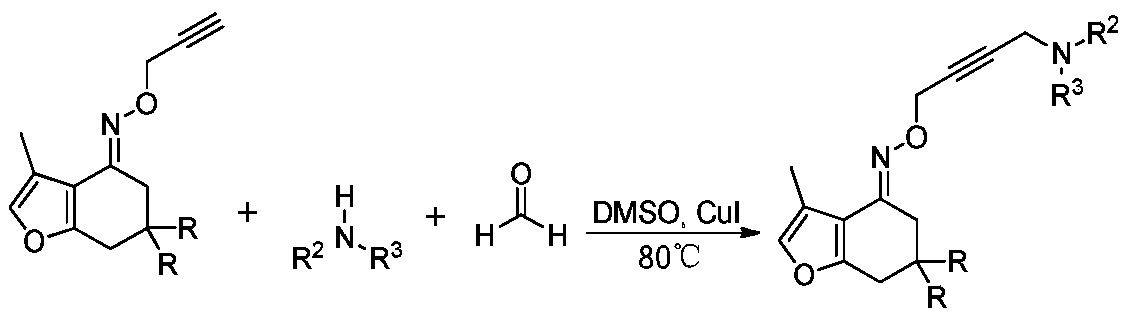
The invention provides tetrahydrobenzofuran Mannich alkali compounds. The structural formula of the tetrahydrobenzofuran Mannich alkali compounds is shown in the specification, wherein R is hydrogen or methyl, R2 and R3 comprise any one of methyl, ethyl, n-propyl, isopropyl, n-butyl, hydroxyethyl, a C4 or C5 methylene chain and an N atom-containing C4 or C5 methylene chain. A preparation method comprises the steps: taking cuprous iodide as a catalyst, dimethyl sulfoxide as a solvent, and a formaldehyde aqueous solution (preferably 30% formaldehyde aqueous solution), secondary amine and furanocyclohexanone oxime ethers as raw materials, and preparing a series of long-chain tetrahydrobenzofuranone Mannich alkalis through a one-pot reaction. The reaction product is single, no side reaction occurs, good functional group tolerance is achieved, and secondary amine containing hydroxyl and heteroatoms has no influence on the reaction. The prepared product is applied to anti-gastric cancer drugs, and a remarkable effect is achieved.
Owner:CHINA THREE GORGES UNIV
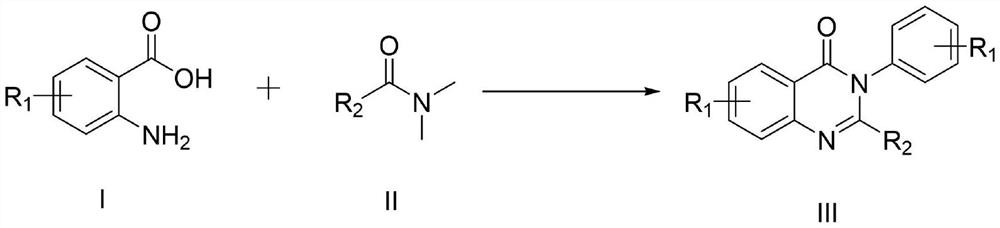
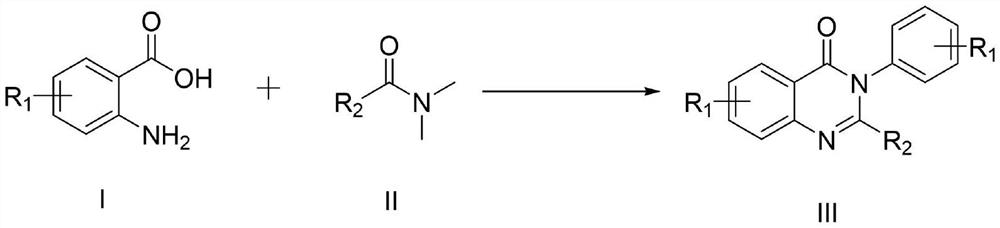
Synthesis method of 4-3 (H) quinazolinone and derivative thereof
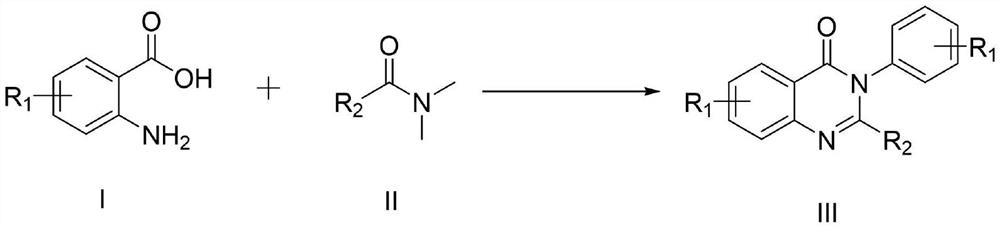
ActiveCN114230526ANo protectionGood functional group toleranceOrganic chemistryBenzoic acidPtru catalyst
The invention provides a synthesis method of 4-(3H) quinazolinone and a derivative thereof (formula III), which is characterized in that imidazole hydrochloride is used as a catalyst, and 2, 3-disubstituted quinazolinone and the derivative thereof are synthesized from anthranilic acid and a derivative thereof and a DMF (Dimethyl Formamide) derivative by a one-pot method in the absence of other catalysts or additives. The synthesis method disclosed by the invention has good functional group tolerance, and 4-(3H) quinazolinone and derivatives thereof can be synthesized for research in medicines or other fields. According to the synthetic method, dichloromethane, corrosive concentrated hydrochloric acid and a metal catalyst are not used, harsh reaction conditions, gas protection and an autoclave are not needed, the reaction time is short, and the synthetic method has certain value in industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Chiral vicinal diamine derivative and catalytic asymmetric synthesis method thereof
ActiveCN107253928AWide range of substratesGood functional group toleranceOrganic chemistry methodsDiamineNitrogen
