Patents
Literature
71 results about "Oxazepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxazepines are a family of unsaturated heterocycles containing seven atoms, with a nitrogen replacing a carbon at one position and with an oxygen replacing a carbon at one position.
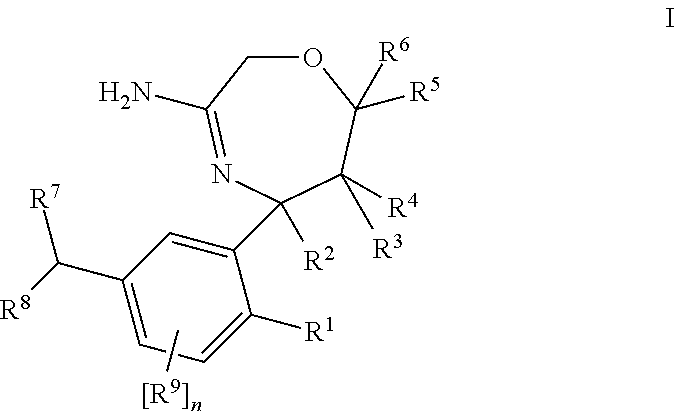
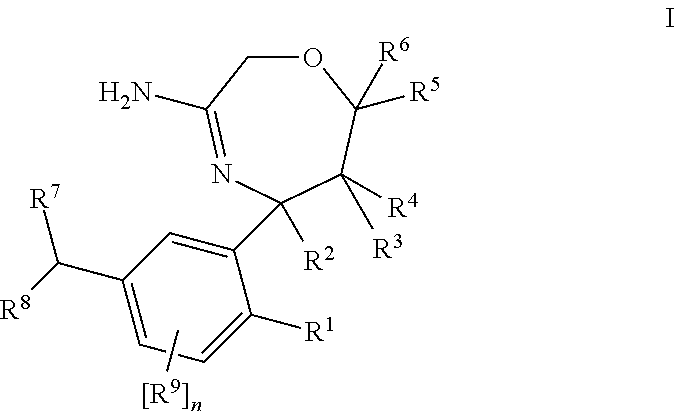
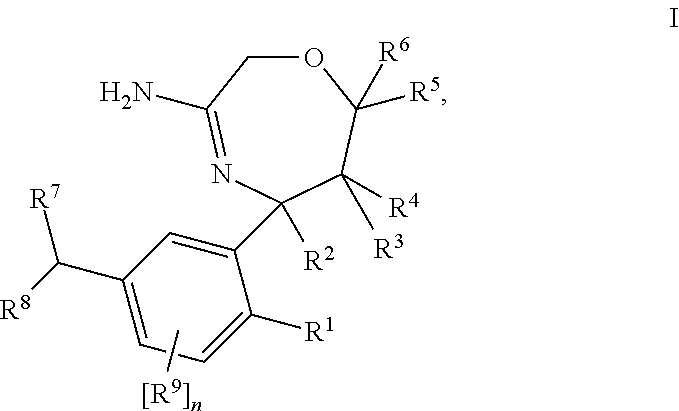
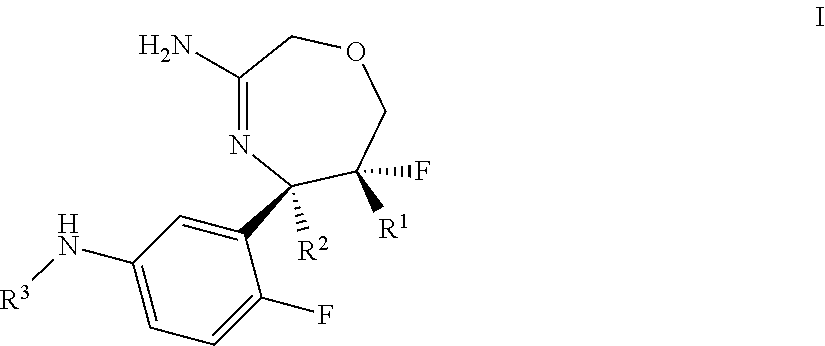
Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections
Owner:GILEAD SCI INC
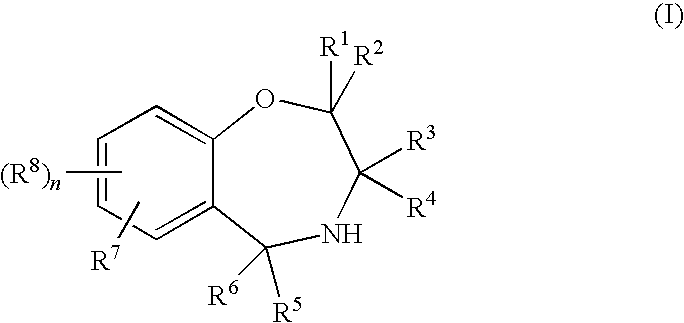
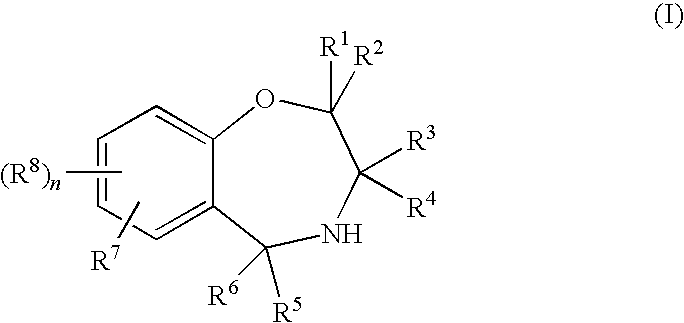
1,4-oxazepines as bace1 and/or bace2 inhibitors
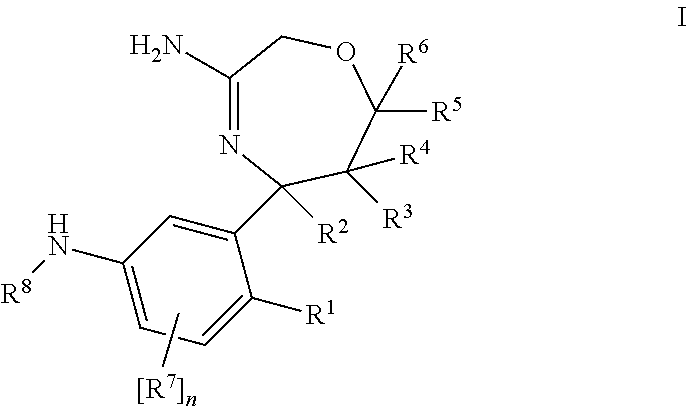
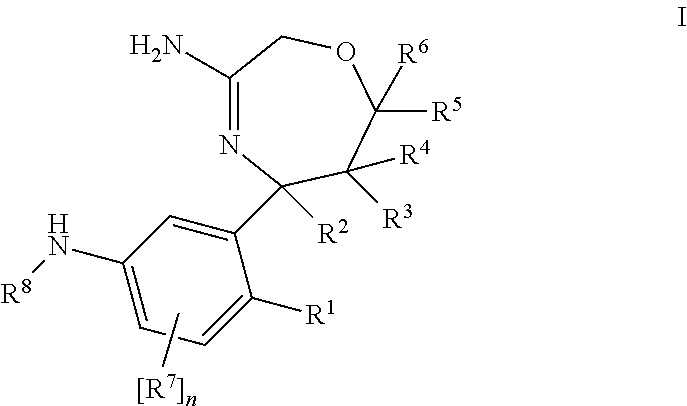
InactiveUS20120253035A1Good pharmacological propertiesImprove propertiesOrganic active ingredientsNervous disorderDiseaseProphylactic treatment
The present invention relates to 1,4 Oxazepines of formula Ihaving BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
Owner:F HOFFMANN LA ROCHE INC
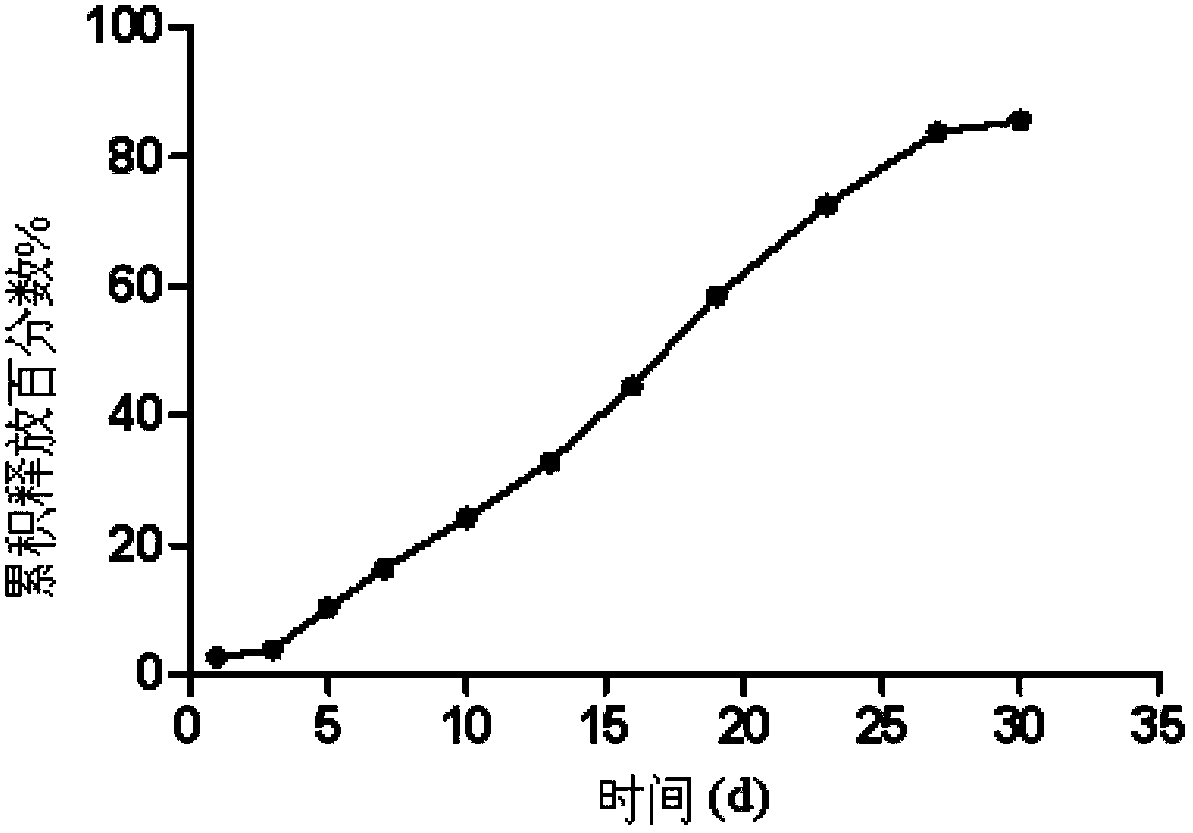
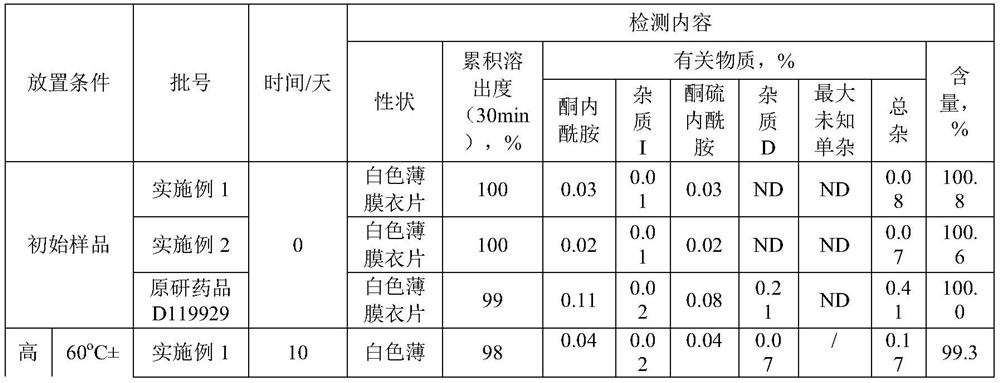
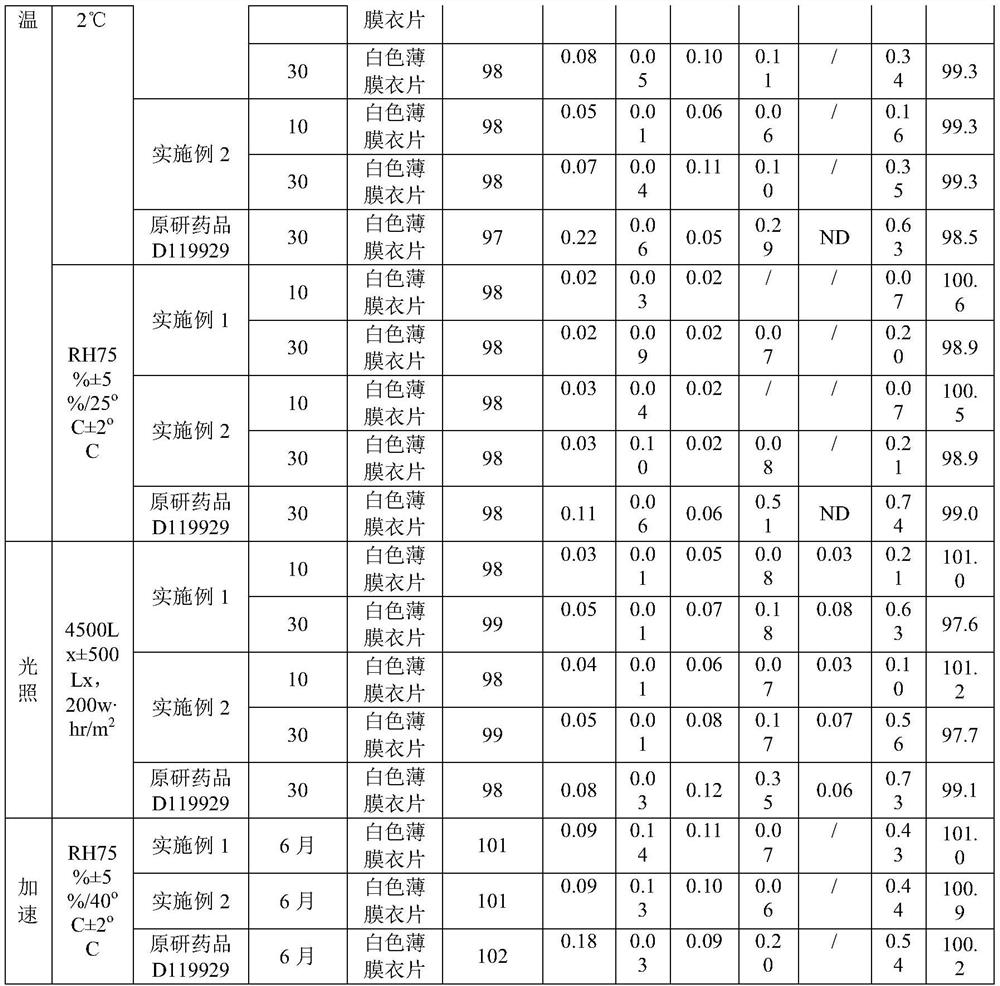
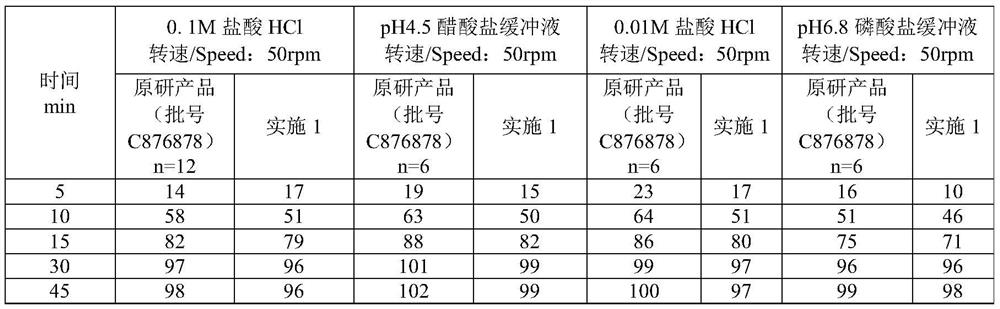
Olanzapine-containing biodegradable microsphere preparation and preparation method thereof
InactiveCN103417492AHigh drug loadingImproved particle size distributionOrganic active ingredientsNervous disorderBiodegradable microspherePharmaceutical drug
