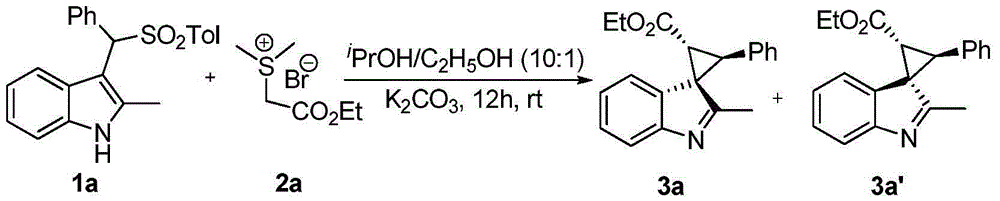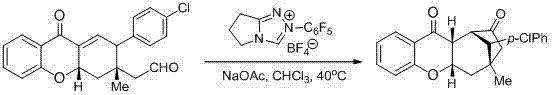Patents
Literature
107results about How to "Good diastereoselectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
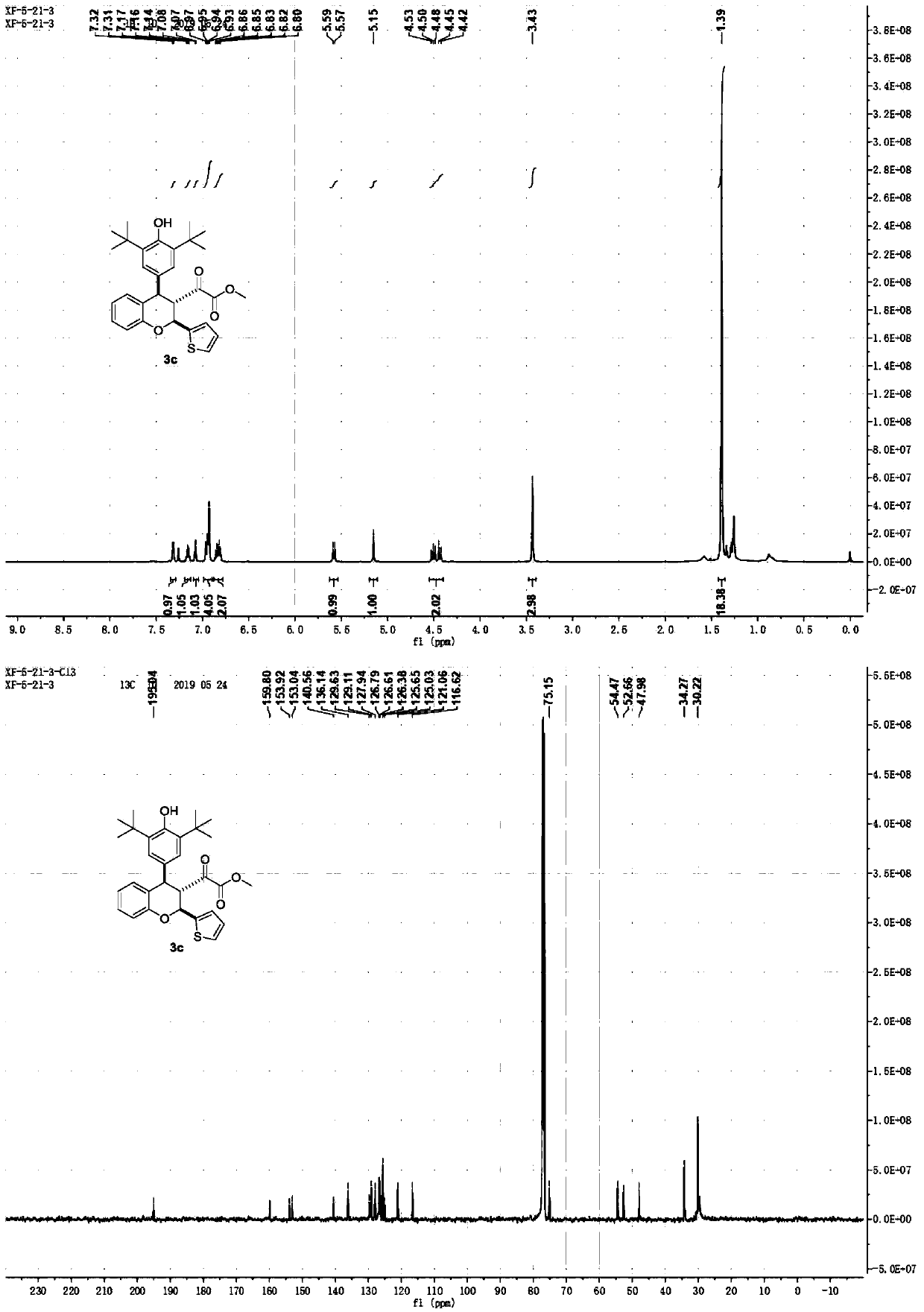
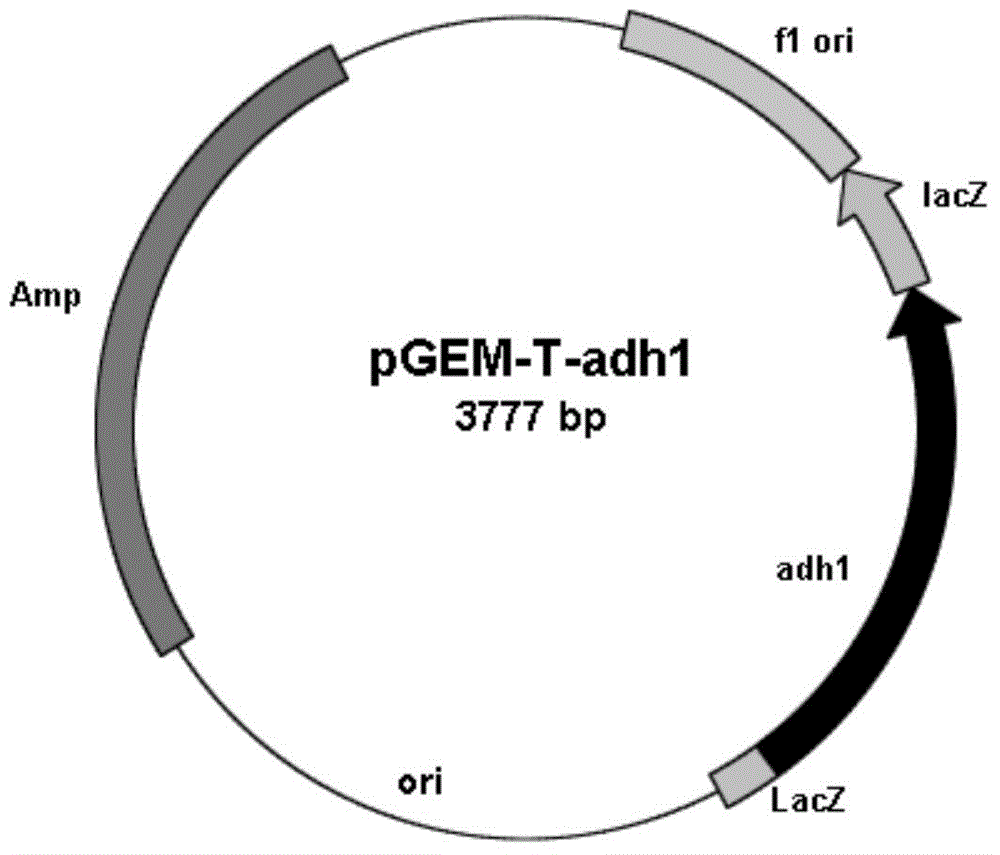
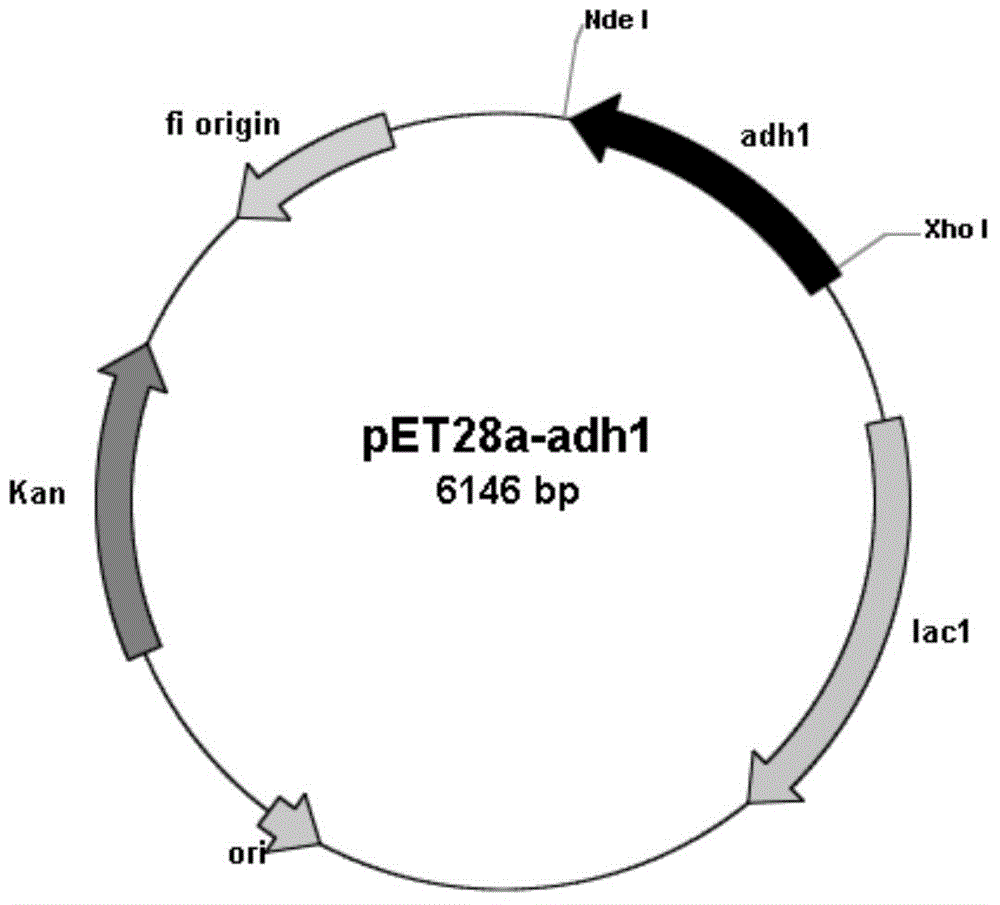
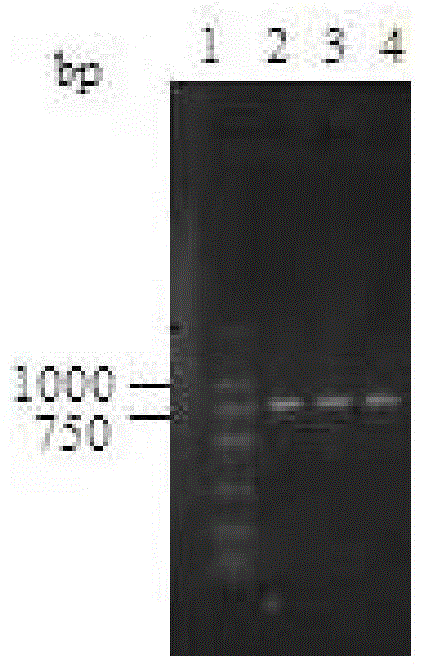
Carbonyl reductase gene, enzyme, vector and engineering bacterium as well as application of carbonyl reductase gene in asymmetrically reducing prochiral carbonyl compounds
ActiveCN104630243AHigh enantioselectivityImprove conversion rateBacteriaOxidoreductasesKetoneCarbonyl group
The invention discloses a recombinant carbonyl reductase sourced from Burkholderia gladioli ZJB-12126 as well as an encoding gene of the recombinant carbonyl reductase, a recombinant vector containing the gene, a recombinant gene engineering bacterium obtained by transforming the recombinant vector, and an application of the recombinant carbonyl reductase in asymmetrically reducing prochiral carbonyl compounds. According to the recombinant carbonyl reductase disclosed by the invention, by respectively taking 2-benzamidomethyl-3-ketone butyrate, 4-chloroacetoacetic acid ethyl ester (COBE), (R)-6-cyano-5-hydroxyl-3-carbonyl caproate tert-butyl ester and (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone as substrates, (2S, 3R)-2-benzamidomethyl-3-polyhydroxybutyrate, (S)-4-chloro-3-ethyl hydroxybutyrate, 6-cyano-(3R, 5R)-dihydroxyhexanoic acid tert-butyl ester and (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxyl valeryl]-4-phenyl-1,3-oxo-azacyclopentane-2-ketone with high optical purity are prepared by virtue of biocatalytic reaction, wherein the biocatalytic reaction can be performed by using recombinant escherichia coli as a biocatalyst, and thus optional new enzyme sources can be provided for biocatalytic synthesis of chiral intermediates of medicines.