The invention discloses a chiral vicinal diamine derivative. The chiral vicinal diamine derivative is a compound as shown in a formula I, or tautomer, enantiomer and diastereoisomer thereof, wherein Ar1 and Ar2 are selected from aryl and ceteroary respectively; the aryl and ceteroary do not have substituent group or have one or more substituent groups; R1 is selected from hydrogen, alkyl, phenyl, esteryl and methylene; when R1 is methylene, an A ring is formed through 0 to 5 methylene; X is selected from N3, NH2, NHBoc and N(Bn)2; B rings are 4 to 7 saturated or unsaturated heterocyclic rings; M is carbon or nitrogen; when M is carbon, G is selected from hydrogen, alkyl, alkoxy and esteryl; when M is nitrogen, G is selected from Ms, Ts, Boc and Bn. The invention further discloses a catalytic asymmetric synthesis method of the chiral vicinal diamine derivative. The method has a wide substrate range, excellent functional group tolerance and mild reaction conditions, is high in yield and good in enantioselectivity, and belongs to a convenient and practical method.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
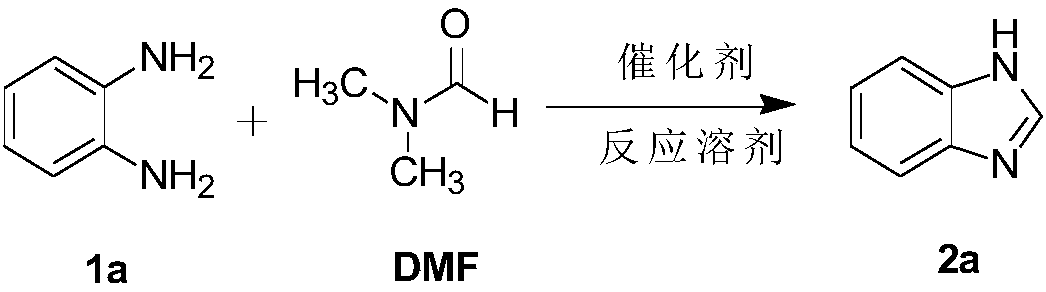
Method for synthesizing benzimidazole and derivative
InactiveCN109265403AGood functional group toleranceGood yield and purityOrganic chemistryBenzimidazoleChloride
The invention provides a method for synthesizing benzimidazole and its derivative. The synthesis of polyfunctional benzimidazole and 2-substituted benzimidazole is achieved by imidazole chloride catalyzed o-phenylenediamine cyclization. The method is simple and economical and strong in practicability. No catalyst or additive is added. The synthesis method has good functional group tolerance, excellent yield and purity and short reaction time, does not require harsh reaction conditions and is suitable for industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
1,1-diarylalkane derivative preparation method
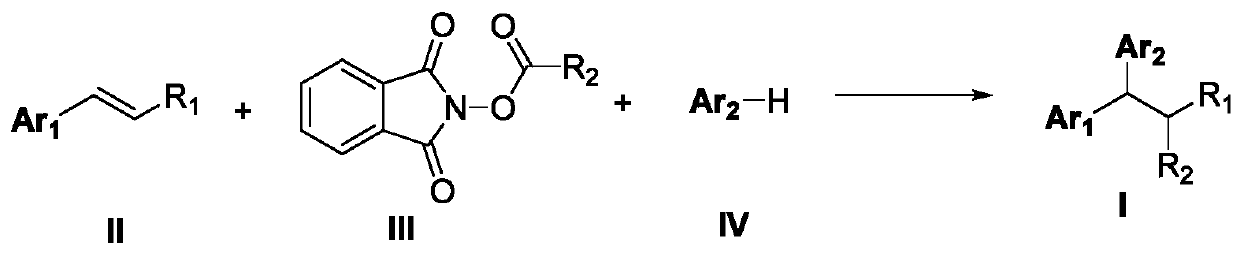
ActiveCN110256211AGood functional group toleranceEfficient new bond formation capabilityOrganic compound preparationOrganic additionAlkanePhotoredox catalysis
The invention discloses a 1,1-diarylalkane derivative preparation method, wherein a C(sp3)-C(sp3) / C(sp3)-C(sp2) bond is constructed under light oxidation-reduction catalytic conditions by using an olefin-based compound, (hetero) aromatic hydrocarbon and a N-hydroxy phthalimide ester compound as raw materials so as to achieve the 1,2-difunctionalization reaction of olefin, such that a series of 1,1-diaryl alkane derivatives are prepared.