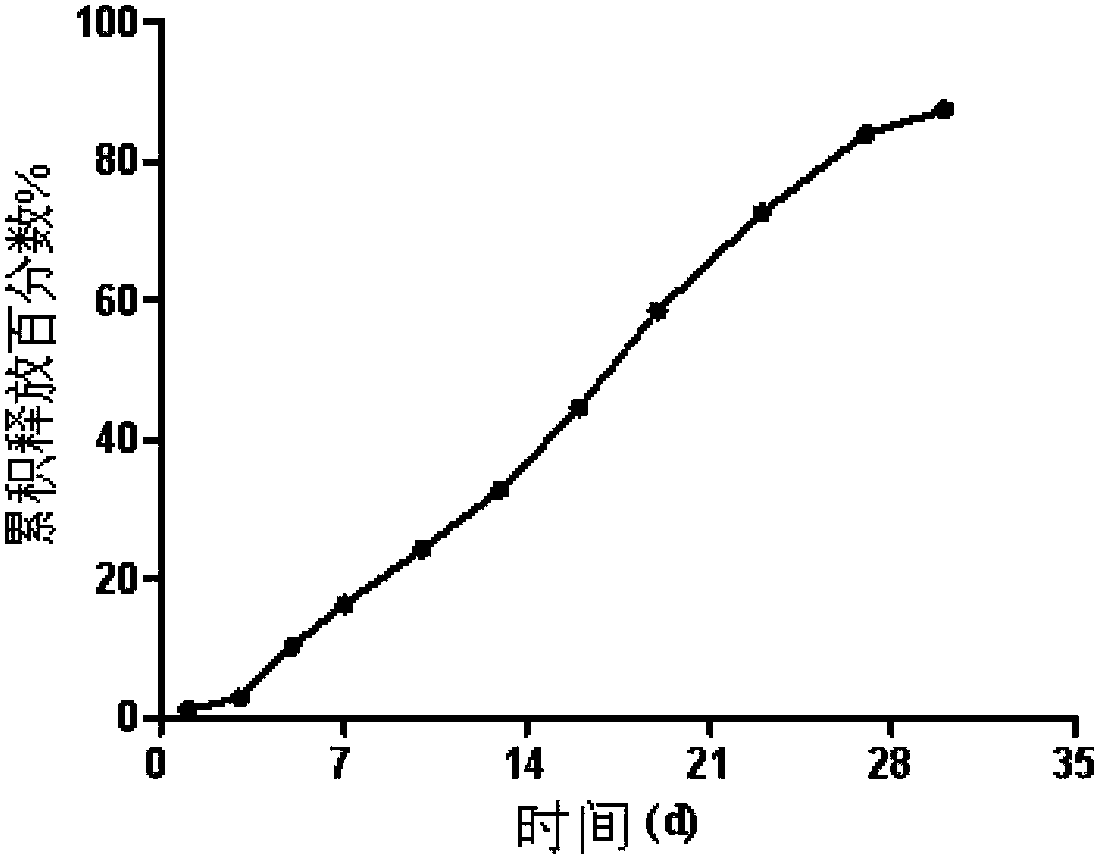
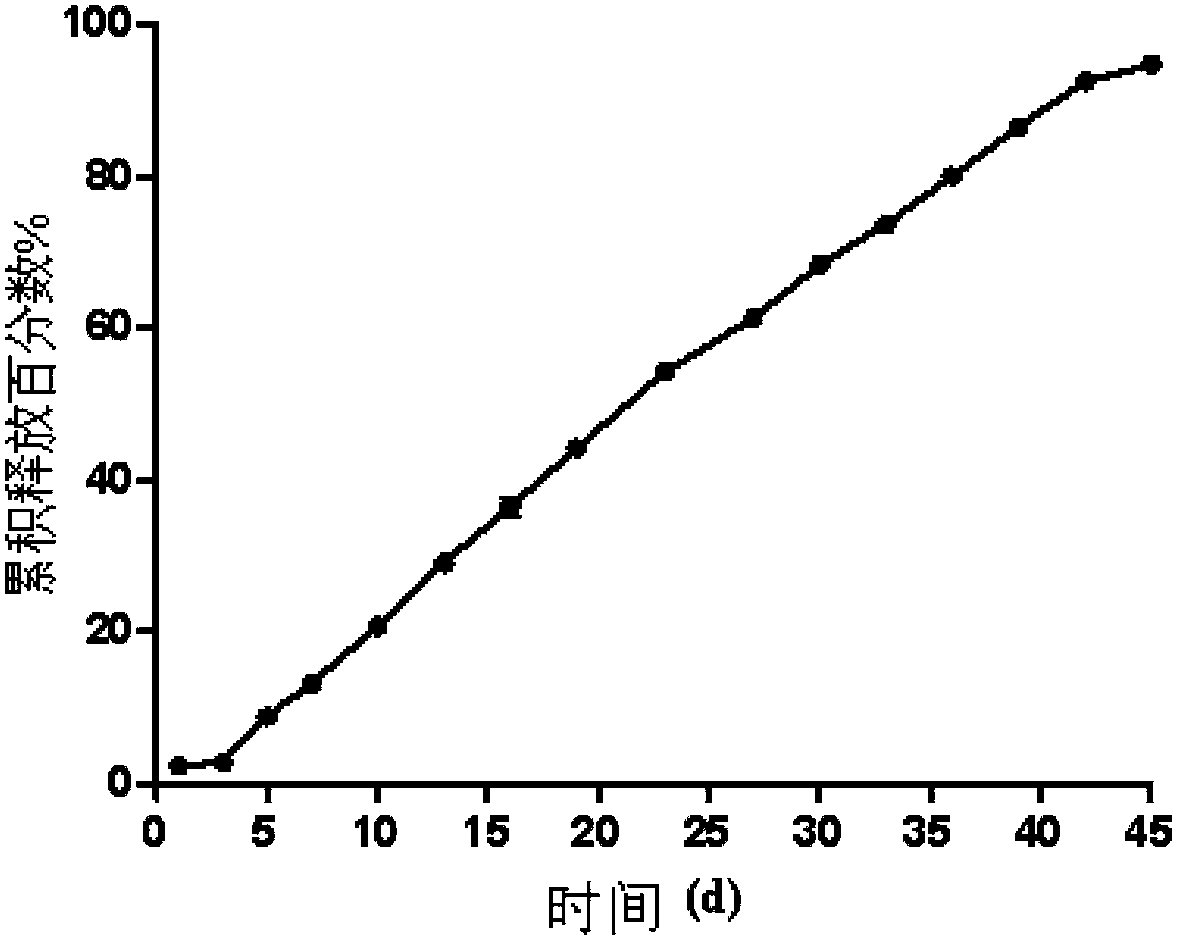
The invention discloses an olanzapine-containing biodegradable microsphere preparation and a preparation method thereof. The olanzapine-containing biodegradable microsphere preparation comprises 10-50wt% of olanzapine or its salt, 0.5-20wt% of release adjusting agents and 30-89.5% of PLGA. In the invention, the PLGA having a proper monomer LA:GA ratio is selected as a matrix material, and above one release adjusting agents are added, so the olanzapine loading is improved to above 30%, and the particle size distribution and the in-vitro drug release of microspheres are improved. The utilization of a biodegradable material to encapsulate olanzapine to prepare the microspheres realizes a good slow release effect and the maintenance of an in-vivo relatively-stable drug concentration for above 15-45d or more.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
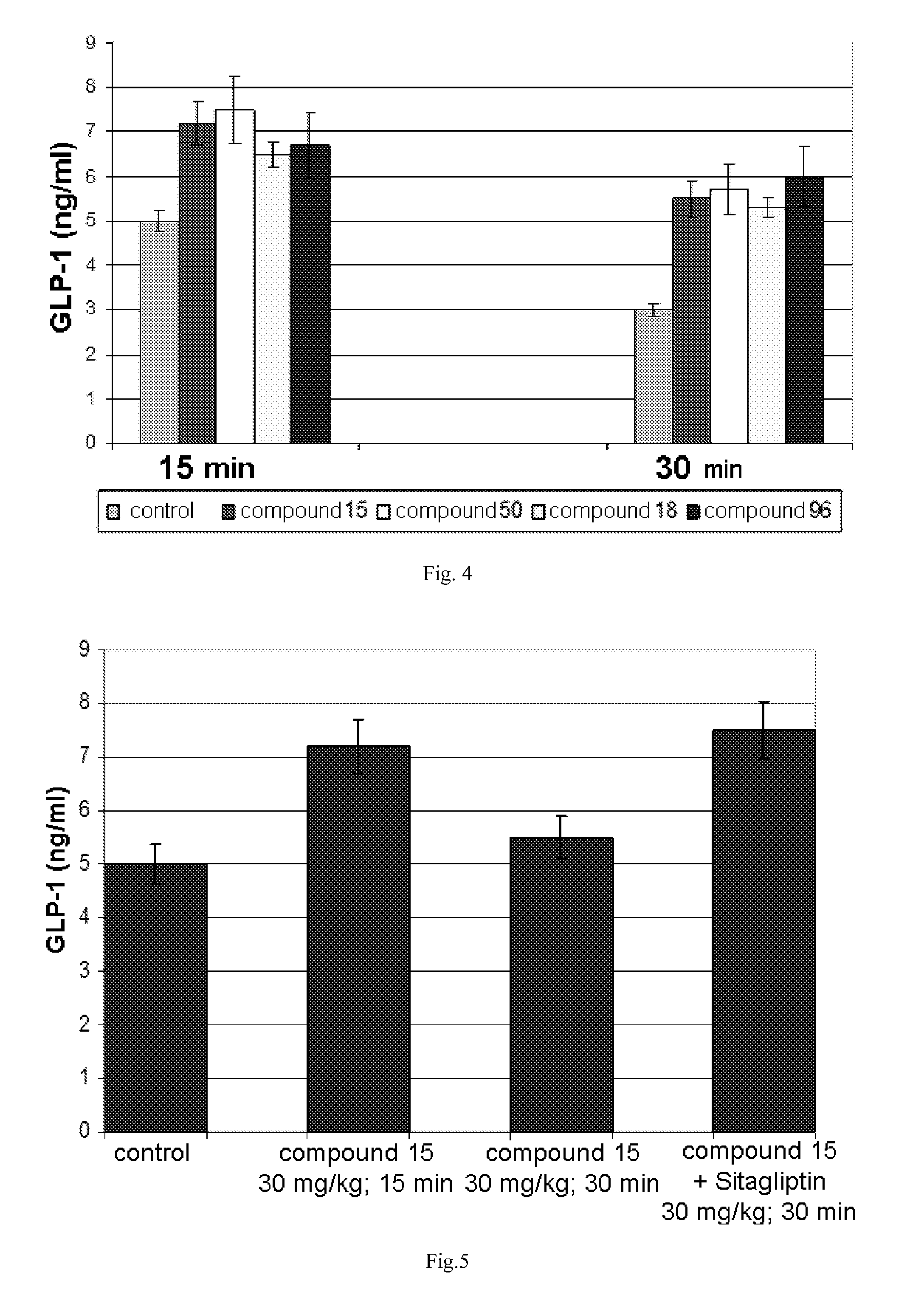
Stimulators of incretin hormones secretion, method for preparation and use thereof
InactiveUS20140100216A1Improve treatment efficiencySimplifies direct careOrganic active ingredientsBiocideDiseaseTreatment effect
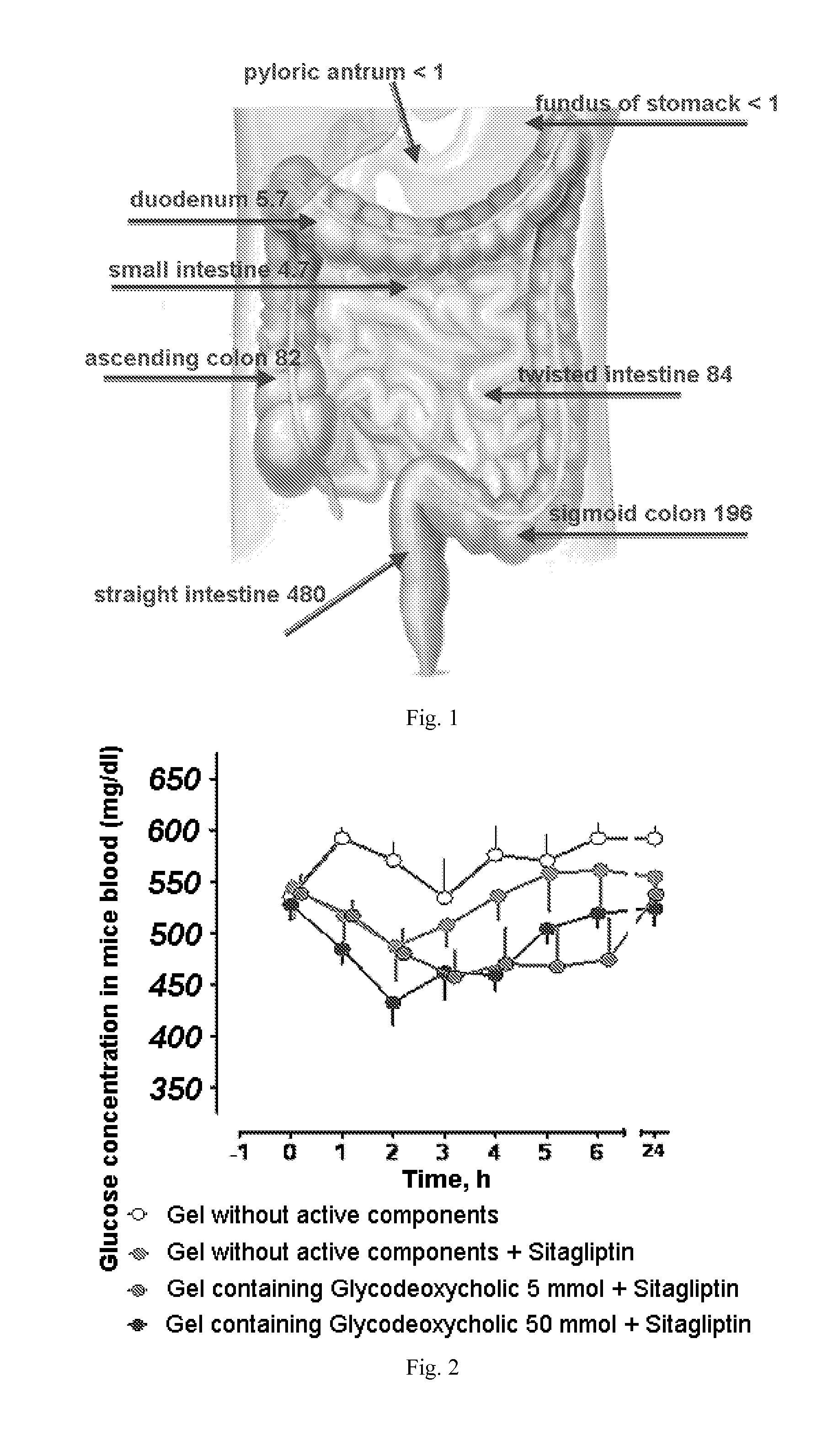
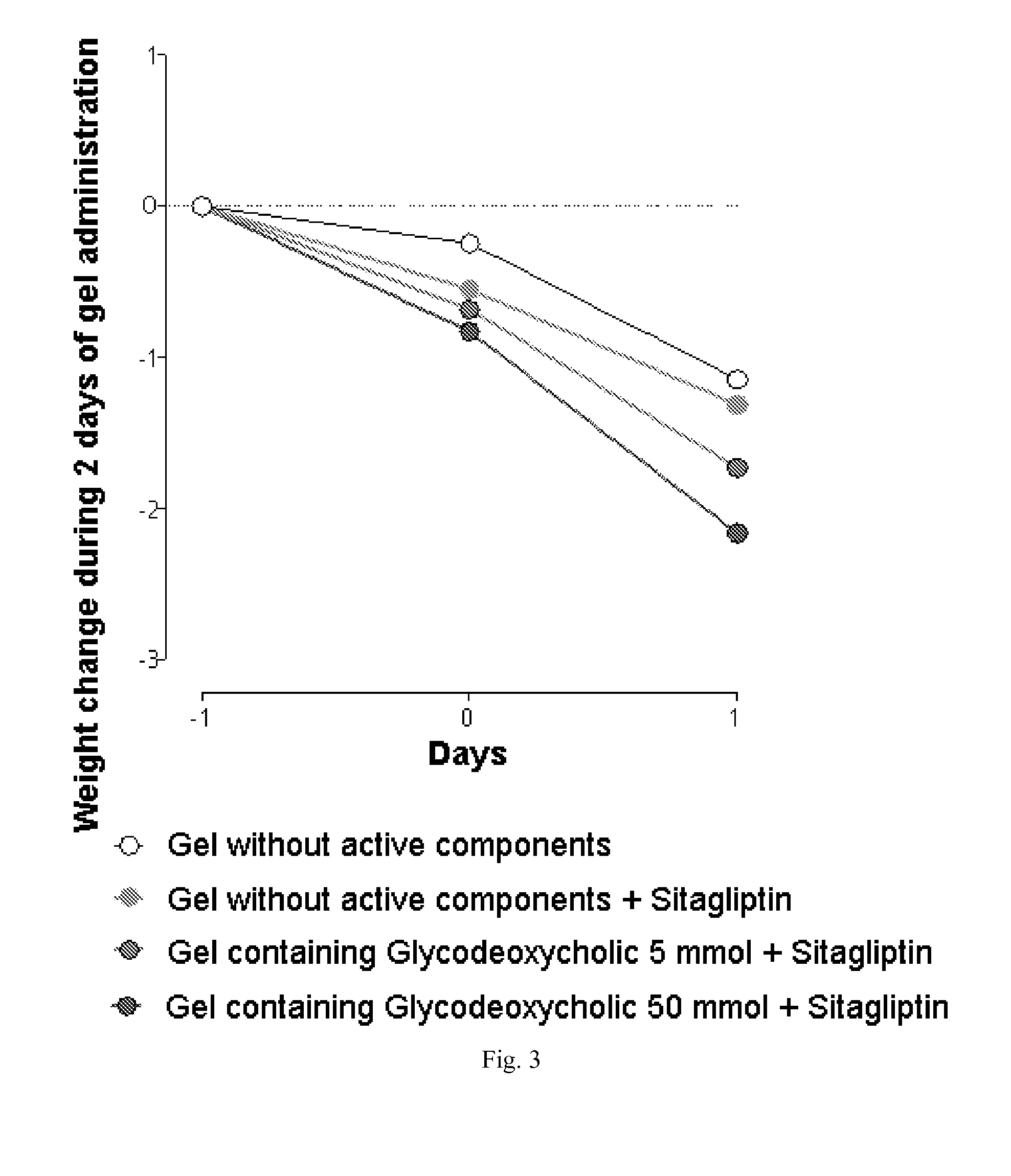
