Preparation method of polysubstituted pyrroloquinoline derivate
A technology of pyrrole derivatives and multi-substitution, applied in the direction of organic chemistry, etc., can solve the problems of long synthesis route, harsh reaction conditions, and increased synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
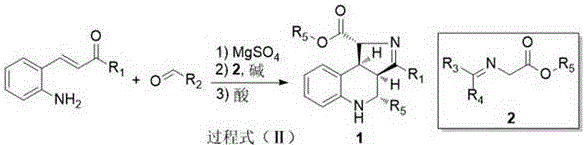
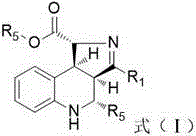
[0024] Example 1 Preparation of tert-butyl 3,4-diphenyl-3a,4,5,9b-tetrahydro-1H-pyrrolo[3,4-c]quinoline-1-carboxylate.
[0025] .
[0026] Weigh E-3-(2-aminophenyl)-1-phenyl-2-propen-1-one (1.0mmol), benzaldehyde (1.0mmol), magnesium sulfate (1.176g), add to the round bottom flask , add 2 mL of organic solvent, and stir overnight at room temperature. After filtration, concentrate the filtrate, add N-dibenzylidene-glycine tert-butyl ester (1.0 mmol), triethylamine (1.0 mmol), add organic solvent 10 mL, and stir at room temperature for 24 hours. Then concentrate the filtrate, add an organic solvent to dissolve the reactant, adjust the pH to neutrality, add 10% citric acid aqueous solution, stir and react at room temperature for 24 hours, and remove the solvent by rotary evaporation, then pass through column chromatography (eluent: sherwood oil: acetic acid ethyl ester = 10:1) to obtain quinolinopyrrole derivative A with a yield of 82% and dr > 20:1. 1 H NMR (600 MHz, CDCl ...
example 2
[0027] Example 2 Preparation of tert-butyl 4-p-fluorophenyl-3-phenyl-3a,4,5,9b-tetrahydro-1H-pyrrolo[3,4-c]quinoline-1-carboxylate.
[0028] .
[0029] Weigh E-3-(2-aminophenyl)-1-phenyl-2-propen-1-one (1.0mmol), p-fluorobenzaldehyde (1.0mmol), magnesium sulfate (1.176g), add to the round bottom Add 2 mL of organic solvent to the flask, and stir overnight at room temperature. Next, after filtration, the filtrate was concentrated, N-dibenzylidene-glycine tert-butyl ester (1.0 mmol), triethylamine (1.0 mmol) were added, and 10 mL of organic solvent was added, and stirred at room temperature for 24 hours. Then concentrate the filtrate, add an organic solvent to dissolve the reactant, adjust the pH to neutrality, add 10% citric acid aqueous solution, stir and react at room temperature for 24 hours, and remove the solvent by rotary evaporation, then pass through column chromatography (eluent: sherwood oil: acetic acid Ethyl ester = 10:1) to obtain quinolinopyrrole derivative B ...
example 3
[0030] Example 3 Preparation of tert-butyl 4-m-methoxyphenyl-3-phenyl-3a,4,5,9b-tetrahydro-1H-pyrrolo[3,4-c]quinoline-1-carboxylate .
[0031] .
[0032] Weigh E-3-(2-aminophenyl)-1-phenyl-2-propen-1-one (1.0mmol), m-methoxybenzaldehyde (1.0mmol), magnesium sulfate (1.176g), add In a round bottom flask, add 2 mL of organic solvent, and stir overnight at room temperature. Next, after filtration, the filtrate was concentrated, N-dibenzylidene-glycine tert-butyl ester (1.0 mmol), triethylamine (1.0 mmol) were added, and 10 mL of organic solvent was added, and stirred at room temperature for 24 hours. Then concentrate the filtrate, add an organic solvent to dissolve the reactant, adjust the pH to neutrality, add 10% citric acid aqueous solution, stir and react at room temperature for 24 hours, and remove the solvent by rotary evaporation, then pass through column chromatography (eluent: sherwood oil: acetic acid Ethyl ester = 10:1) to obtain quinolinopyrrole derivative C with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com