Owner:NANCHANG HANGKONG UNIVERSITY
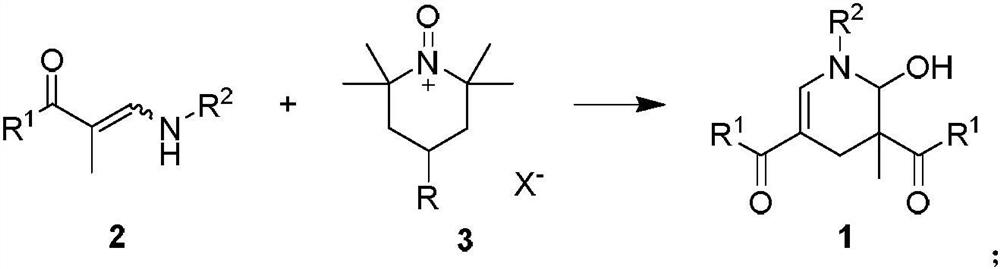
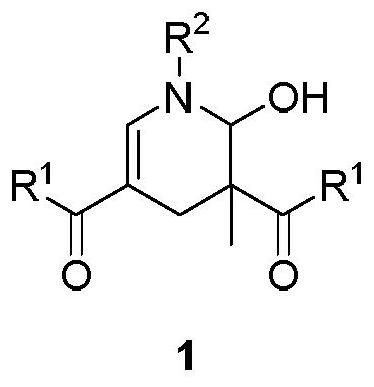
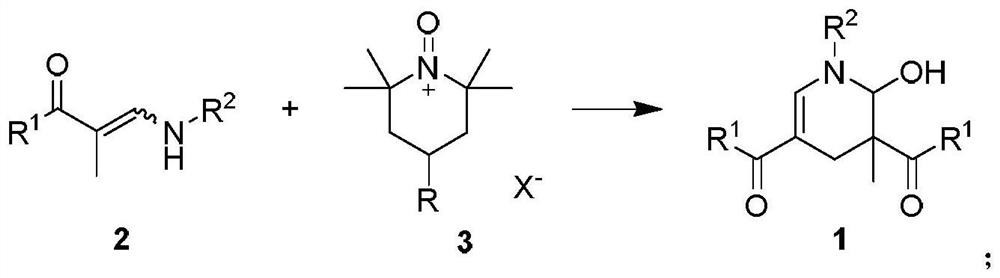
2-hydroxypyridine compound and synthesis method thereof
ActiveCN112592310AGood functional group toleranceWide applicabilityOrganic chemistry methodsOrganic synthesisCombinatorial chemistry
The invention discloses a 2-hydroxypyridine compound and a synthesis method and application thereof. According to the method, enaminone is used as a starting raw material, tempo salt is used as an oxidizing agent, and the 2-hydroxypyridine compound is generated through an oxidation reaction and a coupling cyclization reaction under a heating condition. The reaction functional group is high in compatibility, the yield is as high as 80%, and the product has functional group diversity. The compound can be used as an organic synthesis precursor, and 2-hydroxyl and 6-hydrogen atoms in the structurecan be further functionalized to obtain a drug molecular skeleton or a compound with potential biological activity and the like.
Owner:NANJING UNIV OF TECH
Carbon based materials as solid-state ligands for metal nanoparticle catalysts
ActiveUS10661251B2High catalytic activityStrong anchoringSilicon organic compoundsCatalystsPtru catalystGraphene
High activity metal nanoparticle catalysts, such as Pd or Pt nanoparticle catalysts, are provided. Adsorption of metal precursors such as Pd or Pt precursors onto carbon based materials such as graphene followed by solventless (or low-solvent) microwave irradiation at ambient conditions results in the formation of catalysts in which metal nanoparticles are supported on i) the surface of the carbon based materials and ii) in / on / within defects / holes in the carbon based materials.
Owner:VIRGINIA COMMONWEALTH UNIV
A kind of polyimidazoline compound and preparation method thereof
Owner:SOUTH CHINA UNIV OF TECH
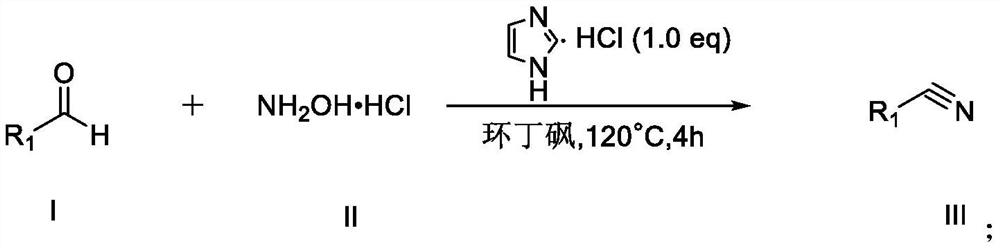
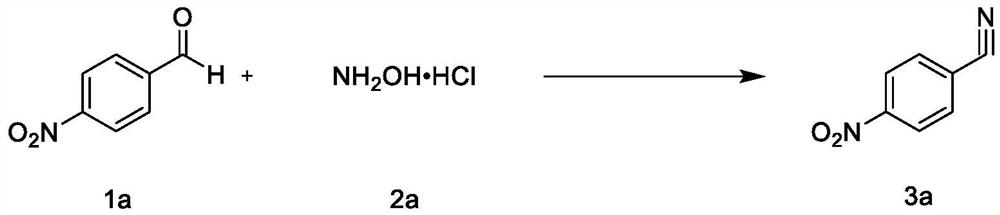
Synthesis method of nitrile compound
PendingCN114409571AGood functional group toleranceShort reaction timeCarboxylic acid nitrile preparationOrganic compound preparationHydroxylamine HydrochlorideAldehyde
Owner:CHONGQING MEDICAL UNIVERSITY
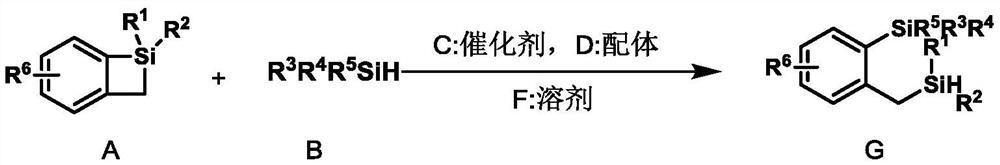
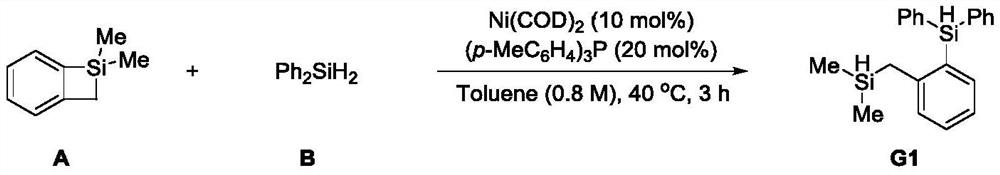
A kind of double silicon compound, its preparation method and application
ActiveCN112174995BRealize the ring-opening metathesis reactionSimple reaction conditionsSilicon organic compoundsArylOrganic synthesis
The present invention relates to the technical field of organic synthesis, in particular to a double silicon compound, its preparation method and application. The molecular structure of the double silicon compound is as follows: wherein, R 1 , R 2 are aryl or alkyl, which can be the same or different; R 3 , R 4 , R 5 Any one of aryl group, alkyl group and hydrogen atom, which can be the same or different, R 3 , R 4 , R 5 Up to two hydrogen atoms at the same time, R 6 Any of aryl group, alkyl group, heteroatom and hydrogen atom. The preparation method of the present invention realizes the ring-opening metathesis reaction of four-membered ring silicon and silane, is a new reaction mode, and provides a simple and efficient method for synthesizing double silicon compounds, especially two silicons containing silicon hydrogen It can be used to synthesize polymers under circumstances, and has very great application prospects.