The invention relates to the area of medicinal chemistry, pharmacology and medicine and includes description of pharmaceutical compositions and combined medicaments on the base of secretion stimulators and protectors of incretin hormones for treatment of metabolic diseases (among them, diabetes, obesity, metabolic syndrome and the like). The invention consists in that that pharmaceutical composition or combined medicament comprises a derivative of tetrahydrobenzo[f][1,4]oxazepine—either nonsteroidal agonist of bile aids receptor TGR5, or one of endogenous bile acids which stimulate incretin hormones secretion, and also one of the known inhibitors of DPP-IV proteinase. In this case administration of TGR5 agonists is carried out peroral, and administration of endogenous bile acids is exercised rectal in the form of suppository or gel. As proteinase DPP-IV inhibitors could be used Vildagliptin, Saxagliptin, Sitagliptin, Teneligliptin, Linagliptin, Dutogliptin, Alogliptin, Gemigliptin, Carmegliptin and the like. Besides, the invention includes description of novel tetrahydrobenzo[f][1,4]oxazepine derivatives—nonsteroidal agonist of bile aids receptors TGR5, and also methods for their preparation. The invention provides enhancement of therapy effectiveness owing to synergetic action of the components, thus making possible simultaneous treatment of diabetes, and obesity, other metabolic diseases and their cardiovascular and renal complications.