Owner:ZHEJIANG UNIV OF TECH
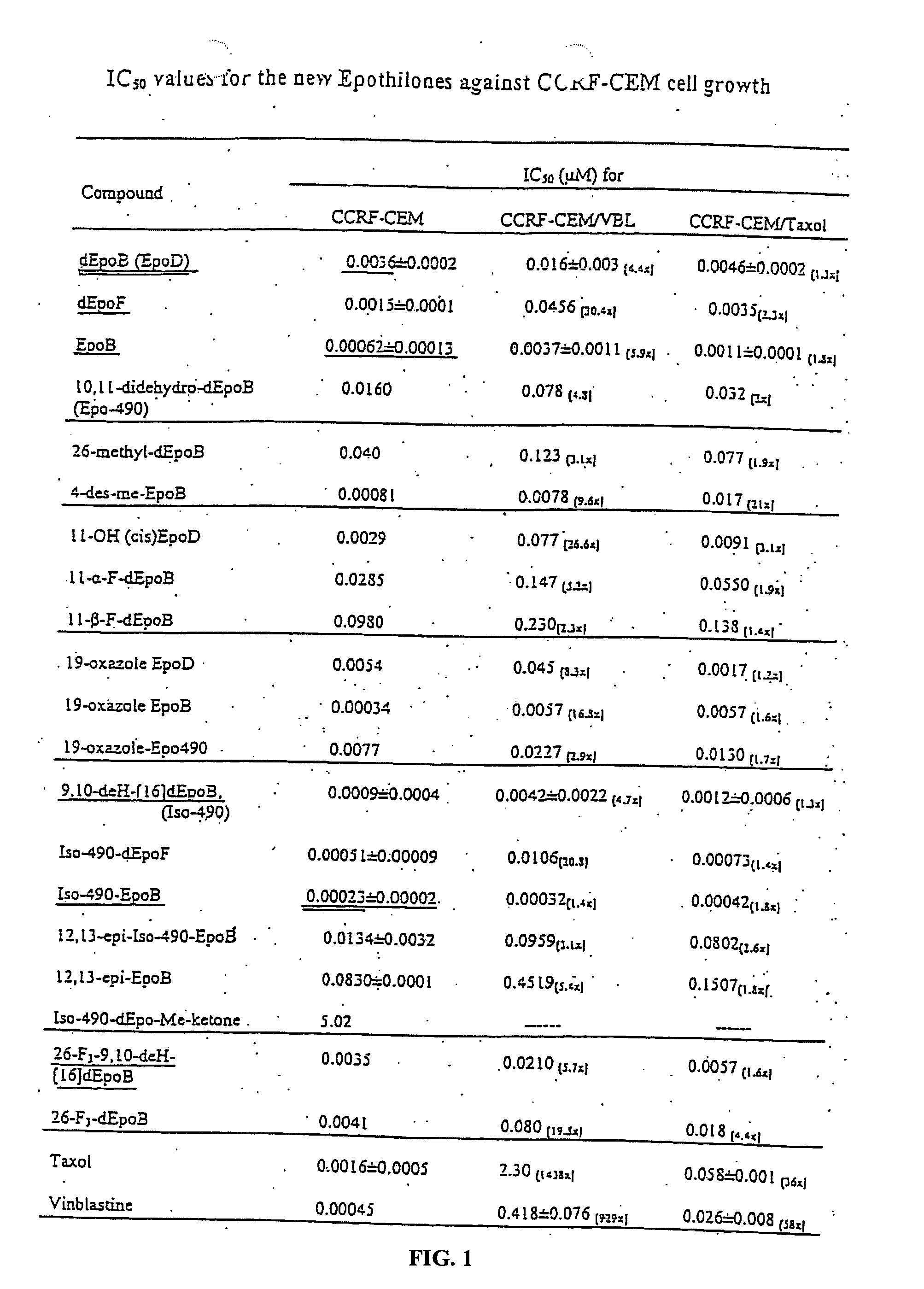
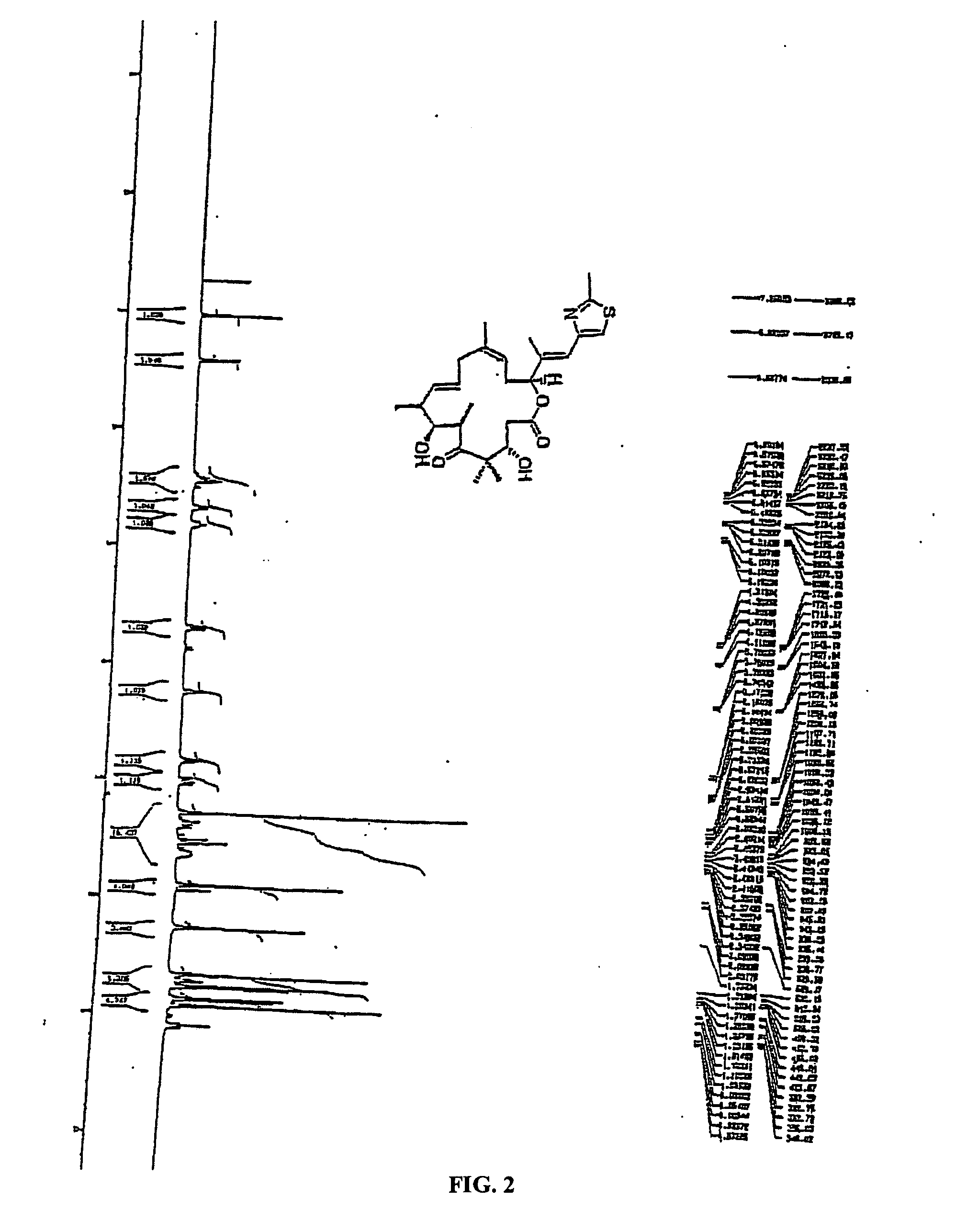
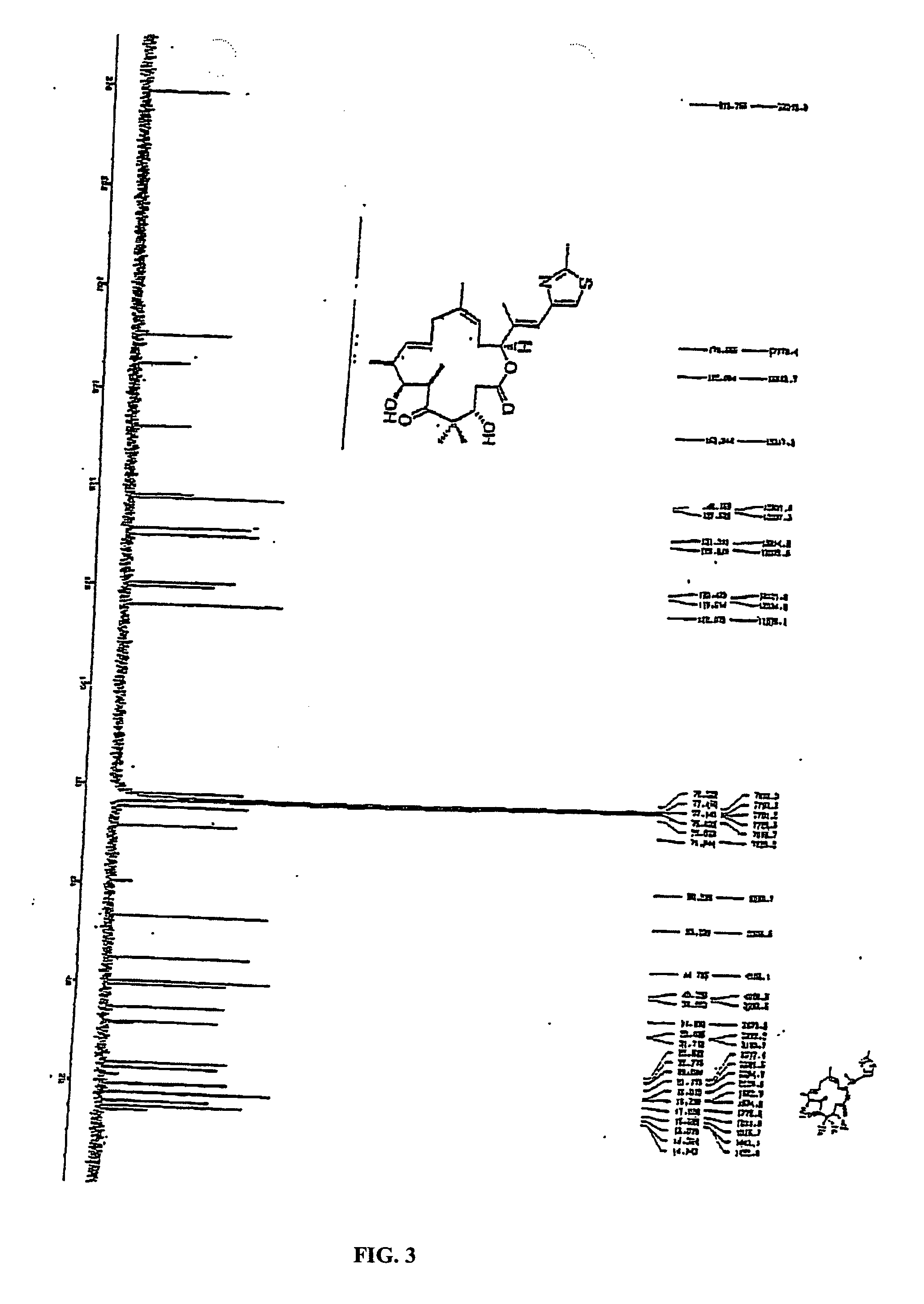
Synthesis of epothilones, intermediates thereto, analogues and uses thereof
InactiveUS20050143429A1Reduce the burden onDoubled survival timeBiocideOrganic chemistryEpothilonePharmaceutical drug
The present invention provides compounds of formula (I): as described generally and in classes and subclasses herein. The present invention additionally provides pharmaceutical compositions comprising compounds of formula (I) and provides methods of treating cancer comprising administering a compound of formula (I).