Owner:WUHAN UNIV
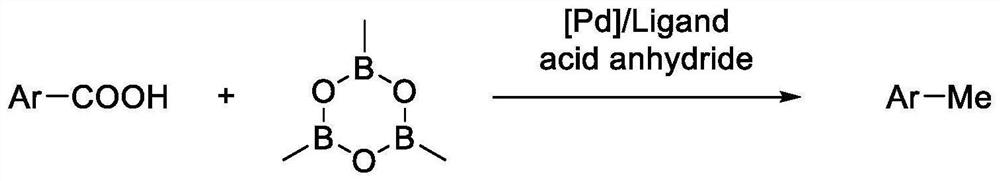
Method for preparing methyl (hetero) arene through decarbonylation coupling of (hetero) aryl formic acid and trimethylcyclotrioxane under catalysis of transition metal
PendingCN114249625AReduce usageAvoid it happening againCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystGrignard reagent
The invention discloses a method for preparing methyl (hetero) arene through decarbonylation coupling of (hetero) aryl formic acid and trimethylcyclotrioxane under catalysis of transition metal. According to the method, (hetero) aryl formic acid, trimethylcycloborane, a palladium-containing catalyst, a phosphine ligand, an anhydride additive and a solvent are mixed and react in an inert gas atmosphere to obtain a methyl (hetero) aromatic hydrocarbon product. The method uses stable, cheap and easily available (hetero) aryl formic acid as a raw material to synthesize methyl (hetero) aromatic hydrocarbon, can make up for the defects existing in the traditional coupling reaction of transition metal catalyzed halogenated (hetero) aromatic hydrocarbon and a nucleophilic methylation reagent, and has the following advantages: (1) use of expensive and difficult-to-prepare halogenated (hetero) aromatic hydrocarbon is avoided, and generation of halogen-containing waste is avoided; (2) the use of unstable raw materials such as a methyl Grignard reagent and methylboronic acid is avoided, and the reaction has better functional group tolerance; and (3) the (hetero) aryl formic acid raw material and the product have large polarity difference, and are easy to separate through column chromatography.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Poly(enamine nitrile) compound and preparation method thereof
ActiveCN113754883AImprove machinabilityImprove thermal stabilityPolymer sciencePotassium tert-butoxide
The invention discloses a preparation method of a poly(enamine nitrile) compound, wherein the preparation method comprises the following steps: carrying out polymerization reaction on a binary nitrile compound, a binary isonitrile compound and a polymerization catalyst in an organic solvent to obtain the poly(enamine nitrile) compound, wherein the polymerization catalyst comprises cuprous iodide and potassium tert-butoxide. A polymerization reaction implementation process is simple in process, and reaction raw materials are easy to obtain and can be directly purchased or prepared through a simple reaction; polymerization reaction conditions are mild, polymerization can be carried out at room temperature, and energy is saved; the polymerization efficiency is high, and a polymer with relatively high molecular weight can be obtained after 4 hours of reaction; and no by-product is generated in the polymerization process, and the atom economy is met. The invention also discloses the poly(enamine nitrile) compound which has good processability and high thermal stability.
Owner:SOUTH CHINA UNIV OF TECH
Mass spectrometry method for compound containing carbon-carbon double bonds
PendingCN114720545AImprove mass spectrometry identificationImprove compatibilityComponent separationMaterial analysis by electric/magnetic meansTetrafluoroborateIsotopic labeling
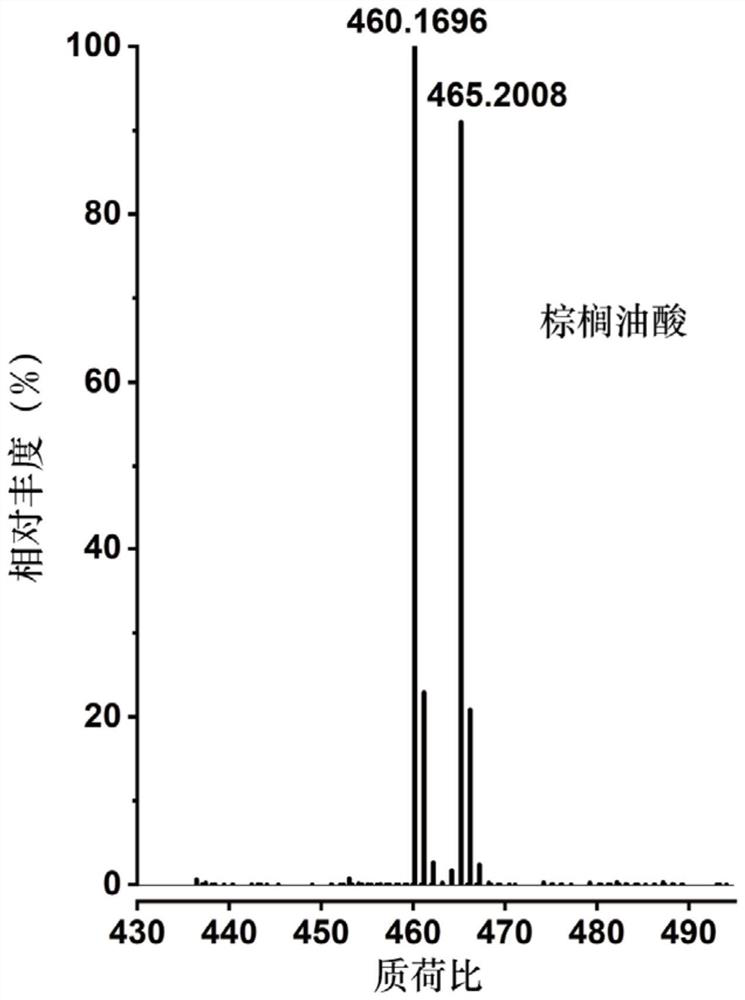
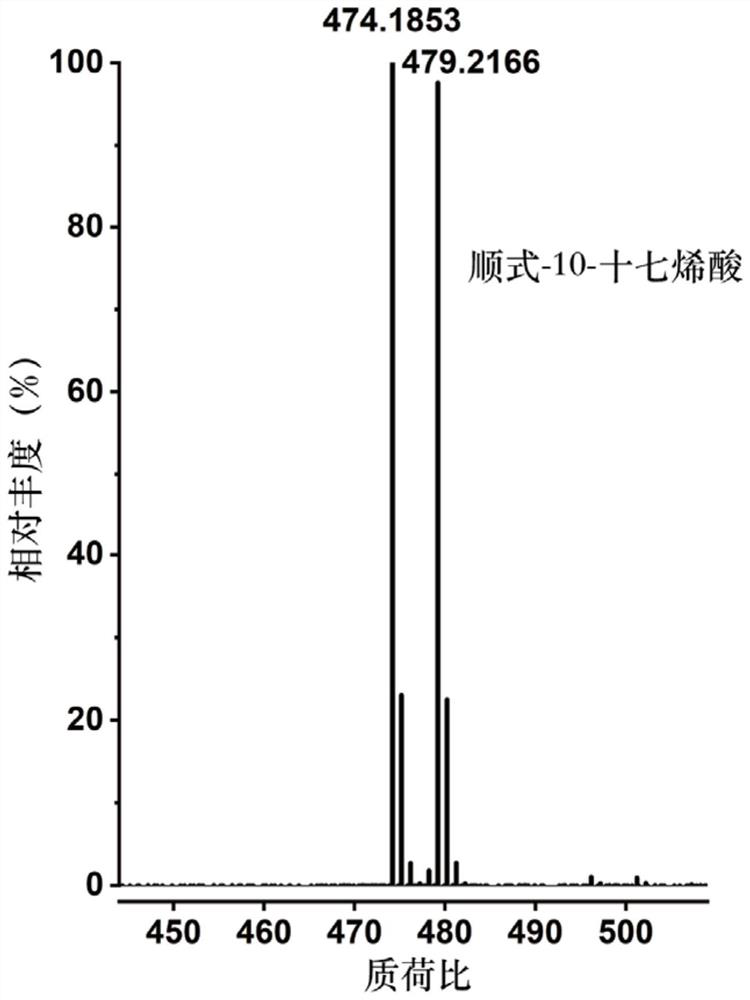
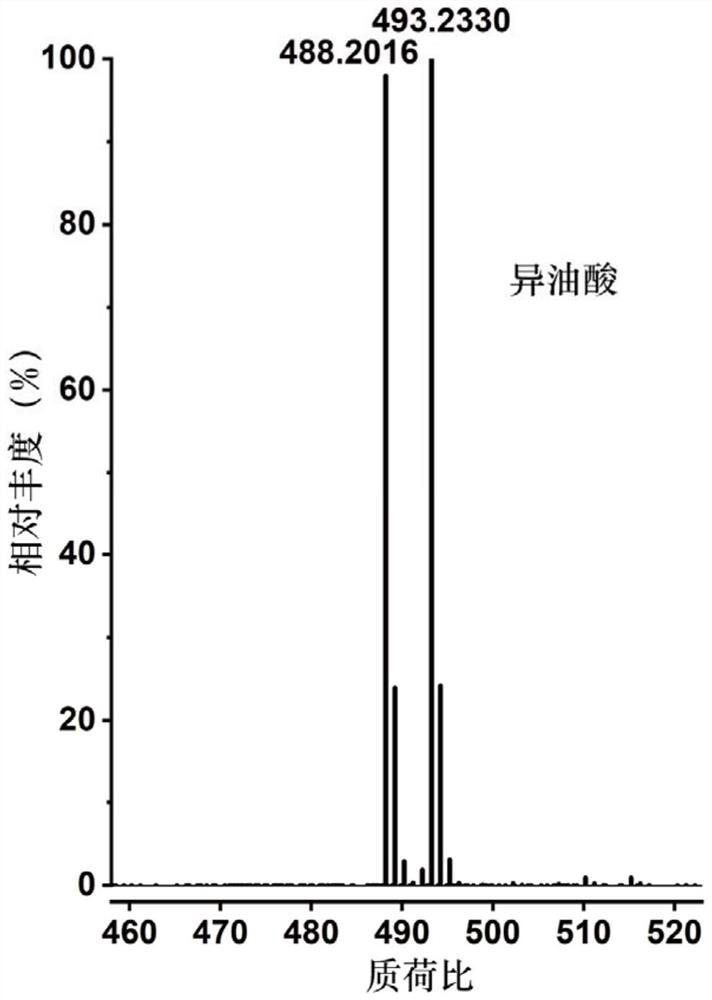