Owner:SAVCHUK NIKOLAY FILIPPOVICH +2
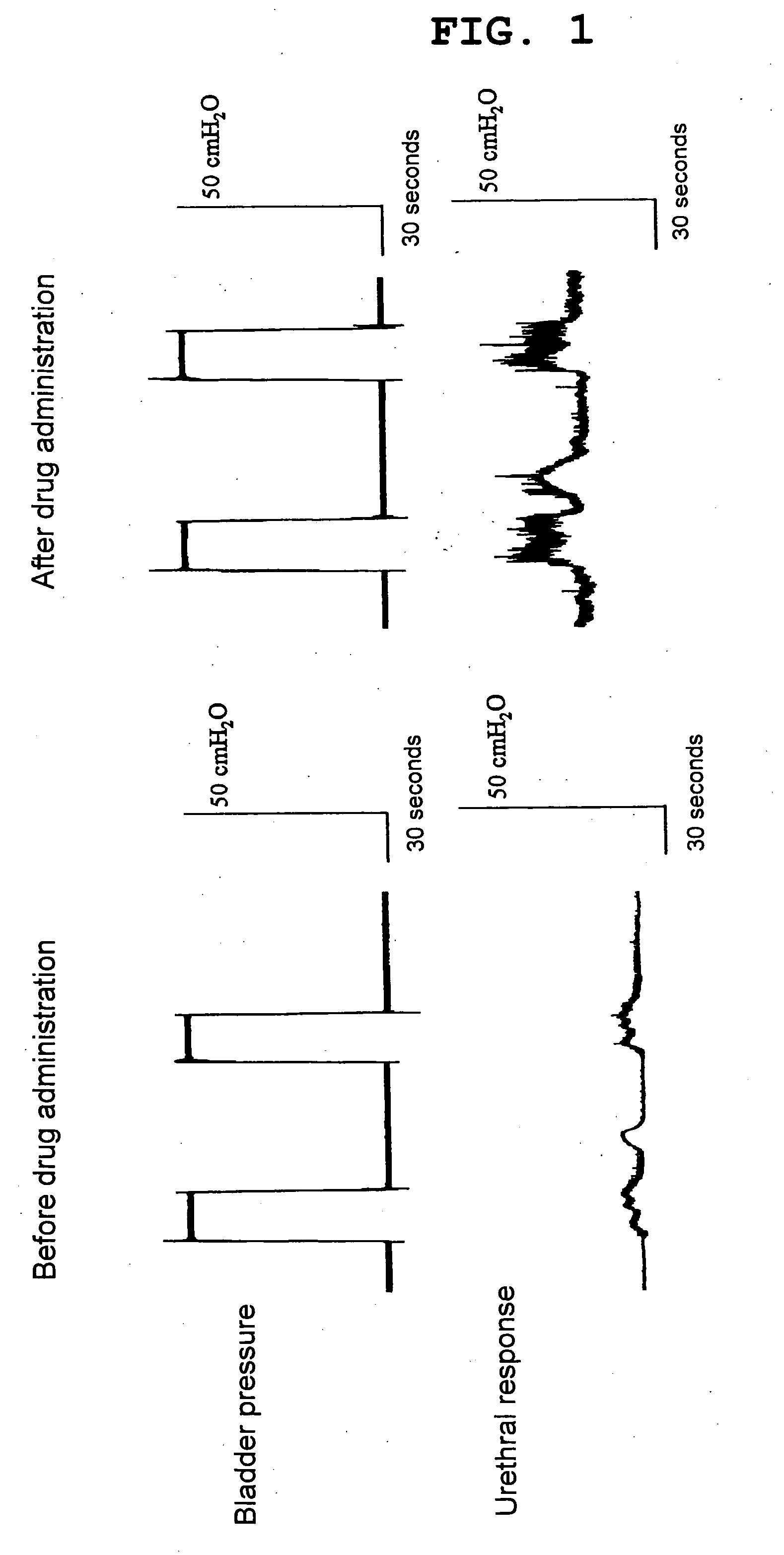
Benzoxazepine derivatives and use thereof
InactiveUS20100087418A1Superior serotonin -HT2C receptor activation actionLow toxicityBiocideSenses disorderSerotoninMedicine
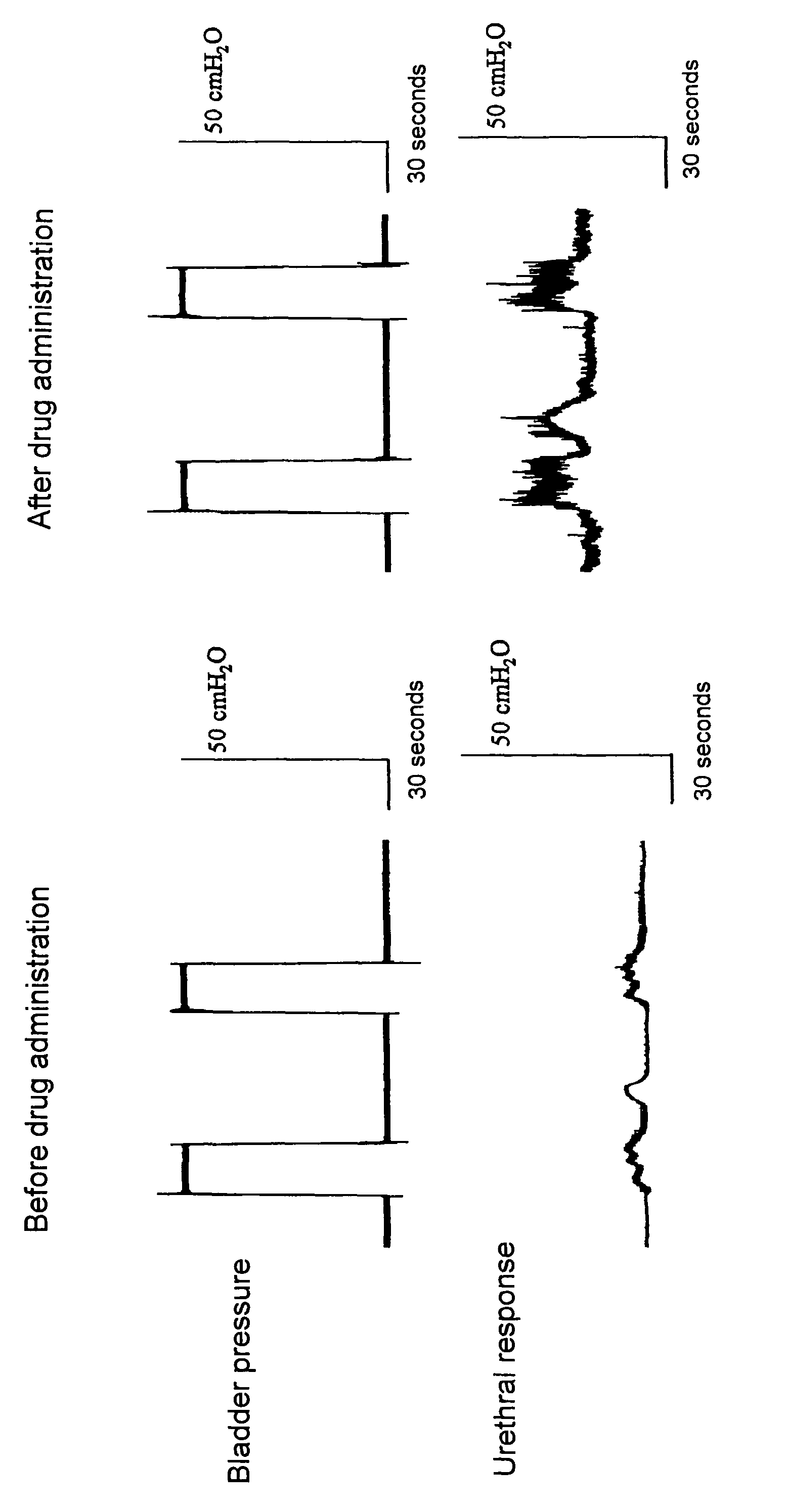
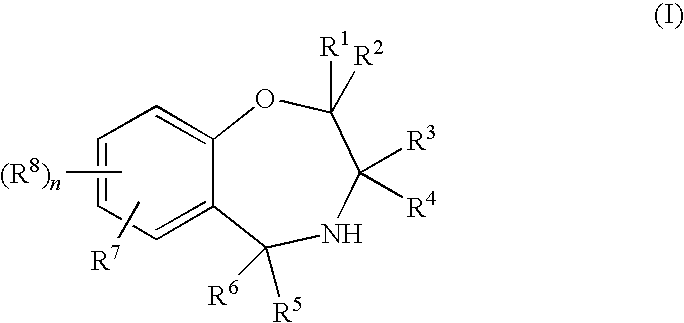
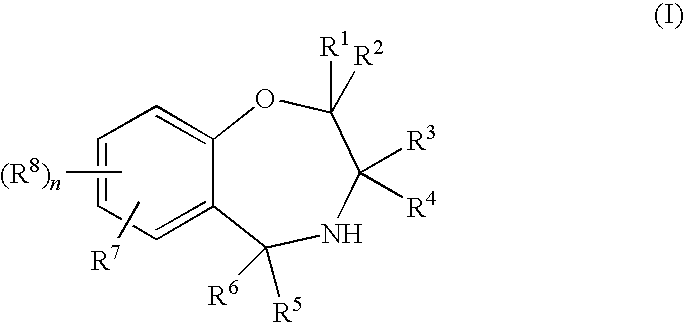
Compounds represented by the general formula (I):wherein each symbol is as defined in the description [with the proviso that 9-chloro-7-(1,1-dimethylethyl)-2,3,4,5-tetrahydro-1,4-benz-oxazepine and N-[[(5S)-2-oxo-3-(2,3,4,5-tetrahydro-1,4-benz-oxazepin-7-yl)-5-oxazolidinyl]methyl]acetamide are excluded], salts of the same, and prodrugs thereof have selective activation effect on serotonin 5-HT2C receptor and are useful as preventive and therapeutic agents for lower urinary tract diseases, obesity, and / or pelvic organ prolapse.
Owner:TAKEDA PHARMA CO LTD
Disintegrants for deodoring effectively and their preparation
An oral disintegrating tablet for effectively shielding the odor of oral cavity is prepared from the granulare odor-shielding medicine (estazolam, etc) and medicinal additive. Its advantages are short disintegrating time (less than 45 S) and high effect.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4