Owner:SLOAN KETTERING INST FOR CANCER RES
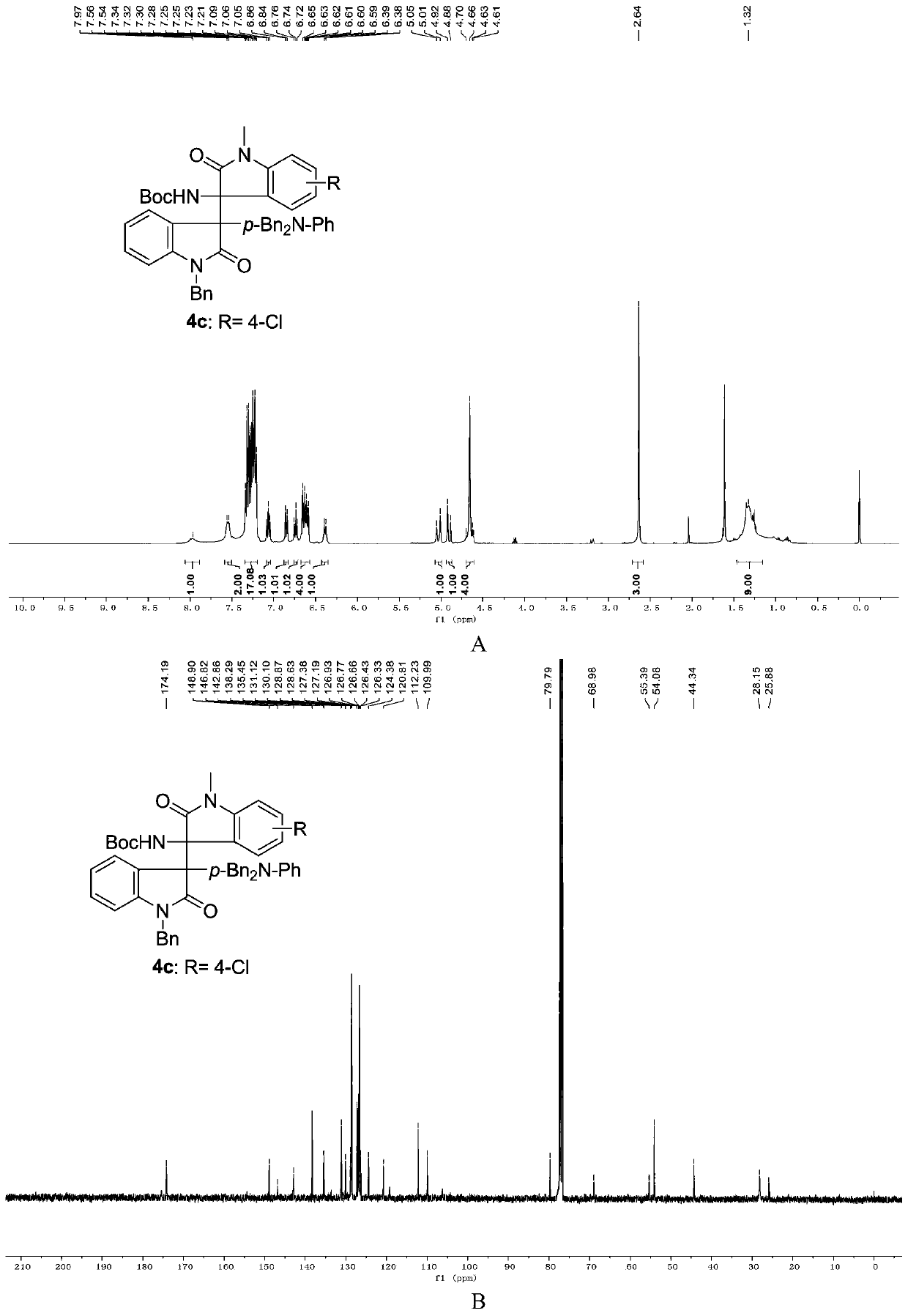
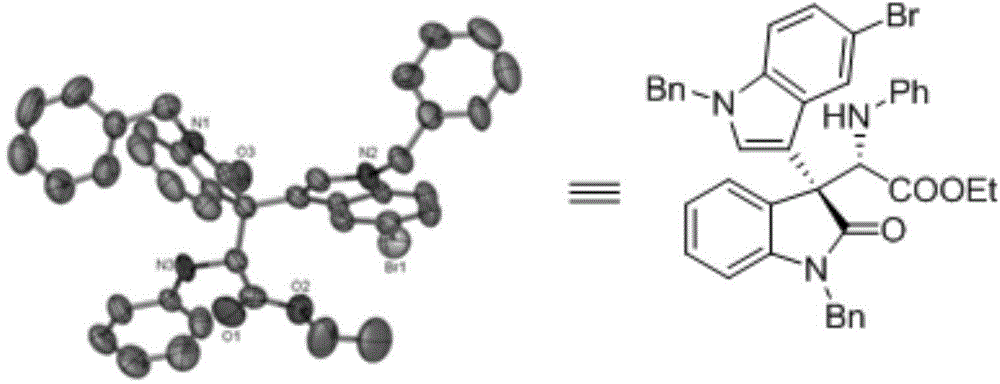
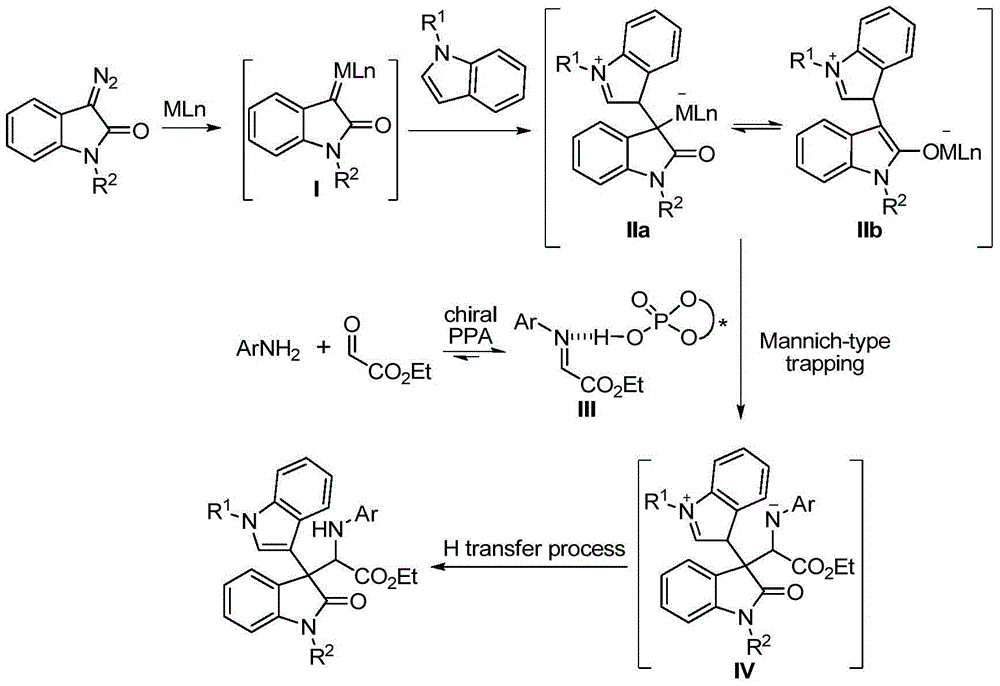
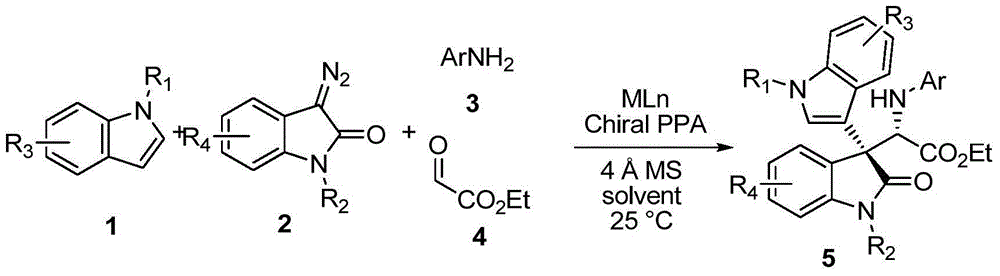
Chiral 3,3-disubstituted oxoindole derivative, and synthetic method and application thereof
ActiveCN104693092AHigh yieldGood diastereoselectivityOrganic chemistryMetabolism disorderChemical synthesisSynthesis methods
The invention discloses a chemical synthetic method of a novel chiral 3,3-disubstituted oxoindole derivative with PTP1B inhibiting action. With diazoisatin, indole, arylamine and aldehyde ester as raw materials, a metal catalyst as catalyst, chiral phosphoric acid as a co-catalyst, an organic solvent as a solvent, and molecular sieve shown in the specification as an additive, a one-step reaction is performed under the condition of 25 degrees centigrade, and the product undergoes column chromatography purification to obtain the target product. The synthetic method has the advantages of high step economy, strong atom economy, high diastereoselectivity and enantioselectivity, high yield, mild reaction condition, and simple and safe operation. The optically pure chiral 3,3-disubstituted oxoindole derivative with two chiral centers synthesized in the invention is an important intermediate in chemical engineering, chemistry and medicines, and has a wide application prospect in the fields of medicines and the chemical engineering.
Owner:广东和博制药有限公司
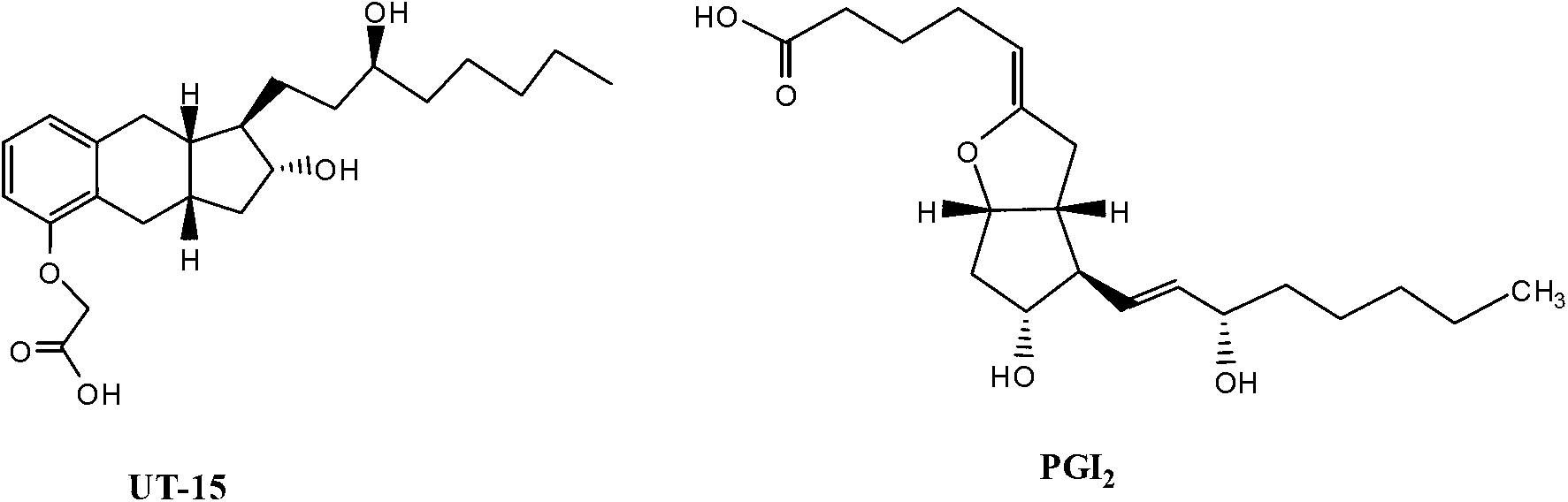
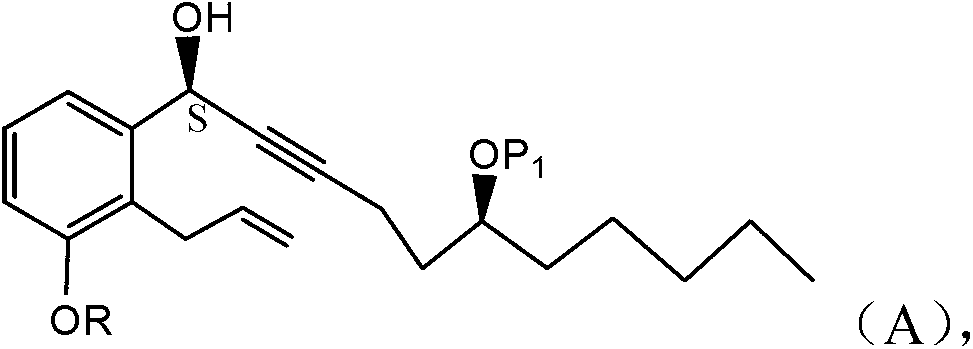
Treprostinil production
ActiveCN103261142AGood diastereoselectivityReduce the numberGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationTreprostinilAlcohol
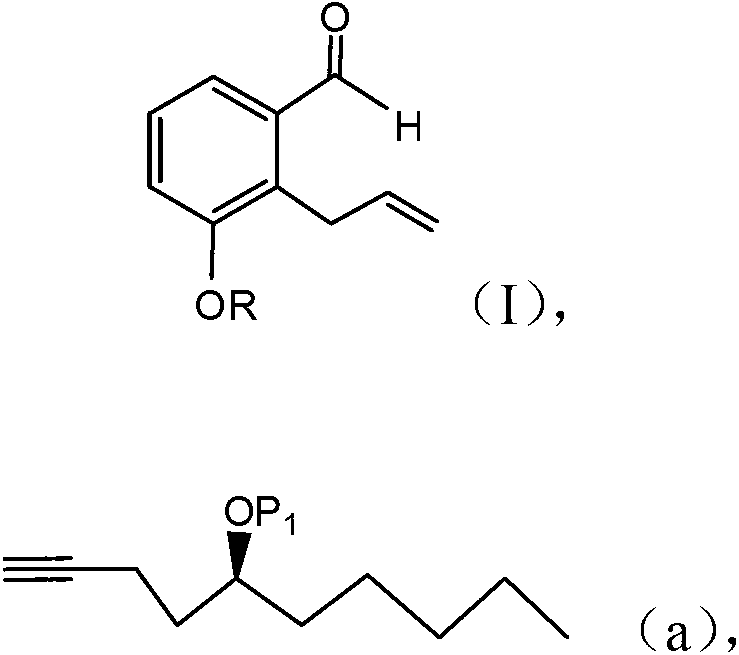
A method is disclosed for preparing a synthetic intermediate for treprostinil via a stereoselective alkyne addition reaction using a chiral inducing agent. Also described are methods of preparing treprostinil or a pharmaceutically acceptable salt thereof comprising the alkyne addition reaction as well as novel intermediates useful for synthesis prostacyclin derivatives. A functional alcohol protecting group protects the alcohol group from participating in reactions that are occurring in other parts of the molecule. The intermediate is later deprotected prior to conversion and hydrolyzing to obtain the final treprostinil product.