The invention discloses a mass spectrometry method for a compound containing a carbon-carbon double bond. The mass spectrometry method comprises the following steps: a) carrying out labeling reaction on light and heavy isotope labeling reagents and the compound containing the carbon-carbon double bond in a target analyte; b) carrying out post-treatment on a product after the marking reaction and then carrying out mass spectrometry; wherein the light isotope labeling reagent is [d0]-bis (pyridine) iodine tetrafluoroborate, and the heavy isotope labeling reagent is [d10]-bis (pyridine) iodine tetrafluoroborate. Experiments prove that the method not only can significantly improve the mass spectrum recognition of the compound containing the carbon-carbon double bond, but also has universality and wide application range, can be suitable for mass spectrum direct analysis, can also be suitable for liquid chromatography and mass spectrum combined analysis, can be used for quantitative analysis of the compound containing the carbon-carbon double bond in a complex system, and has wide application prospects. And the method has important value and practicability for realizing rapid, efficient and accurate mass spectrometry of compounds containing carbon-carbon double bonds in complex samples.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method using one-step method to synthesize 2-amino-5-chloropyridine in high-selectivity manner
InactiveCN109134357AMild reaction conditionsGood functional group toleranceOrganic chemistryBromineDistillation
The invention belongs to the technical field of intermediate synthesis and particularly relates to a method using a one-step method to synthesize 2-amino-5-chloropyridine in a high-selectivity manner.The method includes: adding 2-aminopyridine serving as the raw material into an organic solvent, dropwise adding bromine to serve as the catalyst under the irradiation of a blue LED lamp, and feedingchlorine after solution fading to perform chlorination reaction; subjecting obtained reaction liquid to reduced pressure distillation to obtain a crude product, and performing further recrystallization to obtain high-purity 2-amino-5-chloropyridine. The method has the advantages that the bromine is used as the catalyst, the bromine is irradiated by the blue light LED lamp to generate bromine freeradicals combining with the 5 site of a 2-aminopyridine ring, then chlorination is performed, product 2-amino-5-chloropyridine cascade reaction, which generates polychloro compounds, caused by directchlorination is avoided, good chlorination selectivity is achieved, and the high-content 2-amino-5-chloropyridine is obtained; compared with the prior art, the method is simplified in production process, mild in reaction condition, high in product purity, low in production cost and low in wastewater quantity.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
A method for synthesizing 2,3-dihydrobenzofuran compounds
InactiveCN108329285BNo special handling requiredLow priceGroup 4/14 element organic compoundsSteroidsOrganic solventIodide
The invention provides a method for synthesizing a 2,3-dihydrobenzofurans compound. The method comprises the following steps: dissolving aromatic iodide, an epoxy compound, a palladium catalyst, a phosphine ligand and a norborene derivative in an organic solvent together; then carrying out stirring reaction at the temperature of 30-120 DEG C; and carrying out separation and purification after reaction to obtain the 2,3-dihydrobenzofurans compound. By the method, the 2,3-dihydrobenzofurans compound can be synthesized efficiently, economically and environmentally friendly. The method is gentle in condition, good in substrate universality and high in yield, and the prepared 2,3-dihydrobenzofurans compound is widely applied to the fields of medicinal chemistry and organic chemistry.