Substituted piperazines of azepines, oxazepines and thiazepines
InactiveUS20060270656A1Improved adverse event profileGood dopamine D bindingBiocideNervous disorderThiazepineHalogen
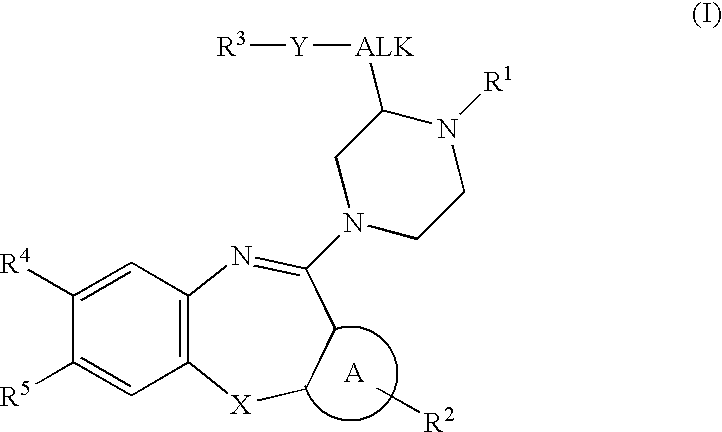
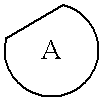
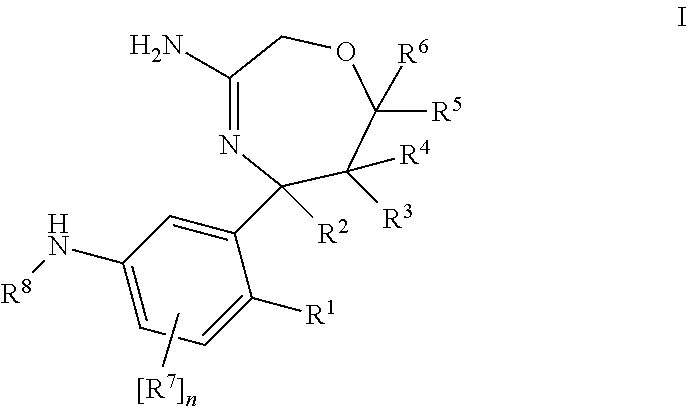
Described herein are antipyschotic compounds of formula (I) wherein: is an optionally benzo-fused five or six member aromatic ring having zero to three hetero atoms independently selected from N, O, and S; R1 is hydrogen, (C1-6) fluoroalkyl, (C3-6) cycloalkyl, or (C1-4) alkyl, wherein the (C1-4) alkyl is unsubstituted or substituted with hydroxy, methoxy, ethoxy, OCH2CH2OH, —CN, imidazolidin-2-one, phenyl, or tetrazole wherein tetrazole is unsubstituted or substituted with (C1-4) alkyl; R2 is H, halogen, (C1-6) fluoroalkyl, (C3-6) cycloalkyl, OR6, SR6, NO2, CN, COR6, C(O)OR6, C(OH)R6, CONR7R8, phenyl or (C1-6) alkyl, wherein the (C1-6) alkyl is unsubstituted or substituted with a hydroxy; R3 is hydrogen, (C1-6)fluoroalkyl , (C3-6) cycloalkyl, (C2-6) alkenyl, phenyl, monocyclic heteroaromatic, bicyclic heteroaromatic, or (C1-4)alkyl wherein (C1-4) alkyl is unsubstituted or substituted with a phenyl; R4 and R5 are independently selected from hydrogen, halogen, (C1-6) alkyl, (C1-6) fluoroalkyl, OR9, SR9, NO2, CN, or COR9; R6 is hydrogen, (C1-6) fluoroalkyl, or (C1-6) alkyl; R7 