Owner:UNITED THERAPEUTICS CORP
Fluorescent labeled organic boron/nitrogen Lewis acid and base bifunctional catalyst and preparation method thereof
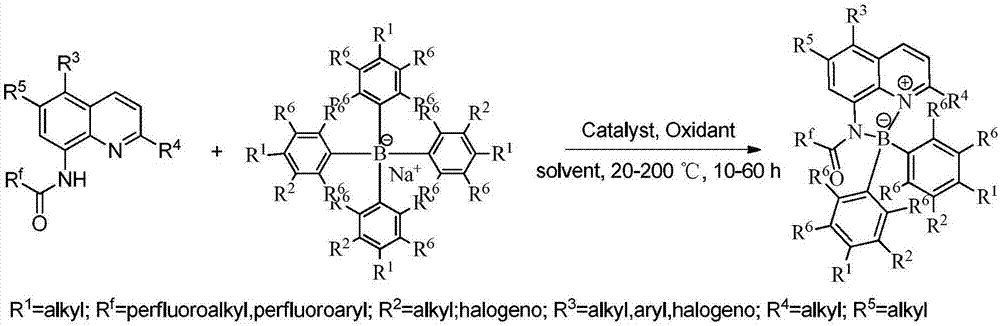
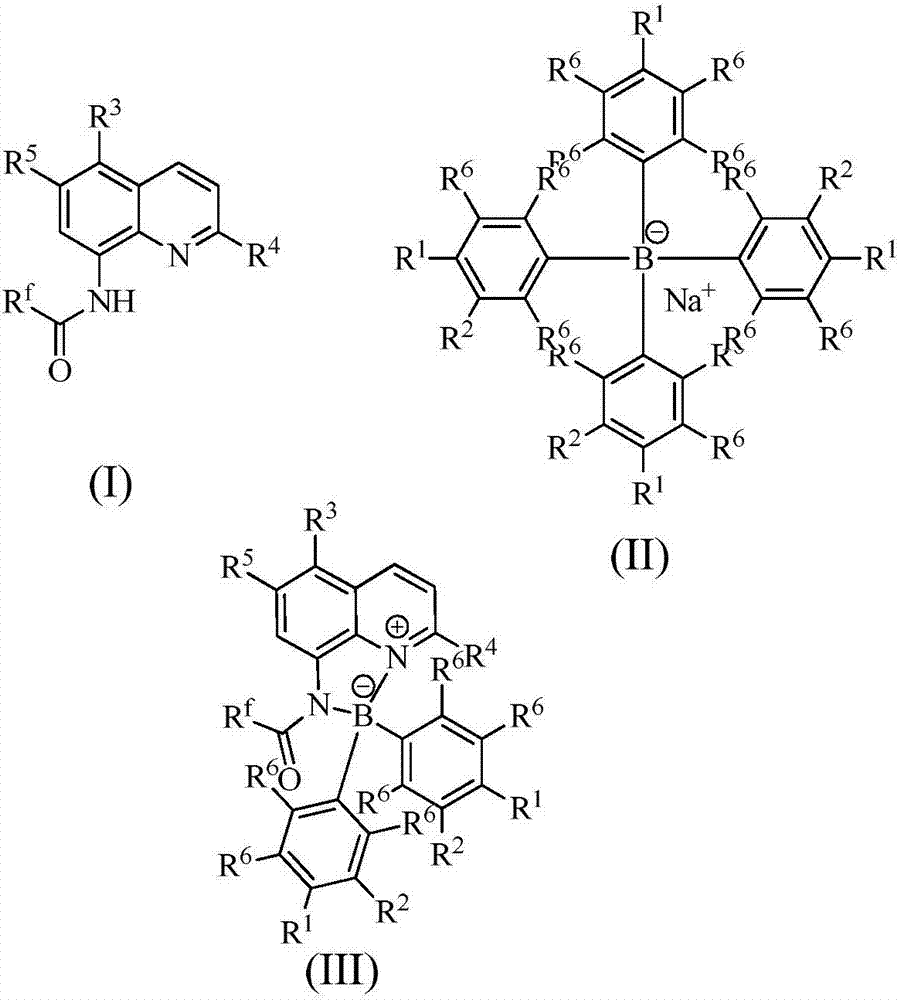
ActiveCN106861757AHigh selectivityGood diastereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFluorescenceMannich reaction
The invention relates to a fluorescent labeled organic boron / nitrogen Lewis acid and base bifunctional catalyst and a preparation method thereof. The catalyst is characterized by being prepared by the following step: by taking a quinoline amide compound and a cheap sodium tetraphenylborate derivative as raw materials, cheap and easily available single element iodine as a catalyst and a common organic solvent as a reaction solvent, performing a reaction for a while at a certain temperature to obtain the bifunctional organic boron / nitrogen Lewis acid and base catalyst with a high yield and high selectivity. The catalyst is good in stability and recyclable, and has very good diastereoselectivity in a Mannich reaction. The method has the advantage s of being relatively low in cost, high in yield, simple and convenient to operate, pollution-free and the like, and has certain feasibility for realizing industrial production.
Owner:HUNAN UNIV
Novel method for synthesizing chiral 4-nitryl-3, 5-diaryl cyclohexanone
InactiveCN102153477ASimple ingredientsRaw materials are easy to getOrganic chemistryOrganic compound preparationSolventPotassium hydroxide
The invention belongs to the field of asymmetric synthesis, and particularly relates to a novel method for catalyzing and synthesizing a chiral 4-nitryl-3, 5-diaryl cyclohexanone compound by chiral thiocarbamide as a catalyst. The method comprises the following steps of: (1) reacting by taking diketene and nitromethane as a reaction substrate, quinine-derived thiocarbamide as a chiral catalyst and methylbenzene as solvent at the temperature of between 20 and 25 DEG C for 24 to 72 hours to generate a Michael addition product; and (2) reacting by taking the Michael addition product obtained in the step (1) as a reactant, potassium hydroxide as a catalyst and ethanol as solvent at the temperature of between 0 and 10 DEG C for 0.5 to 2 hours to obtain a chiral 4-nitryl-3, 5-diaryl cyclohexanone derivative. The novel method for synthesizing the chiral 4-nitryl-3, 5-diaryl cyclohexanone compound has the advantages of readily available materials and mild reaction conditions and is easy and convenient to operate, and a reaction system is insensitive to air, aqueous vapor and the like and aftertreatment is convenient, so the chiral 4-nitryl-3, 5-diaryl cyclohexanone compound is suitable for industrial production. Simultaneously, the synthetic method has the characteristics of high yield and excellent diastereoselectivity and enantioselectivity.
Owner:SUZHOU UNIV
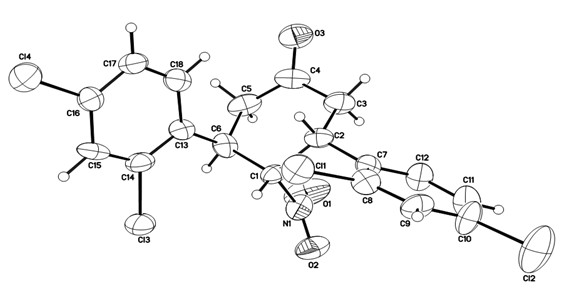
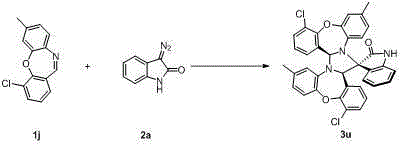
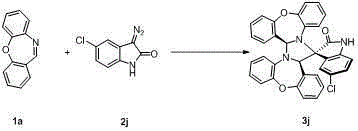
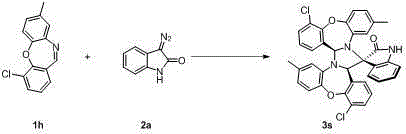
Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof
InactiveCN102432526ASimple ingredientsRaw materials are easy to getOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesUnsaturated ketoneBis indole
The invention belongs to the field of asymmetric catalysis, and specifically relates to a method for synthesizing a novel chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile compound. According to the invention, chiral primary amine derived from quinine is adopted as a catalyst, and chiral or achiral Brownst acid is adopted as a co-catalyst, such that the compound is synthesized under catalyzing. According to the invention, the chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile compound is synthesized through a dual-Michael addition reaction of an isatin malononitrile condensation substance and methyl aryl alpha,beta-unsaturated ketone. The synthesizing method is advantaged in simple raw materials which are easy to acquire, convenient operation, and good substrate compatibility. A plurality of chiral centers can be established with a one-kettle method. Under a high-temperature reaction condition, high yield and high conversion frequency are realized, and excellent enantioselectivity and excellent diastereoselectivity can be realized.
Owner:SUZHOU UNIV
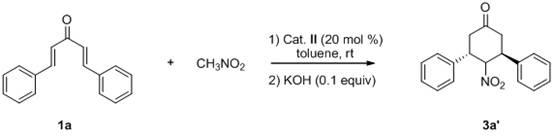
Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone
InactiveUS20110046389A1Good diastereoselectivityHigh chemoselectivityOrganic chemistryBulk chemical productionKetoneSolvent
A method for the preparation of (S)-alcohol oxazolidides of general formula II, in which PG represents hydrogen or a hydroxyl protecting group, such as trimethylsilyl, tert-butyldimethylsilyl, benzyloxycarbonyl, tert-butoxycarbonyl, benzyl, benzhydryl or trityl, in which a ketal oxazolidide of general formula III, where PG has the same meaning as above and R means an alkyl with 1-4 carbon atoms, linear or branched, such as methyl, ethyl, isopropyl or butyl, or R+R together represents a divalent alkyl, or substituted with 1 or 2 alkyl groups, e.g. 1,2-ethylene, 1,2-propylene, 1,2-butylene, 1,3-propylene or 2,2-dimethyl-1,3-propylene, is deprotected by the action of acidic reagents in a mixture of water and a water-miscible solvent in the temperature range of 0 to 100° C. (stage A), and the obtained ketone oxazolidide of general IV, in which PG has the same meaning as above, is reduced with asymmetrical reagents in an inert organic solvent in the temperature range of −30 to +40° C. (stage B).
Owner:ZENTIVA AS
Organoaluminum compound
InactiveUS8329931B2Good diastereoselectivityHigh yieldPreparation by isomerisationOrganic compound preparationArylCompound a
A catalyst for obtaining isopulegol of high diastereoselectivity by highly selective cyclization reaction of citronellal is provided. The present invention relates to an organoaluminum compound obtained by reacting at least one organoaluminumoxy compound selected from the group consisting of chain aluminoxanes, cyclic aluminoxanes and bis(dialkylaluminumoxy)alkylboranes, with at least one hydroxy compound selected from the group consisting of diarylphenols, bis(diarylphenol) compounds, biaryldiols, dimethanols and silanols.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Organoaluminum compound
InactiveUS20110295031A1Good diastereoselectivityHigh yieldPreparation by isomerisationOrganic chemistry methodsArylCompound a
A catalyst for obtaining isopulegol of high diastereoselectivity by highly selective cyclization reaction of citronellal is provided. The present invention relates to an organoaluminum compound obtained by reacting at least one organoaluminumoxy compound selected from the group consisting of chain aluminoxanes, cyclic aluminoxanes and bis(dialkylaluminumoxy)alkylboranes, with at least one hydroxy compound selected from the group consisting of diarylphenols, bis(diarylphenol) compounds, biaryldiols, dimethanols and silanols.
Owner:TAKASAGO INTERNATIONAL CORPORATION
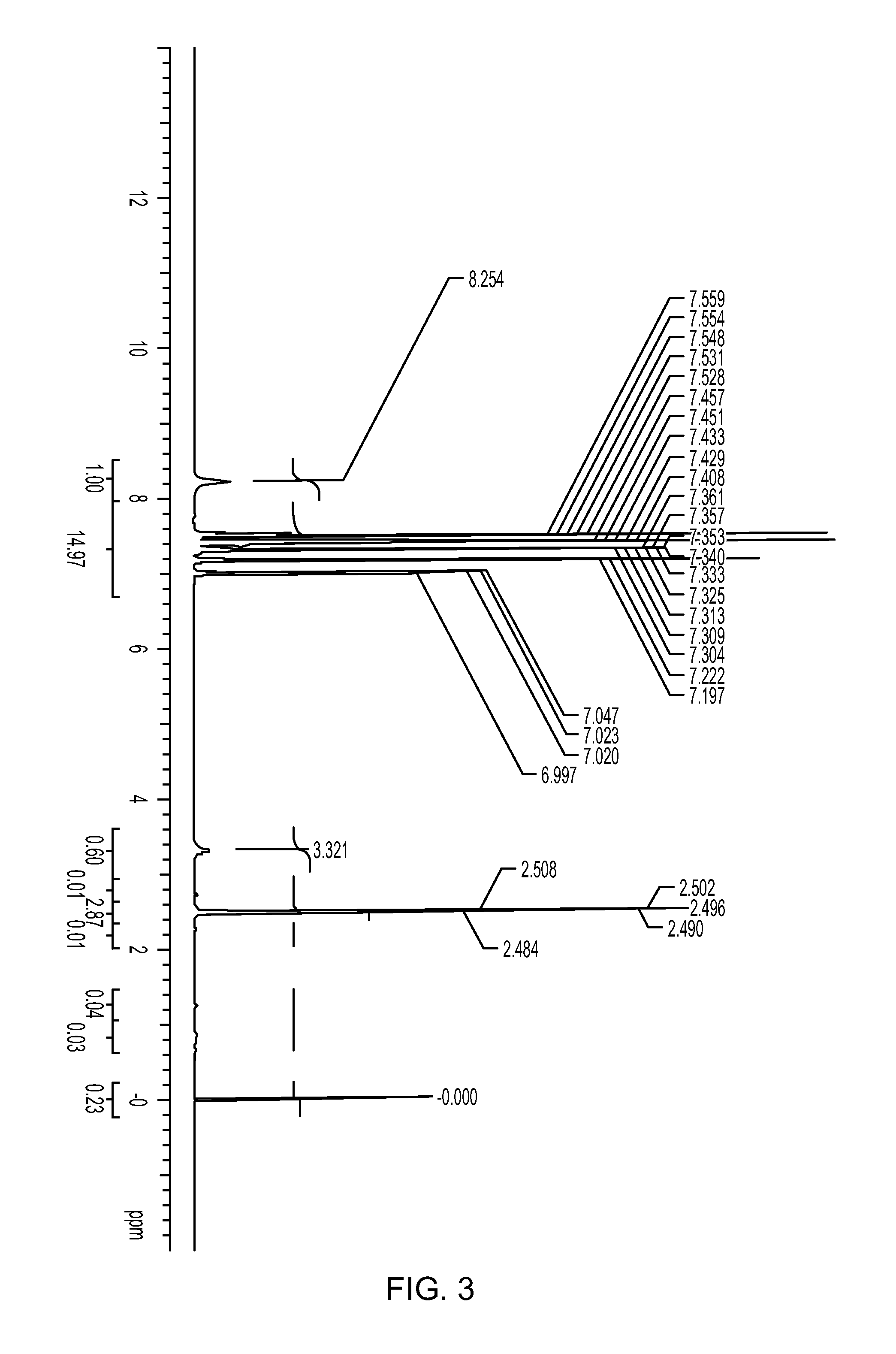
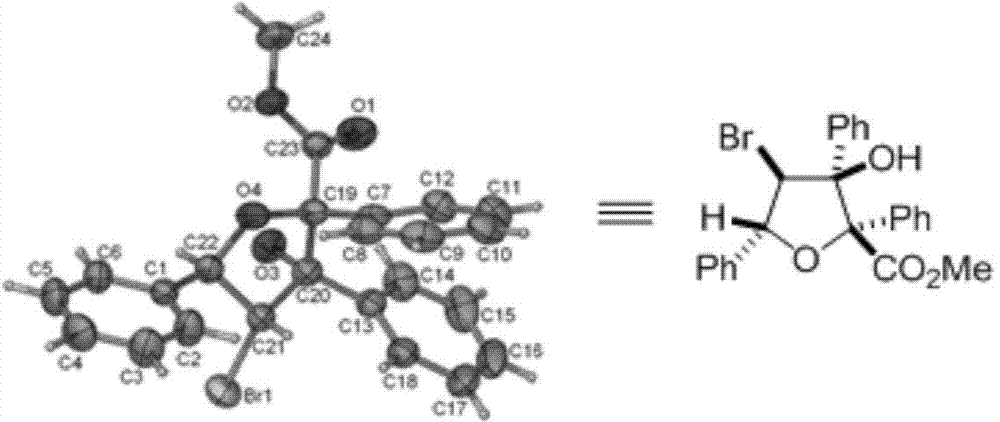
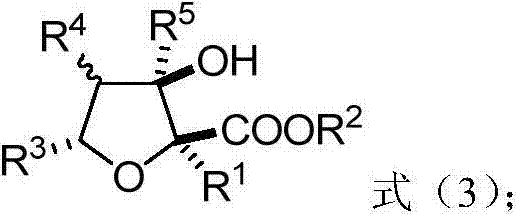
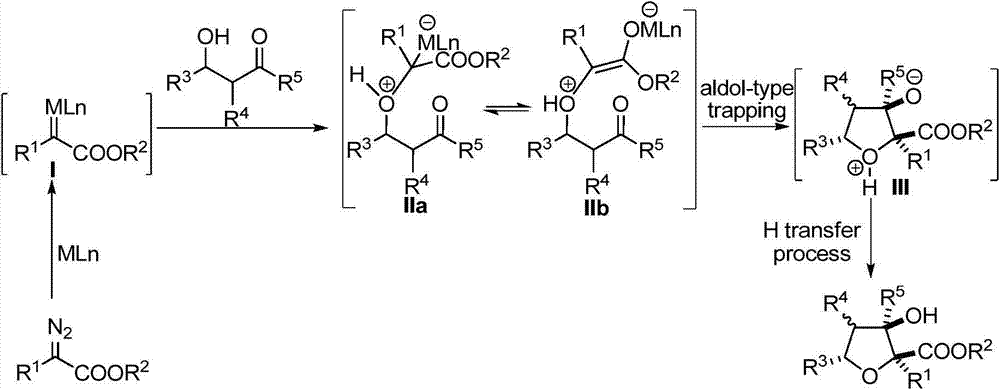
Polysubstituted tetrahydrofuran derivatives as well as synthesis method and application thereof
ActiveCN104744410AHigh yieldGood diastereoselectivityOrganic chemistryMetabolism disorderMolecular sieveCarbonyl group
The invention relates to polysubstituted tetrahydrofuran derivatives and a chemical synthesis method thereof. The chemical synthesis method comprises the following steps: carrying out one-step reaction on raw materials (a diazo compound and a 3-hydroxyl-carbonyl flavone compound) in an organic solvent in the presence of a metal catalyst and an additive which is a molecular sieve shown in the specification; and carrying out column chromatography purification to obtain the target product. The chemical synthesis method of the polysubstituted tetrahydrofuran derivatives has the advantages of environmental benefit, step economy, atom economy, diastereoselectivity and high yield; in addition, the reaction conditions are mild, the applicability of the substrate range is wide and the operation is simple and safe. The invention discloses that the polysubstituted tetrahydrofuran derivatives with multiple chiral centers have PTPlB inhibiting effect; and therefore, the polysubstituted tetrahydrofuran derivatives are important chemical engineering, chemical and medical intermediates and have a wide prospect in the field of pharmaceutical chemicals.