Owner:WUHAN UNIV
Method for synthesizing halogenated alkane by high-selectivity halogenated saturated carbon-hydrogen bonds
PendingCN114438521AIncrease profitSimple methodElectrolysis componentsElectrolytic organic productionElectrochemical responseAlkane
The invention discloses a synthesis method for highly selectively chlorinating or brominating a saturated carbon-hydrogen bond in alkane into corresponding chlorinated or brominated alkane under an electrochemical condition, and relates to the technical field of medicine synthesis. The synthesis method comprises the following steps: in an electrochemical reaction tank, dissolving alkane, a catalyst, an electrolyte and a halogen source in a solvent, connecting an electrode, and electrifying and reacting at room temperature, thereby selectively halogenating saturated carbon-hydrogen bonds in the alkane into corresponding halogenated alkane. The method can be widely applied to large-scale synthesis of various medicines.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
A method for synthesizing alkyne sulfone compounds from terminal alkynes and sulfonyl hydrazides under electrochemical conditions
ActiveCN111139494BAtom economy is highGood functional group toleranceElectrolysis componentsElectrolytic organic productionSulfohydrazideAlkyne
The invention discloses a method for synthesizing alkyne sulfone compounds with terminal alkyne and sulfonyl hydrazide. This method synthesizes a series of alkynyl sulfone compounds through the oxidative cross-coupling reaction of terminal alkynes and sulfonyl hydrazides. Functional group tolerance and other advantages. The compounds synthesized by this method were screened for anti-tumor activity in vitro, and the experimental results showed that most of the compounds had strong inhibitory activity on tumor cells.
Owner:GUANGXI NORMAL UNIV
A kind of 4-perfluoroalkyl substituted pyrimidine compound and its preparation method and application
ActiveCN113024470BExpand the range of prepared substratesMild reaction conditionsOrganic chemistryAir atmospherePtru catalyst
The invention discloses a 4-perfluoroalkyl substituted pyrimidine compound, a preparation method and application thereof. In the present invention, alkali additives and solvents are added to the reaction raw materials mixed with perfluoroalkyl substituted alkyne compounds and amidine hydrochloride, stirred and reacted for 1 to 24 hours in an air atmosphere at 70°C, and determined by TLC detection The reaction process, the reaction product is obtained after the reaction is completed, the reaction product is washed, extracted and dried, and then separated by column chromatography to obtain a 4-perfluoroalkyl substituted pyrimidine compound. The preparation method of the present invention has mild reaction conditions, does not need expensive transition metal catalysts, only uses inorganic alkali cesium carbonate as an additive, has the characteristics of simple post-treatment, green steps, low pollution, and high economic benefits, and the prepared compound is a novel multi-substituted pyrimidine molecule. It has good application prospects in the fields of new medicine and new material development.