and R8 are independently hydrogen, or (C1-6) alkyl; R9 is hydrogen, (C1-6) fluoroalkyl, (C1-6) alkyl; Alk is (C1-4) alkylene unsubstituted or substituted with a hydroxy; Y is oxygen, sulfur, SO2, or a bond; X is CH2, C═O, S, O, or SO2; Z is hydrogen, halogen, (C1-6) alkyl, (C1-6)fluoroalkyl, —OH, (C1-6) alkoxy, (C1-6) fluoroalkoxy, (C1-6) alkylthio, (C1-6) acyl, (C1-4)alkylsulfonyl, —OCF3, —NO2, —CN, carboxamido which may be substituted on the nitrogen by one or two (C1-4) alkyl groups, and —NH2 in which one of the hydrogens may be replaced by a (C1-4) alkyl group and the other hydrogen may be replaced by either a (C1-4) alkyl group, a (C1-6) acyl group, or a (C1-4) alkylsulfonyl group; the phenyl of R1, R2 or R3 is independently unsubstituted or substituted with one to three substituents independently selected from Z; the monocyclic heteroaromatic of R3 is unsubstituted or substituted with one to three substituents independently selected from Z; the bicyclic heteroaromatic of R3 is unsubstituted or substituted with one to three substituents independently selected from Z; and salts, solvates, and crystal forms thereof. Also described are the use of the compounds of formula (I) as antagonists of the dopamine D2 receptor and as agents for the treatment of psychosis and bipolar disorders, and pharmaceutical formulations of the compounds of formula (I).