Owner:EAST CHINA NORMAL UNIVERSITY
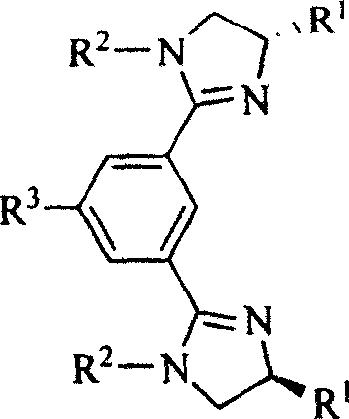
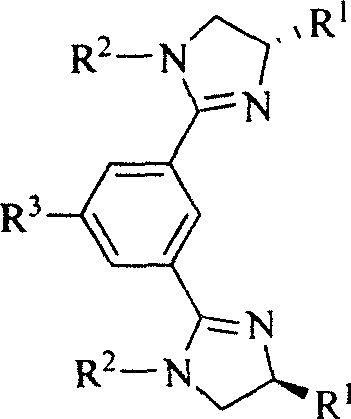
Chiral and non-chiral diimidazolinylbenzene compounds and synthesis method
InactiveCN101012198AEasy to reuseGood diastereoselectivityOrganic chemistryChromatographic separationBenzene
The invention discloses a chirality and non-chirality diimidazoline benzene compound and synthesizing method with general formula as right formula (R1 is H, i-Pr or Bn; R2 is p-phenylmethyl, p-tolyloxy, cyclohexyl or isopropyl; R3 is H, OH, NO2, NH2 or CH3COO), which comprises the following steps: refluxing bishydralkamine in the thionyl chloride; evaporating excessive thionyl chloride; dissolving in the ether; filtering; adding trimethylamine and different substituted ammonia; stirring under indoor temperature; adding sodium hydroxide solution; extracting; drying; condensing; proceeding film chromatographic separation; obtaining the product.
Owner:ZHENGZHOU UNIV
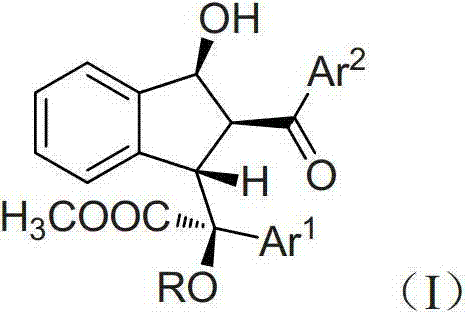
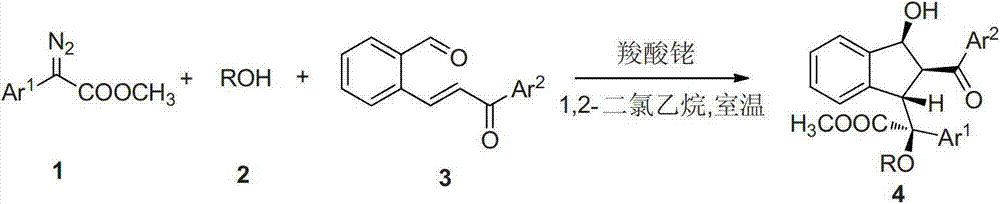
Preparation method of multi-substituted indanol derivatives
InactiveCN102887808ALow costThe synthetic route is simpleOrganic compound preparationCarboxylic acid esters preparationSolventMolecular sieve
The invention discloses a preparation method of multi-substituted indanol derivatives. The diazo compound, alcohol and o-formyl chalcone are used as raw materials, a metal catalyst is used as a catalyst, an organic solvent is used as a solvent, and a molecular sieve is used as a water absorbent; and the multi-substituted indanol derivatives are obtained by one-step reaction. According to the preparation method disclosed by the invention, the raw materials are easily available, the operation is simple and safe, and the method is suitable for industrial production.
Owner:EAST CHINA NORMAL UNIVERSITY
Trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative and synthetic method
ActiveCN105037298AImprove adaptabilityWide adaptabilityOrganic chemistryChemical industryVacuum evaporation
The invention discloses a trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative and a synthetic method, belonging to the technical field of synthesis of medicine chemical industry. The synthetic method comprises the following steps: adding N-tosyl hydrazone, trifluoromethyl ketone, alkali, a phase transfer catalyst and a solvent into a reactor, carrying out reaction under stirring at 70 to 90 DEG C for 12 to 24 hours, after the reaction is completed, carrying out cooling to room temperature, filtering obtained reaction liquid, carrying out vacuum evaporation and removing the solvent so as to obtain a crude trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative, and carrying out purifying through column chromatography so as to obtain the trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative. The method provided by the invention avoids using a transition metal catalyst and uses non-toxic, cheap and easily-available raw materials; the reaction has good adaptability to functional groups and wide adaptability to a substrate, has high product yield and good non-reflect selectivity, can be enlarged to gram-grade scale production and synthesis, and is favorable for industrial production; meanwhile, the obtained trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative has extensive application in the fields of pesticides, medicines and materials.
Owner:SOUTH CHINA UNIV OF TECH
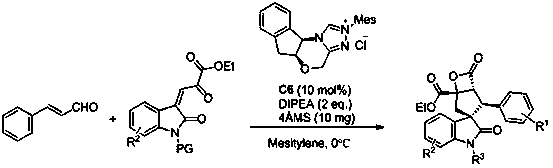
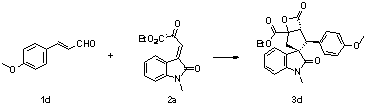
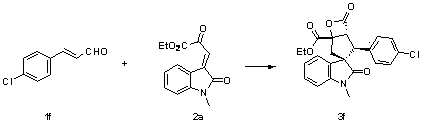
Synthesis method of spiro hydroxyindolocyclopentane beta-exo fat compound
ActiveCN107056795ASimple and fast operationHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsNitrogen
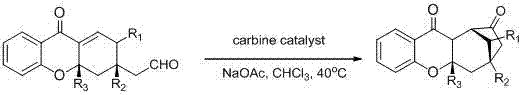
The invention discloses a synthesis method of a spiro hydroxyindolocyclopentane beta-exo fat compound. The synthesis method comprises the following step: by taking phenyl unsaturated aldehyde and unsaturated ketoacid ester as reactants and by taking n-heterocyclic carbene as a catalyst, performing reaction, thus obtaining the spiro hydroxyindolocyclopentane beta-exo fat compound. According to the method disclosed by the invention, the raw materials are simple and readily available; the reaction conditions are mild; post-treatment is simple and convenient; the synthesis method is wide in application substrate range, high in yield and high in diastereoselectivity and enantioselectivity; therefore, a synthesized product can be used for synthesizing an intermediate of a medicine and a pesticide.
Owner:SUZHOU UNIV
Method for synthetizing 2,3-disubstituted dihydrobenzofuran
The invention discloses a method for synthetizing 2,3-disubstituted dihydrobenzofuran. Kinds of substituted 2,3-disubstituted dihydrobenzofurans can be obtained from simple ylide and substituted phenol by reactions under an alkali catalytic condition. The method disclosed by the invention is simple and practical in operation, available in raw materials, high in yield, and good in diastereoselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of polysubstituted pyrroloquinoline derivate
InactiveCN106749235AReduce consumptionRaw materials are easy to getOrganic chemistryChalconeEnantio selectivity
The invention discloses a preparation method of a polysubstituted pyrroloquinoline derivate. The preparation method is characterized in that aminochalcone and aldehyde are condensed to obtain chalcone imine; the chalcone imine and glycinate imine are subjected to series cyclizing through Michael / Mannich and then are subjected to acidizing, and thus the polysubstituted pyrroloquinoline derivate containing four chiral centers, as shown in formula (I), can be generated based on high diastereoselectivity. The preparation method is on the basis of series reaction, and has the advantages that the environmental protection is achieved; the synthesizing steps are simple and convenient; the atom economy is ensured; the diastereoselectivity is high; in addition, the reaction conditions are mild; the substance is wide in applicable scope; the operation is simple and safe; the preparation method is applicable to industrial production.
Owner:SOUTHWEST UNIVERSITY
Spiro oxindole imidazolinyl oxazepine compound and synthesis method thereof
The present invention discloses a spiro oxindole imidazolinyl oxazepine compound and a synthesis method thereof, wherein particularly diazo oxindole and dibenzoxazepine are adopted as reactants, and synthesis is performed in a solvent under catalysis of iron trifluomethanesulfonate (II) or a p-toluenesulfonic acid monohydrate to obtain the product. According to the present invention, the method has characteristics of simple and easily-available raw materials, mild reaction conditions, simple and convenient post-treatment, wide substrate application range, high yield and high diastereoselectivity; and the synthesized product can be used for synthesizing the intermediate of drugs and insecticides.