Owner:NANJING TECH UNIV
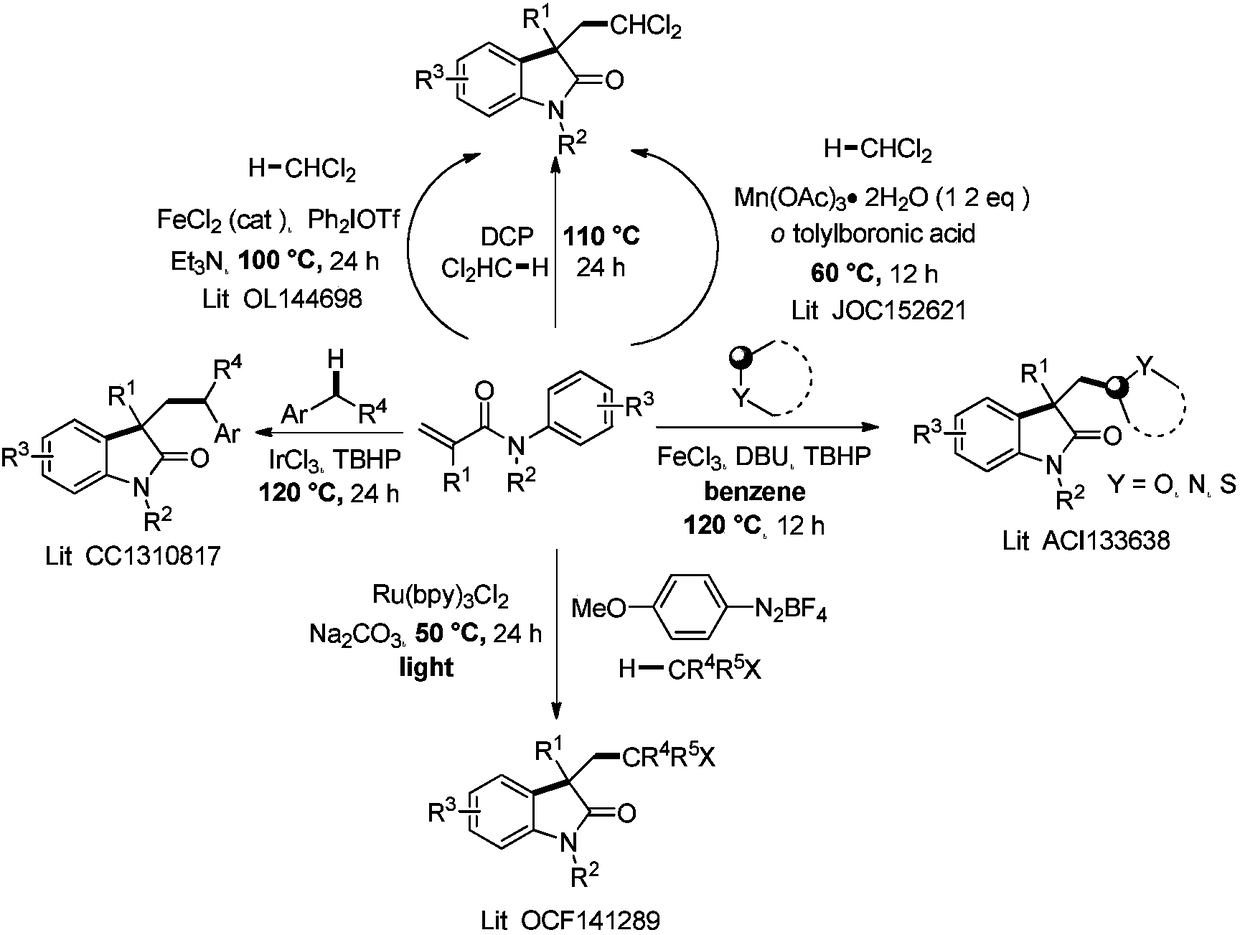
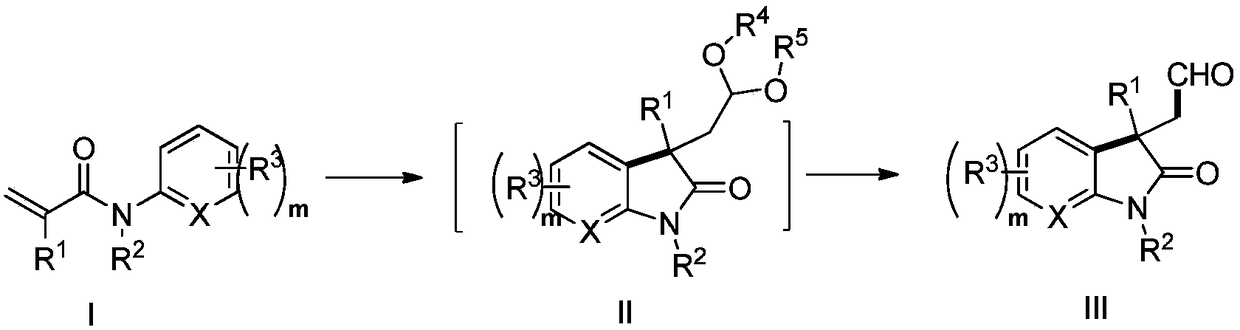
Method for photocatalytic synthesis of alkaloid
ActiveCN108707101ASynthetic brevityInexpensive and easy to operateOrganic chemistrySynthetic alkaloidRoom temperature
The invention provides a method for photocatalytic synthesis of an alkaloid. The alkaloid comprises an indole fused ring compound and an indole spiro compound. The method comprises the following steps: synthesizing a 3-acetal-2-indole ketone compound from azoaryl acrylamide serving as a raw material and a peroxide under photocatalysis; hydrolyzing to obtain a 3-formyl-2-indole ketone compound; further reducing to synthesize the alkaloid. The method is mild in route, and has high efficiency. A reaction can occur under room temperature illumination under mild conditions, and better substrate versatility and functional group tolerance are achieved.
Owner:SHENZHEN UNIV
Application of nitrogen-containing heterocyclic mercaptan cuprous compound in photocatalytic reaction of carbonyl compound
PendingCN112898129ALow priceImprove catalytic performanceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhoto catalyticPhotocatalytic reaction
The invention discloses an application of a nitrogen-containing heterocyclic mercaptan cuprous compound in a photocatalytic reaction of a carbonyl compound, relates to the technical field of application of photocatalysts; in particular, photocatalytic reduction reaction is carried out on the carbonyl compound by adopting the nitrogen-containing heterocyclic mercaptan cuprous compound as a photocatalyst to prepare an alcohol compound. The nitrogen-containing heterocyclic mercaptan cuprous compound is used as the photocatalyst for the photocatalytic reduction reaction of the carbonyl compound, visible light is successfully catalyzed to induce reduction of the carbonyl compound into the alcohol compound, the catalyst is low in price and good in catalytic effect, and the production cost can be reduced.