Owner:ELI LILLY & CO
Tricyclic oxazepines as in vivo imaging compounds
Novel compounds of formula (I): suitable for use as in vivo imaging agents are provided as well as precursors suitable for the preparation of said compounds. The present invention also provides pharmaceuticals comprising the compounds and kits for the preparation of the pharmaceuticals. Furthermore, use of the compounds for imaging peripheral benzodiazepine receptors in a subject is provided, in particular for imaging pathological conditions in which PBR are upregulated, e.g. Parkinson's disease, multiple sclerosis, Alzheimer's disease and Huntington's disease, neuropathic pain, arthritis, asthma, atherosclerosis and cancer.
Owner:ARSTAD ERIK +2
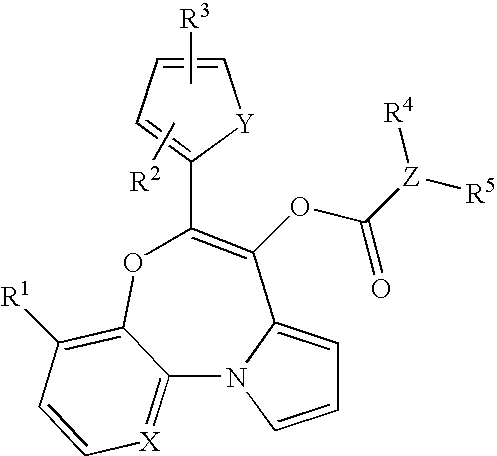
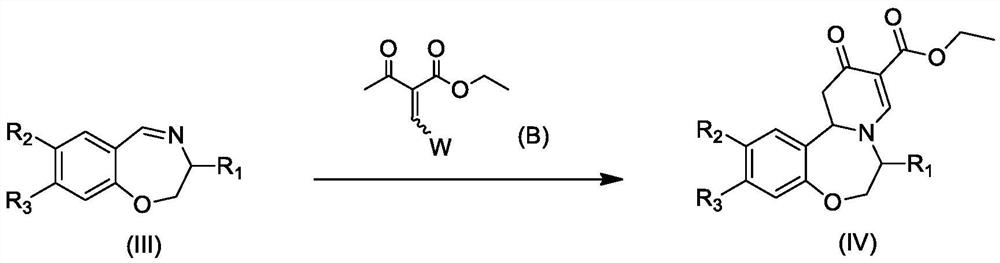
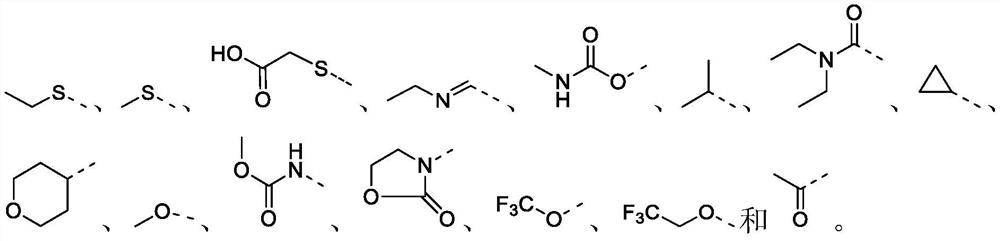
Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof
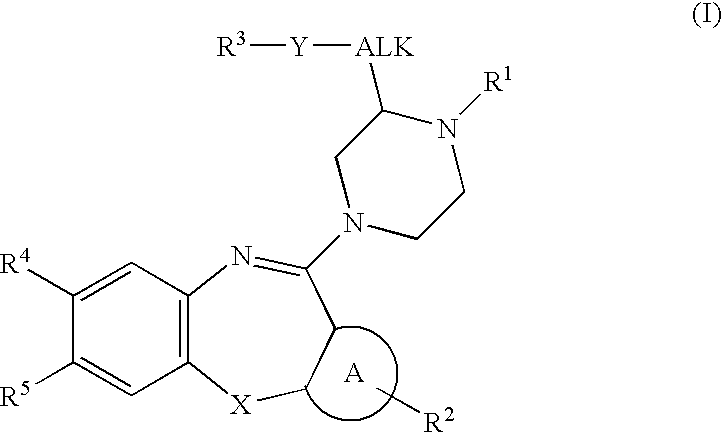
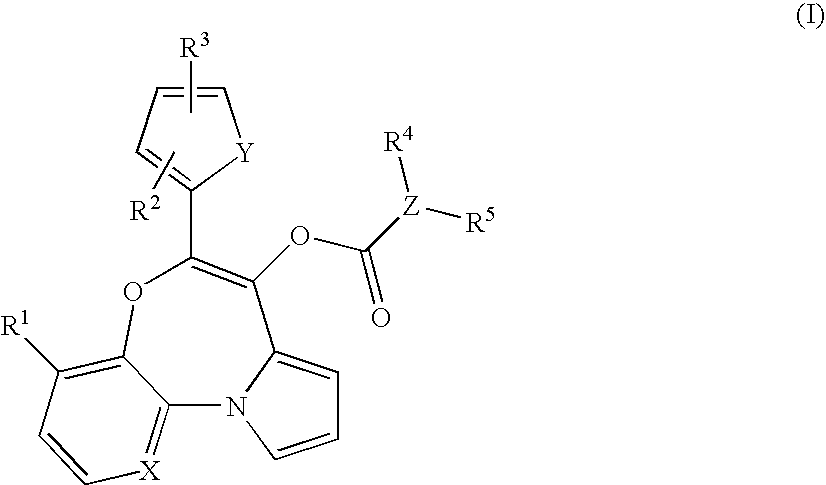
The invention discloses a polysubstituted benzo[b][1,4] oxazepine derivative and a preparation method thereof. The polysubstituted benzo[b][1,4] oxazepine derivative has the structural formula as shown in the description. The benzo[b][1,4] oxazepine derivative with multiple substituents, which cannot be synthesized by other methods, is synthesized, and profound and lasting significance is achieved from the angle of medical chemistry; the method has the advantages of easily available raw materials, high yield, mild reaction conditions, short reaction time, wide substrate range, strong reaction specificity, simple post-treatment and greenness.
Owner:HUAQIAO UNIVERSITY
Oral Formulation of Anhydrous Olanzapine Form I
ActiveUS20080311203A1Improve stabilitySurprising effectBiocideNervous disorderOral medicationFilm-forming agent
The invention relates to a solid formulation for the oral administration of olanzapine that comprises a core of anhydrous olanzapine Form I or a pharmaceutically acceptable salt thereof and, optionally, pharmaceutically acceptable excipients, said core being coated with a functional polymer that acts as filmogenic agent. The method for obtaining it comprises: i) providing anhydrous olanzapine Form I or a salt thereof and, optionally, pharmaceutically acceptable excipients in solid form; ii) providing a functional polymer that acts as filmogenic agent; iii) preparing a dispersion of said functional polymer in an aqueous medium,—and applying the dispersion obtained in step iii) onto the solid form of step i).