Owner:SUZHOU UNIV
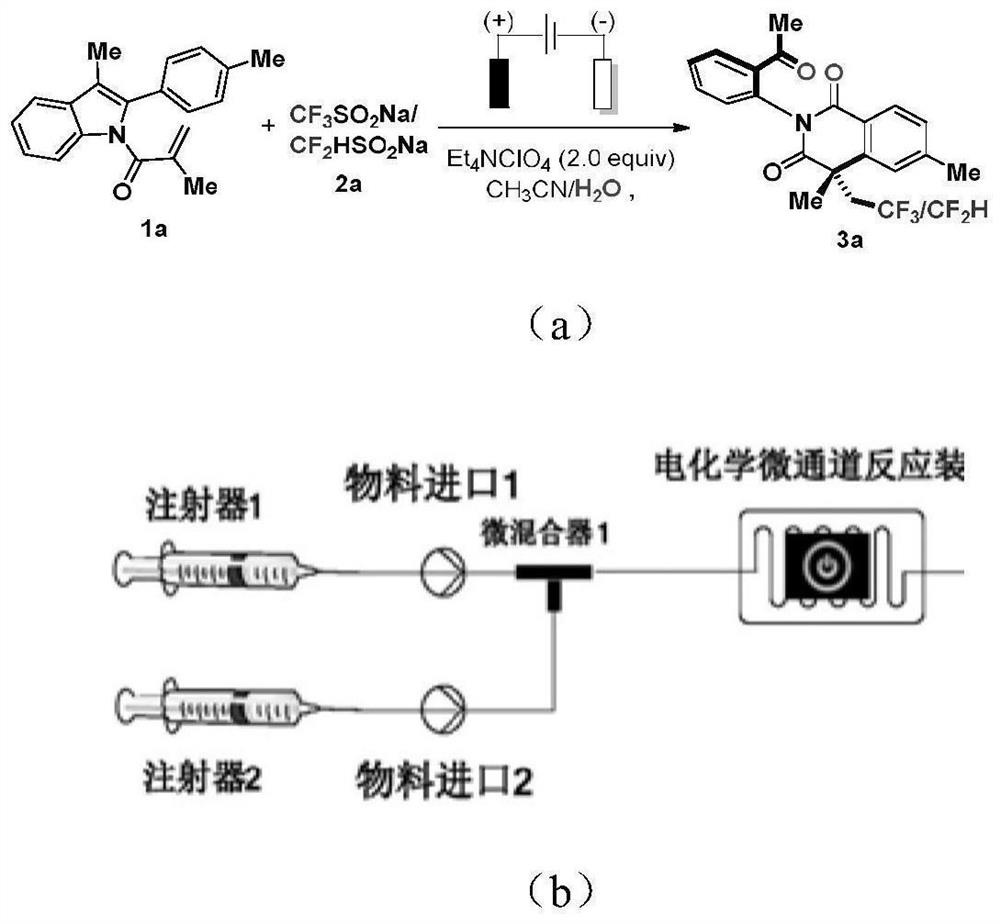
Method for synthesizing fluorine-containing quinolinone compound by electro-catalyzing indole by using electrochemical micro-channel reaction device
ActiveCN112410809AControl reaction rateShort reaction timeCellsElectrolytic organic productionSide productElectrochemistry
The invention discloses a method for synthesizing a fluorine-containing quinolinone compound by electro-catalyzing indole by using an electrochemical micro-channel reaction device, which is characterized by comprising the following steps of respectively and simultaneously pumping a first solution containing an N-substituted 2-aryl indole compound shown as a formula I with a second solution containing a fluorine source and an electrolyte, reacting to obtain a reaction solution containing the fluorine-containing quinolinone compound as shown in the formula II. The micro-channel reaction device is used for preparing the fluorine-containing quinolinone compound, the reaction rate can be effectively controlled, the reaction time is shortened, continuous production is achieved, high diastereoselectivity is achieved, generation of by-products is reduced, the maximum yield can reach 98.7%, the refining process is simpler, and the product quality is improved; basically no amplification effect exists, and industrial amplification is facilitated.
Owner:NANJING UNIV OF TECH
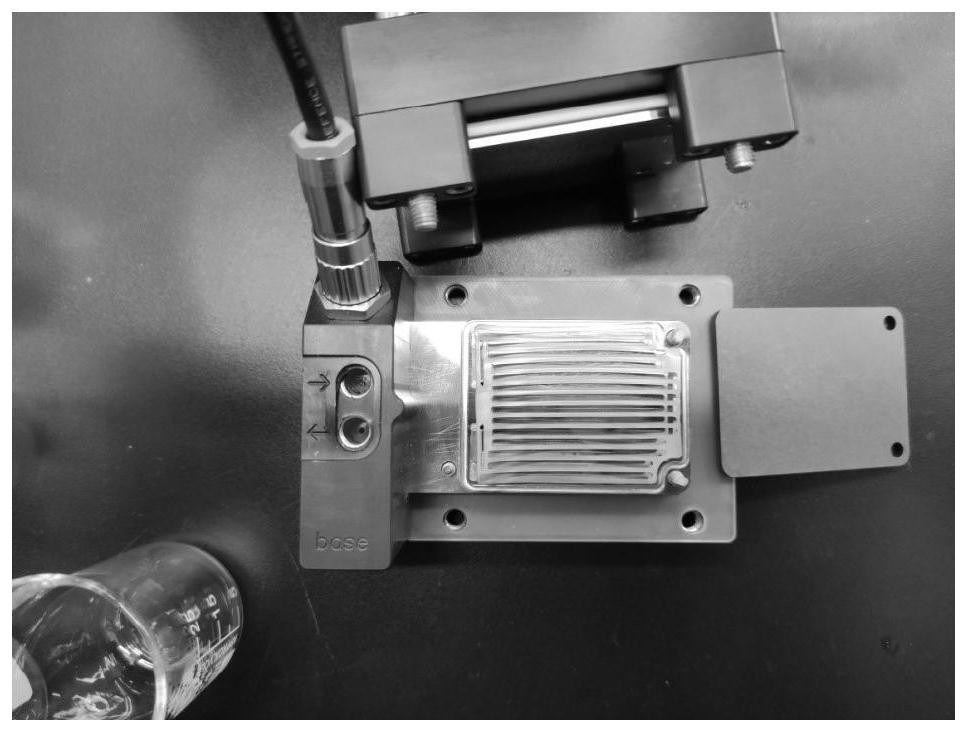
Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system
ActiveCN113072548AMild reaction conditionsSimple and fast operationOrganic chemistryFixed bedQuinoline
The invention provides a method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on a micro-reaction system. A first solution and a second solution are introduced into a first micro-mixer through a feeding pump to be mixed; a mixture of an arylethylamine solution and an aryl aldehyde solution is pumped into a first fixed bed reactor through the first micro-mixer to be subjected to a dehydration condensation reaction; the mixture subjected to the dehydration condensation reaction is introduced into a second fixed bed reactor through a second micro-mixer for catalytic hydrogenation; the mixed material subjected to catalytic hydrogenation and a methanol solution of saturated hydrochloric acid are introduced into a micro-channel reactor through a third micro-mixer for a salt forming reaction, and vacuum concentration, pulping and purification are carried out to obtain secondary amine hydrochloride; and in the presence of acid, a dehydrating agent and an additive, the secondary amine hydrochloride and a glyoxal solution are subjected to Pictet-Spengler reaction and Friedel-Crafts hydroxyalkylation and dehydration cascade reaction, so as to obtain the 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline compound.
Owner:SICHUAN UNIV +1
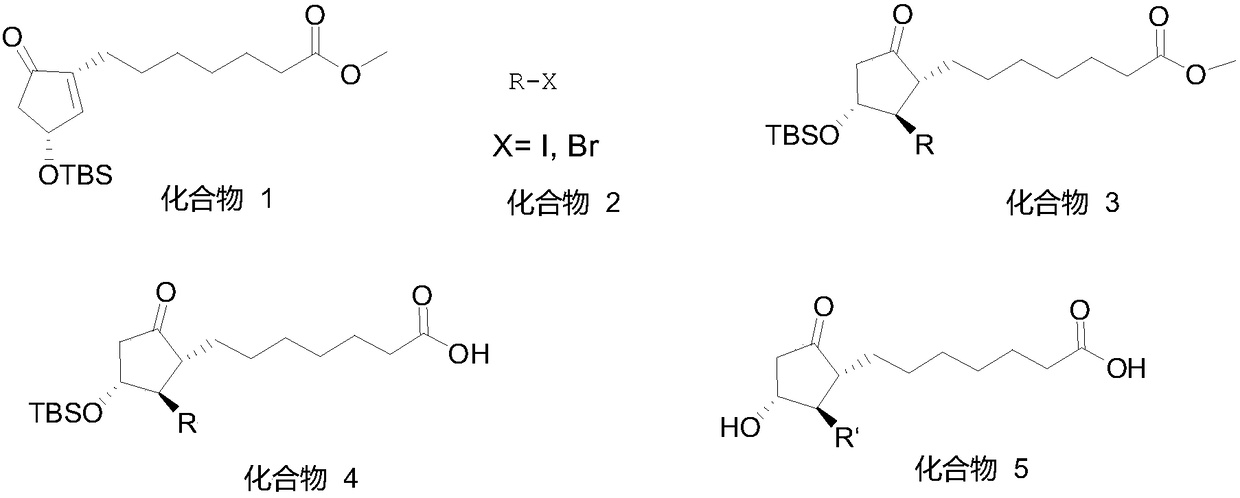
Method for synthesizing 1R,2R,3R-substituted cyclopentanone compound
InactiveCN108503619AOvercome the disadvantage of low purityGood diastereoselectivityOrganic compound preparationCarboxylic compound preparationOrganic solventSynthesis methods
The invention relates to a method for synthesizing a 1R,2R,3R-substituted cyclopentanone compound. The method comprises: carrying out a reaction on a compound 1 and a halogenated alkane in an organicsolvent under the actions of a strong alkali and a cuprous salt to obtain a compound 3, carrying out ester hydrolysis on the compound 3 under the action of an alkali or pancreatin, and carrying out deprotection by using a fluorine reagent to obtain the product. According to the present invention, the synthesis method has characteristics of high yield, high product purity, good diastereoselectivity, easy reaction control and simple post-treatment, is suitable for industrial production, and can synthesize high-purity and high-yield alprostadil, lubiprostone, and the analogs of misoprostol and limaprost. The compound 1 is defined in the specification.
Owner:GUANGZHOU KAIMO BIOTECH CO LTD
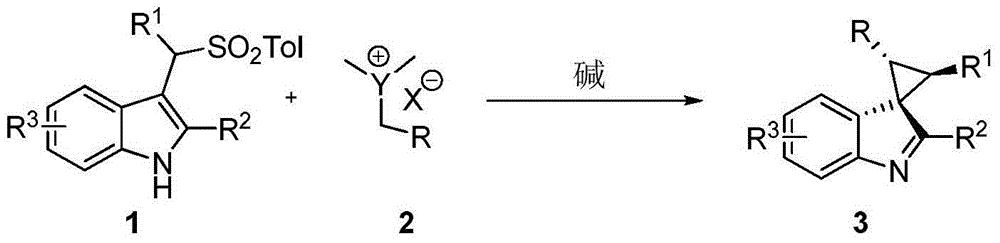
Method for synthesizing substituted cyclopropane compounds through dearomatizing indole
InactiveCN104803907ARaw materials are easy to obtainHigh purityOrganic chemistryCyclopropaneCyclopropanation
A method for synthesizing substituted cyclopropane compounds through dearomatizing indole is characterized in that simple Ylide reacts with substituted indole under alkaline conditions to obtain various the indole-dearomatized substituted cyclopropane compounds. The method has the advantages of simple and practical operation, easily available raw materials, high yield, and good diastereoselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
3-aryl-3'-amino bis(quaternary carbon) bis(oxindole) compounds and synthetic method and applications thereof
ActiveCN109776384AMild reaction conditionsFew reaction stepsNervous disorderOrganic chemistrySolventHigh selectivity
3-aryl-3'-amino bis(quaternary carbon) bis(oxindole) compounds and a synthetic method and applications thereof are disclosed. The compounds are prepared from diazo compounds, N,N-disubstituted anilineand isatin imine compound by adopting rhodium acetate as a catalyst, an organic solvent as a solvent and a 4A molecular sieve as an additive through one-step reaction at room temperature. The methodhas advantages of efficient atom economy, high selectivity, a low dosage of the catalyst, simple and safe operation, and the like. The synthesized bis(quaternary carbon) bis(oxindole) compounds can beadopted as important medical and chemical intermediates and have wide application prospects in the medicinal field.
Owner:EAST CHINA NORMAL UNIV
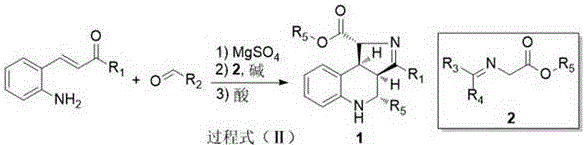
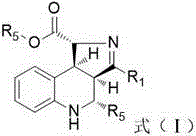
Preparation method of multi-substituted tetrahydroquinoxaline derivative
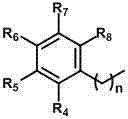
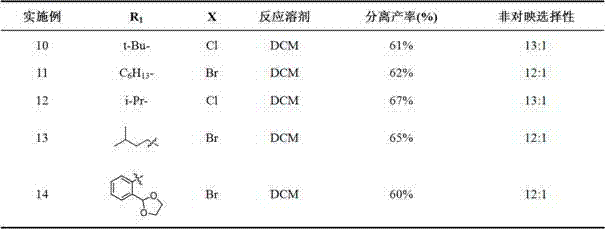
InactiveCN110105294AEasy diastereoselectivityEasy to separate and purifyOrganic chemistryArylEnantio selectivity
The invention discloses a preparation method of a tetrahydroquinoxaline compound shown in the formula (V). Aryl methyl diazoacetate and o-aminophenyl imine compounds are subjected to a [5+1] cyclization reaction under the catalysis of rhodium acetate to generate the tetrahydroquinoxaline compound. The preparation method has the advantages that the raw materials are cheap and easy to obtain, the preparation route is short, the operation is simple and convenient, the reaction conditions are mild, the time is short, and the energy consumption is low; the reaction conversion rate and the atom utilization rate are high, few by-products are generated, the diastereoselectivity is high, and the product is easy to separate and purify; a novel tetrahydroquinoxaline derivative is convenient to prepare, and a diverse compound framework is provided. The formula (V) is shown in the description.