Owner:BOZHOU UNIV
Photocatalytic method for synthesizing alkaloids
ActiveCN108707101BSynthetic brevityInexpensive and easy to operateOrganic chemistryArylSynthetic alkaloid
The invention provides a method for photocatalytically synthesizing alkaloids, the alkaloids include indole fused rings and indole spiro compounds, the process is as follows: nitrogen aryl acrylamide is used as raw material to react with peroxide under photocatalysis Synthesize 3-acetal 2-indolinone compounds, obtain 3-formyl-2-indolinone compounds through hydrolysis, and further reduce and synthesize alkaloids. This method has a mild route and high efficiency. The reaction can occur under light at room temperature, the conditions are mild, and it has good substrate versatility and functional group tolerance.
Owner:SHENZHEN UNIV
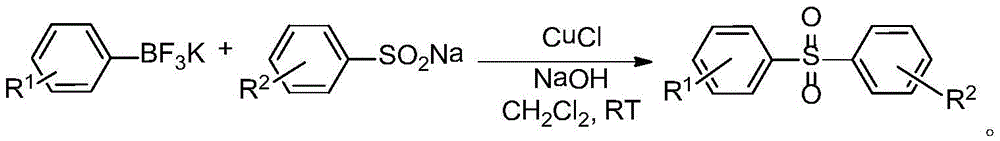
Asymmetric diaryl sulfone compound and its preparation method
InactiveCN103922976BImprove practicalityHigh selectivityOrganic chemistryOrganic compound preparationArylSynthesis methods
The invention relates to an asymmetric diaryl sulfone compound and a preparation method thereof, and discloses diaryl sulfone into which aryl fluoborate catalyzed by cuprous chloride participates at room temperature, and a preparation method thereof. Available nontoxic aryl sulfinic acid sodium is selected as a substrate, and a low-cost reaction system is selected to develop coupling reaction of aryl sulfinic acid sodium and aryl potassium fluoborate catalyzed by cuprous chloride, so as to synthesize asymmetric diaryl sulfone. The diaryl sulfone synthesis method is economic and effective, mild in condition, short in reaction time, high in reaction yield and low in cost, and has good practicability and economic value.
Owner:SHAOXING UNIVERSITY
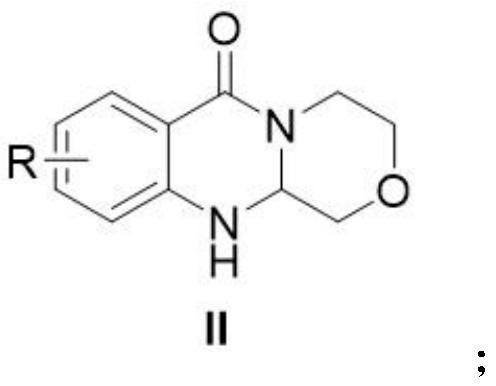
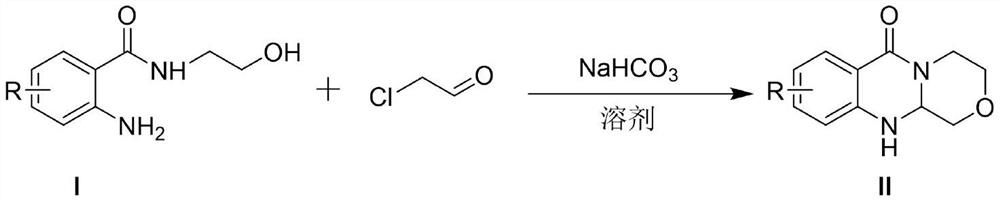
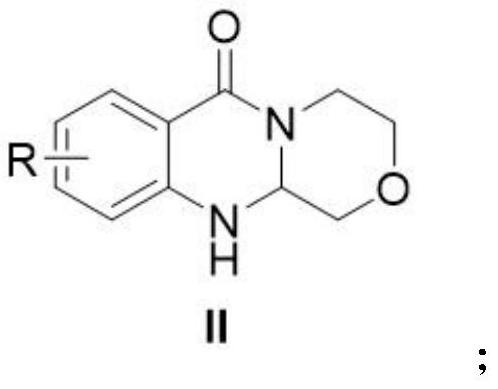
Polycyclic carbamoyl pyridone analogue and preparation method and application thereof
ActiveCN112679519AGood functional group toleranceGood yield and purityOrganic chemistryAmino amidePerylene derivatives
The invention provides a polycyclic carbamoyl pyridone analogue which is used as a polycyclic carbamoyl pyridone analogue and can be used as a medicine raw material or an intermediate for synthesizing a novel anti-influenza virus medicine. The polycyclic carbamoyl pyridone analogue is obtained by cyclization of the o-amino amide derivative and chloroacetaldehyde under the action of alkali without any other catalyst or additive, harsh reaction conditions are not needed, and the method is simple to operate and suitable for industrial production. The invention also discloses application of the polycyclic carbamoyl pyridone analogue in serving as an impurity reference substance of baloxavir dipivoxil analogue drugs.
Owner:CHONGQING MEDICAL UNIVERSITY
2-hydroxypyridine compound and its synthesis method
ActiveCN112592310BWith structural diversityGood functional group toleranceOrganic chemistry methodsOrganic synthesisPharmaceutical drug
The invention discloses 2-hydroxypyridine compounds, their synthesis method and application. Using enaminone as a starting material and tempo salt as an oxidizing agent, 2-hydroxypyridine compounds are generated through oxidation reaction and coupling cyclization reaction under heating conditions. The reaction has strong functional group compatibility, the yield is as high as 80%, and the product has functional group diversity. It can be used as a precursor for organic synthesis, and the 2-position hydroxyl group and 6-position hydrogen atom in the structure can be further functionalized to obtain drug molecular skeletons or compounds with potential biological activity.
Owner:NANJING TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com