Owner:SOUTHWEST UNIV
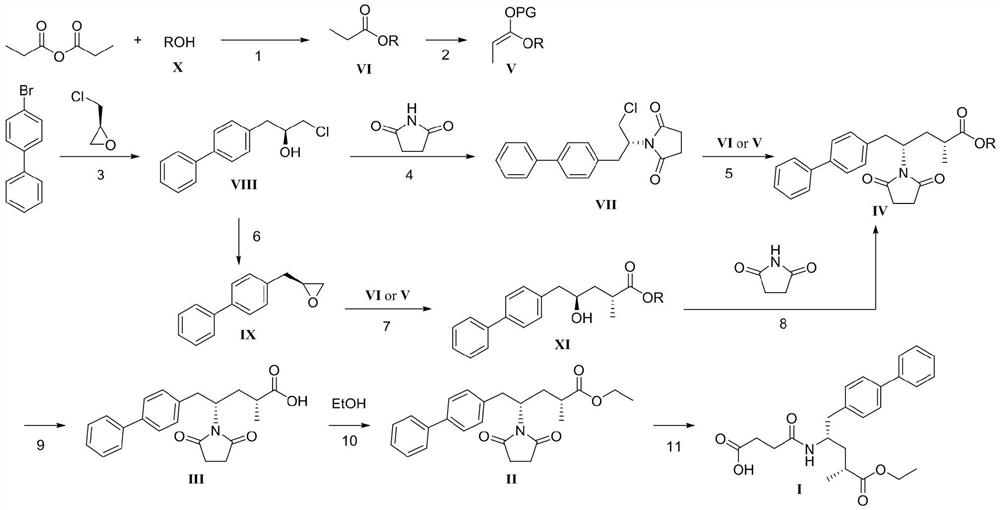
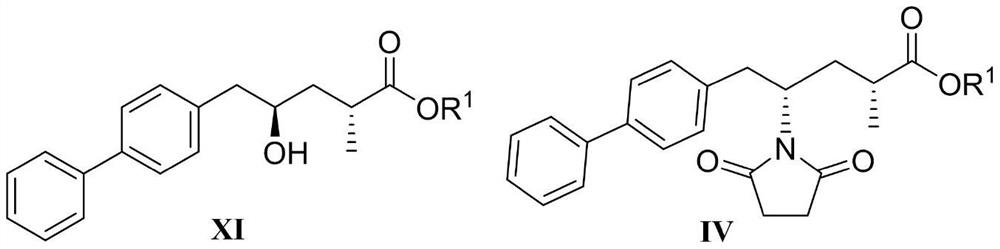
Preparation method of sacubitril
ActiveCN113387829AHigh selectivityEasy to operateGroup 4/14 element organic compoundsOrganic compound preparationSacubitrilChemical compound
The invention discloses a sacubitril, and a method for preparing the sacubitril form sacubitril intermediates and chiral prosthetic groups. The method comprises steps 1-11 in a synthesis route shown in the specification. In the synthesis route, ROH (formula X) is chiral alcohol and an analogue thereof, PG is a hydroxyl protecting group, and a compound of formula VI and a compound of formula V are the chiral prosthetic groups provided by the invention. Cheap and easily available propionic anhydride and chiral alcohol ROH (formula X) are used for preparing chiral prosthetic compounds of formula VI and formula V, high diastereomeric excess sacubitril intermediates of formula XI and formula IV are efficiently prepared through chiral prosthetic groups, and the sacubitril disclosed by the invention can be prepared through three simple operation steps of the formula IV. The whole route is simple in process, safe, environment-friendly, free of special requirements on equipment, low in production cost, high in yield, high in enantiomer selectivity and suitable for industrial production.
Owner:SHENZHEN CATALYS SCI & TECH CO LTD +1
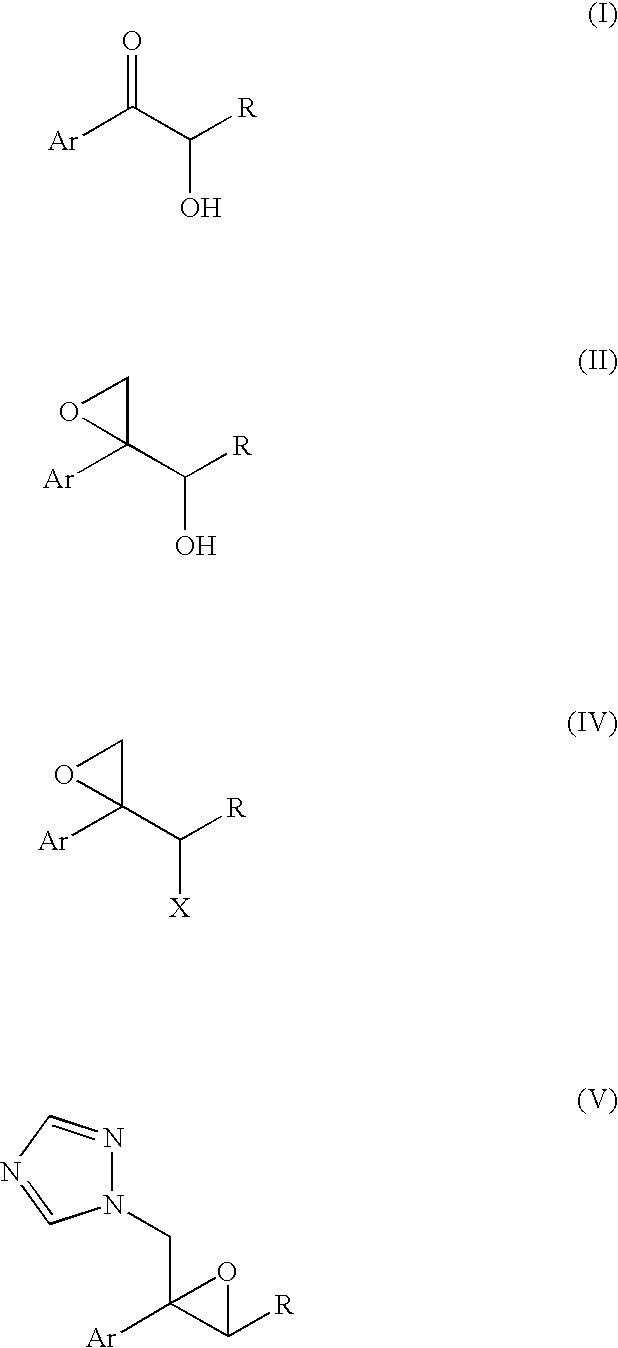
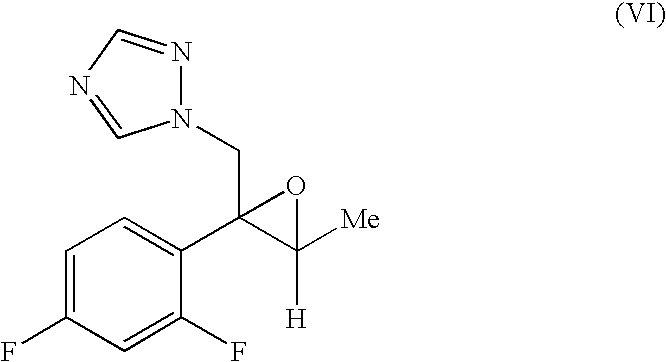
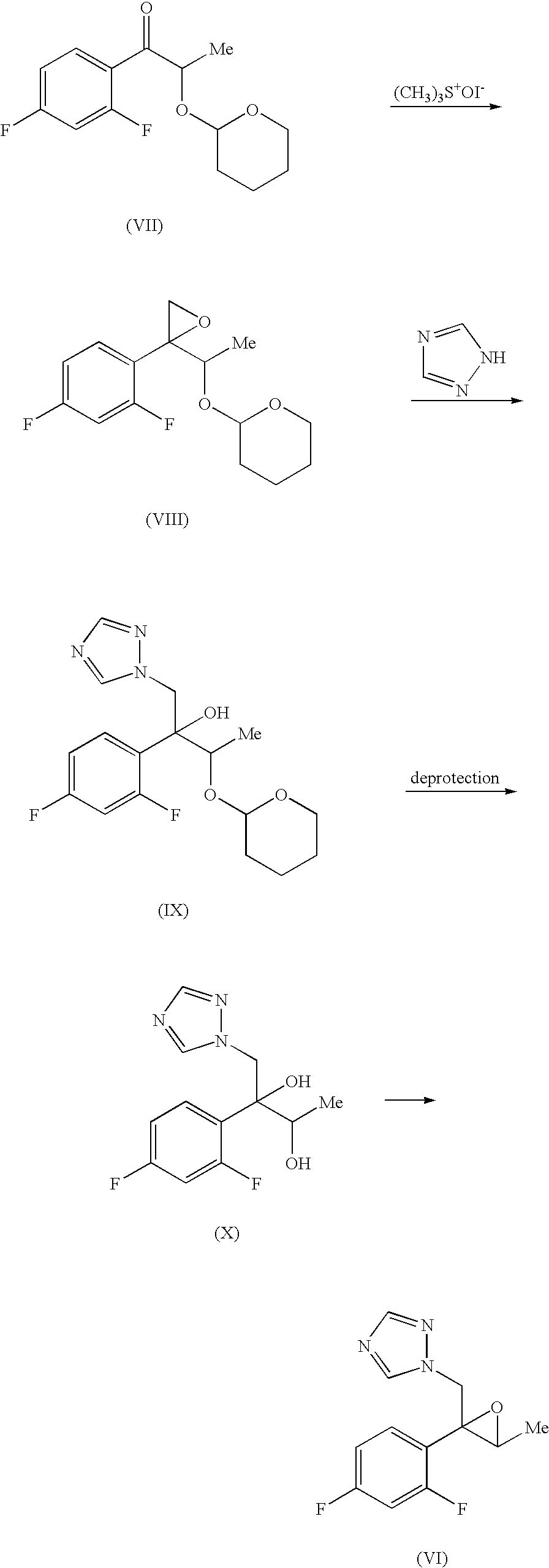
Production methods of epoxytriazole derivative and intermediate therefor
InactiveUS7297802B2Quality improvementProtection deprotectionOrganic compound preparationCarbonyl compound preparationHydrogen atomLeaving group
An epoxytriazole derivative (V) useful as an intermediate for anti-fungal agents and an intermediate therefor having high quality can be produced economically and efficiently by the following industrial means. A compound of the following formula (I) is reacted with trimethyloxosulfonium salt and the like in the presence of a base to give compound (II), this compound is converted to compound (IV), and this compound is reacted with 1,2,4-triazole in the presence of a base.wherein Ar is a phenyl group optionally substituted by 1 to 3 halogen atom(s) or trifluoromethyl group, R is a hydrogen atom or lower alkyl group, and X is a leaving group.
Owner:SUMITOMO CHEM CO LTD
Preparation method of chiral alpha-amino acid derivatives
InactiveCN102408287ASimple processMild reaction conditionsAsymmetric synthesesAmino acid synthesisPeptide drug
The invention discloses a preparation method of chiral alpha-amino acid derivatives, which belongs to the field of organic intermediate synthesis. The preparation method comprises the following steps of: taking chiral sulphinyl ester imine compound and halide metal reagent as raw materials, and carrying out one-step addition reaction to obtain various different substituted chiral alpha-amino acid derivatives. The preparation method has the characteristics of simple process, high non enantioselectivitiy, moderate reaction condition, quick synthesis speed, high production rate and low cost; the used materials are cheap, easy to get and stable for photo-thermal chemical properties; noble metal and highly toxic product can be avoided being used and produced; the preparation method has remarkable operation safety and general applicability and can be used for preparation of various different substituted chiral alpha-amino acids and derivatives thereof; and the preparation method has greatly important application value for organic intermediate synthesis, non natural chiral alpha-amino acid synthesis and multi-peptide drug research and development.
Owner:LANZHOU UNIVERSITY
Preparation method of organic boron-nitrogen fluorescent compound
InactiveCN108164554AHigh yieldHigh selectivityGroup 3/13 element organic compoundsLuminescent compositionsFluorescenceKetone
The invention discloses a preparation method of an organic boron-nitrogen fluorescent compound. The preparation method comprises the following steps of: adopting a quinoline amide compound, cheap sodium-tetraphenylborate type derivatives and carboxylic-acid type derivatives as raw materials, adopting cheap copper bromide or sodium bromide as a catalyst, adopting a common organic solvent as a reaction solvent, reacting for a period of time under a certain temperature, and obtaining a novel chiral organic boron-nitrogen fluorescent compound by high yield and high selectivity. The organic boron-nitrogen fluorescent compound is good in stability, can be recovered, has very good diastereoisomeric selectivity in aldehyde-ketone condensation reaction. The preparation method disclosed by the invention has the advantages of lower cost, high yield, simple operation and no pollution and the like, and has certain feasibility for realizing industrial production.
Owner:汝城县三鑫电化有限公司
Compound containing chiral chromanone skeletons and asymmetric synthetic method thereof
InactiveCN102516217AHigh catalytic activityMild reaction conditionsOrganic chemistryBulk chemical productionKetoneSilyl ether
The invention provides a synthetic method for preparing a compound containing multiple-chiral center chromanone skeletons, which can effectively utilize chiral amine-in particular a, a-diaryl prolinol O-silyl ether--catalytic chromone electron-deficient diene compound and olefine aldehyde to generate Diels-Alder reaction required by asymmetric anti-electrons with high regioselectivity and high stereoselectivity and series vinylogy Aldol or series hemiacetal reaction under the mild reaction conditions and can also be used for synthesizing the compound with the multiple-chiral center dihydro-chromogenic ketone skeletons. The asymmetric synthetic method is reported for the first time and has the various advantages of cheap and easily-obtained catalyst, high catalytic activity, mild reaction condition, simplicity in operation, wide substrate application scope and high product area and enantioselectivity; and more importantly, the compound containing the various multiple-chiral center dihydro-chromogenic ketone skeletons prepared by the method possibly has special physiological activity or becomes an important synthetic intermediate body of a natural product.
Owner:SICHUAN UNIV
Synthesis method of polysubstituted 4-phenyl chroman compounds
The invention belongs to the technical field of organic synthesis and discloses a synthesis method of polysubstituted 4-phenyl chroman compounds. The structural formula of polysubstituted 4-phenyl chroman compounds is shown by formula I. The method involves non-metal catalyzed oxamichael addition and 1,6-tandem reaction conjugated addition under mild condition. The catalyst used in the invention is a commercial nonmetallic alkali, has low cost, is environmentally friendly and is easy to operate; the method disclosed in the invention has high yield, excellent diastereoselectivity, mild conditions, convenient post-treatment and easy separation and purification of products; the method of the invention is easy to produce on a large scale, and the amplified yield and diastereoselectivity can bemaintained.
Owner:QINGDAO UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/7e5e1504-3970-4079-8484-5a3fff8a38f0/BDA0000089398160000021.PNG)
![Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/7e5e1504-3970-4079-8484-5a3fff8a38f0/BDA0000089398160000022.PNG)
![Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof Chiral 2',5-dicarbonyl-3-aryl spiro[cyclohexane-1,3'-indole]-2,2-dinitrile derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/7e5e1504-3970-4079-8484-5a3fff8a38f0/BDA0000089398160000031.PNG)
![Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone](https://images-eureka.patsnap.com/patent_img/4329719b-1b73-4e18-9ef9-6e95a9db0c99/US20110046389A1-20110224-C00001.png)
![Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone](https://images-eureka.patsnap.com/patent_img/4329719b-1b73-4e18-9ef9-6e95a9db0c99/US20110046389A1-20110224-C00002.png)
![Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone](https://images-eureka.patsnap.com/patent_img/4329719b-1b73-4e18-9ef9-6e95a9db0c99/US20110046389A1-20110224-C00003.png)
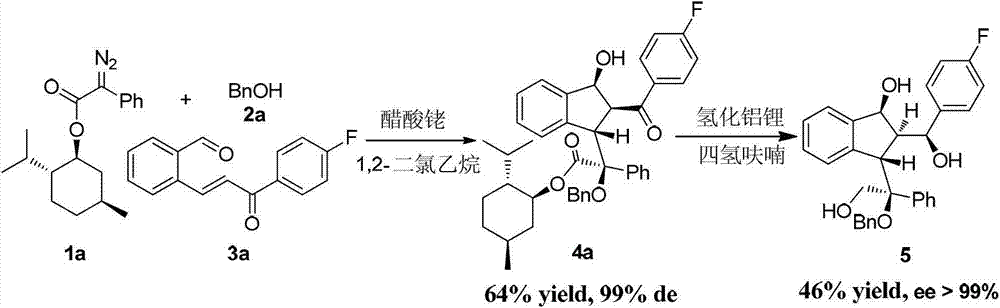
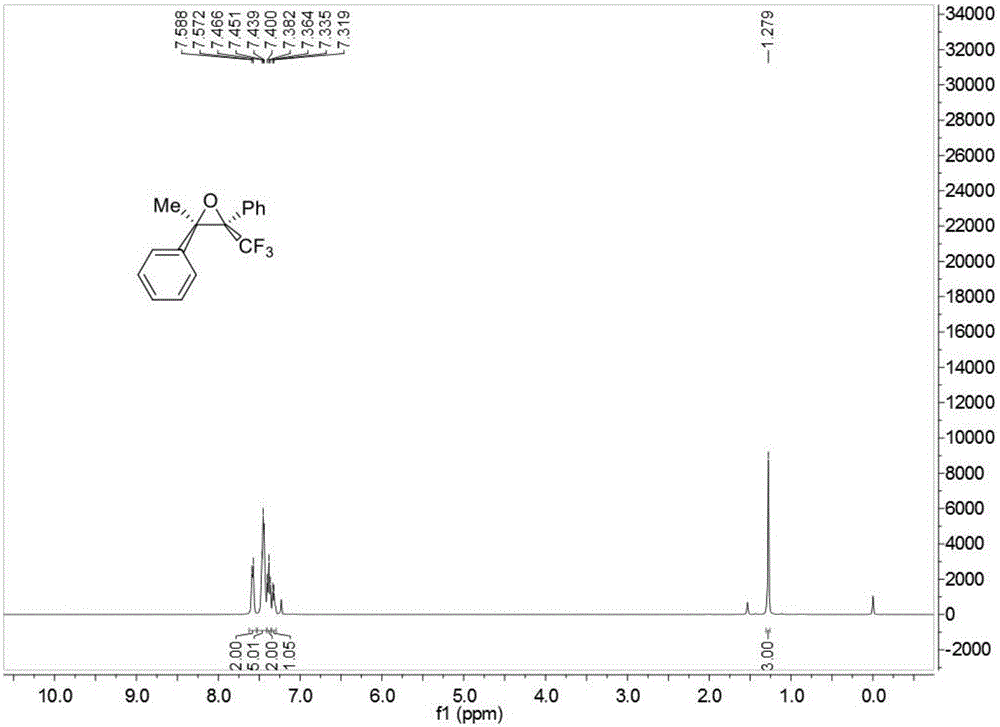
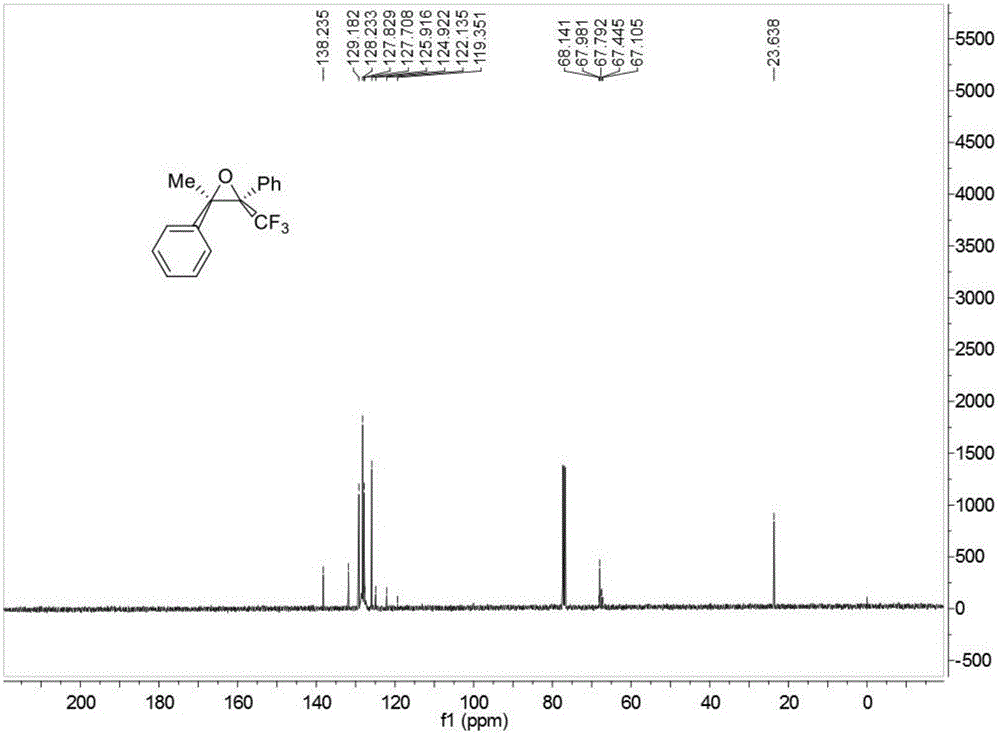
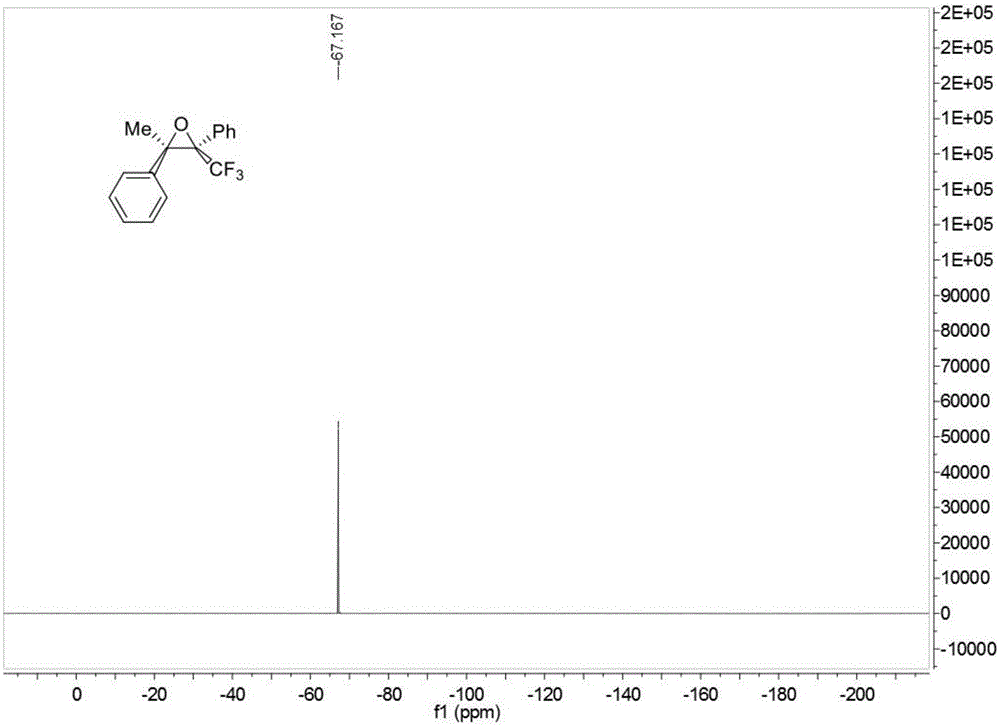
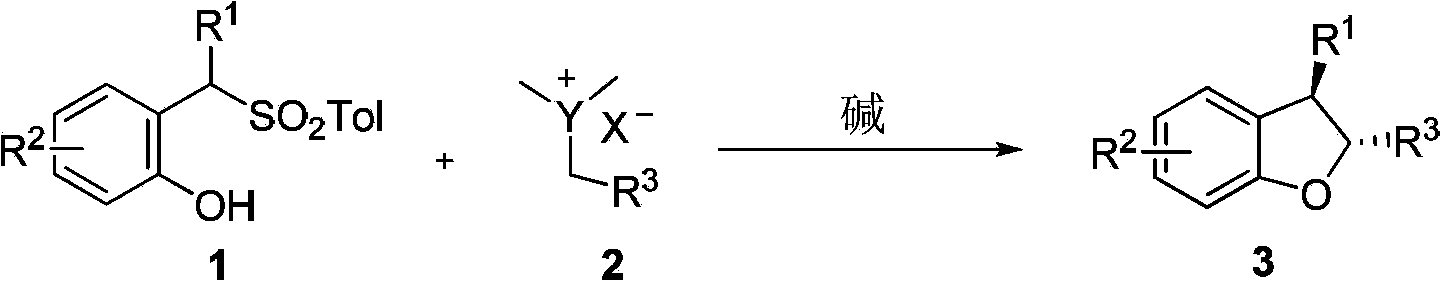
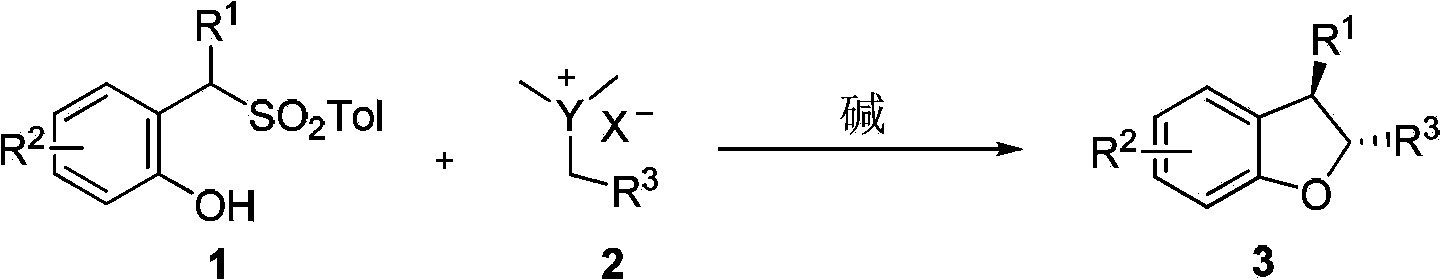
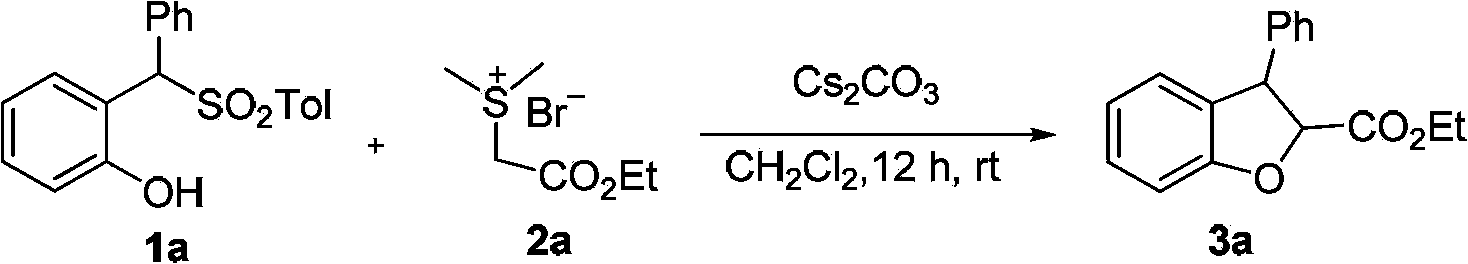

![Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system](https://images-eureka.patsnap.com/patent_img/c2529be1-a9ba-4754-ab57-e9d92c18f5c4/HDA0002984993440000011.png)
![Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system](https://images-eureka.patsnap.com/patent_img/c2529be1-a9ba-4754-ab57-e9d92c18f5c4/FDA0002984993420000011.png)
![Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system Method for preparing 5, 8-dihydro-6H-isoquinoline [3, 2-alpha] isoquinoline based on micro-reaction system](https://images-eureka.patsnap.com/patent_img/c2529be1-a9ba-4754-ab57-e9d92c18f5c4/FDA0002984993420000021.png)