Owner:LAB LESVI SL
1,4-Oxazepines as BACE1 and/or BACE2 inhibitors
The present invention relates to 1,4 Oxazepines of formula Ihaving BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
Novel Process For Preparation of a Pharmaceutically Pure Polymorphic Form I of Olanzapine
InactiveUS20080234479A1Satisfactory color stabilitySatisfactory thermal stabilityOrganic active ingredientsNervous disorderSolventChemistry
The invention is directed to a novel method for making crystalline Form I of Olanzapine, wherein crude olanzapine is dissolved in a water-miscible solvent in which it is freely soluble, from which substantially pure polymorphic Form I of Olanzapine is recovered by precipitation.
Owner:SHASUN CHEM & DRUGS LTD
1,4-Oxazepines as BACE1 and/or BACE2 inhibitors
InactiveUS8815841B2Enhanced therapeutic and pharmacological propertyPreserve and restore β-cell massBiocideOrganic active ingredientsDiseaseProphylactic treatment
This invention relates to compounds of the formulawherein and R1 to R3 are as described herein, or to pharmaceutically acceptable salts thereof. These compounds are BACE1 and / or BACE2 inhibitors and can be used as pharmaceuticals for the therapeutic and / or prophylactic treatment of diseases such as Alzheimer's disease, diabetes, particularly type 2 diabetes, and other metabolic disorders.
Owner:F HOFFMANN LA ROCHE & CO AG +1
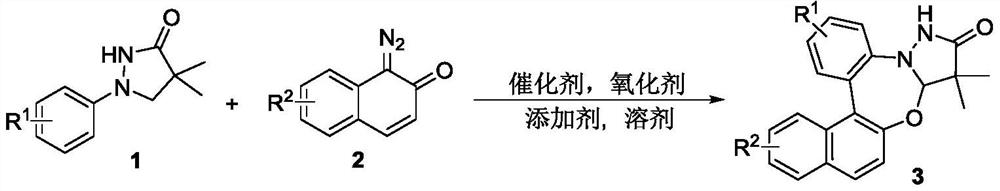
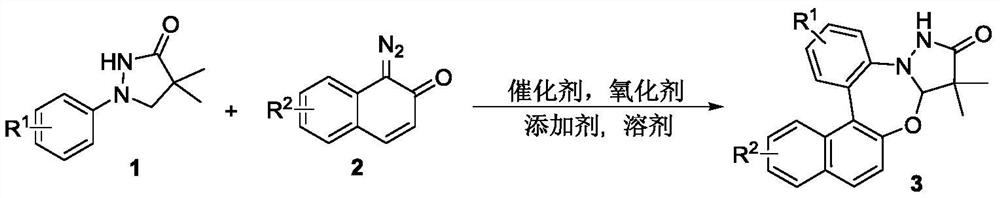
Synthetic method of pyrazolidinone-fused benzo 1,3-oxazepine compound
The invention discloses a synthesis method of a pyrazolidinone-fused benzo 1,3-oxazepine compound, and belongs to the technical field of organic synthesis. 1-aryl pyrazolidinone 1 and a diazonaphthalene ketone compound 2 are used as raw materials, and in the presence of a catalyst, an oxidizing agent and an additive, a heating reaction is performed in an organic solvent to obtain the pyrazolidinone-fused benzo 1,3-oxazepine compound 3. According to the method, the pyrazolidinone-fused benzo 1,3-oxazepine compound is efficiently and regioselectively synthesized through cascade reaction between the 1-aryl pyrazolidinone compound and the diazonaphthalene ketone compound, and the method has the advantages that the raw materials are simple and easy to obtain, the operation is simple and convenient, the condition is mild, the selectivity is good, the substrate application range is wide, and the like.
Owner:HENAN NORMAL UNIV
Olanzapine composition and preparation method thereof
PendingCN113143878ASimple recipeFew accessoriesOrganic active ingredientsNervous disorderPharmacologyLubrication Agents
The invention relates to an olanzapine composition and a preparation method thereof. The olanzapine composition consists of olanzapine, a diluent, a disintegrating agent and a lubricant. The olanzapine composition is simple in formula, few in auxiliary materials, more economical and low in production cost. The preparation method is simple, the powder fluidity of the preparation is good, large-scale production is easy, and the product is more stable.
Owner:杭州新诺华医药有限公司
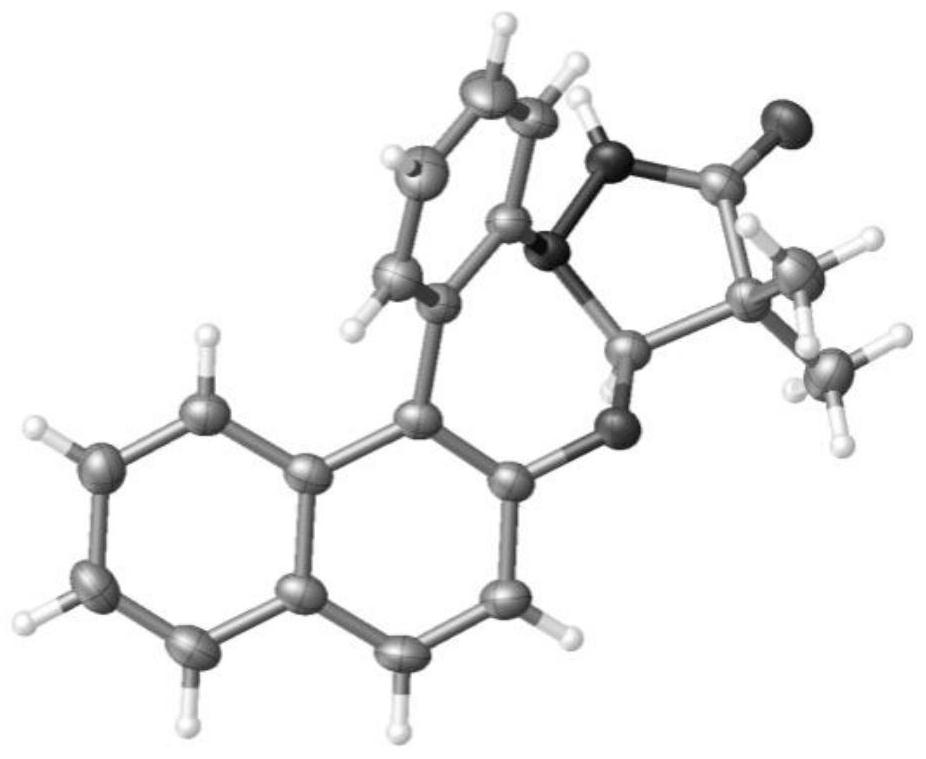
Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof
ActiveCN105294593AInhibitory activityAntibacterial agentsOrganic chemistryAlcoholPhosphatidylcholine-specific phospholipase C
The invention discloses an optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol, wherein the structural formula of an R-type 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol compound is as shown in a formula (I), and the structural formula of an S-type 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol compound is as shown in a formula (II). The invention also discloses application of the compounds in inhibiting the activity of phosphatidylcholine-specific phospholipase C (PC-PLC). According to the optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol provided by the invention, a situation that the R-type / S-type 7-amino-2,3,4,5- tetrahydrobenzo[b] [1,4]oxazepine-3-ol compound as an effective tool lays a foundation for the research on the activity inhibition of bacterial PC-PLC and the research, preparation and development of relevant human disease treatment drugs caused by PC-PLC is found.
Owner:SHANDONG UNIV
1,4-oxazepines as BACE1 and/or BACE2 inhibitors
The present invention relates to 1,4-Oxazepines of formula Ihaving BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
Owner:SIENA BIOTECH +1
Tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives and preparation method and application thereof
The invention discloses tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives of which the structure is shown in a general formula of a figure in the abstract. The invention simultaneously discloses the preparation method of the tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives. The invention further discloses the application of the tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives as a reversible and selective monoamine oxidase inhibitor drug, specifically, the tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine can be applied to drugs or drug compositions for treating and preventing diseases related to aging, alzheimer's disease and parkinson's disease.
Owner:汤文建
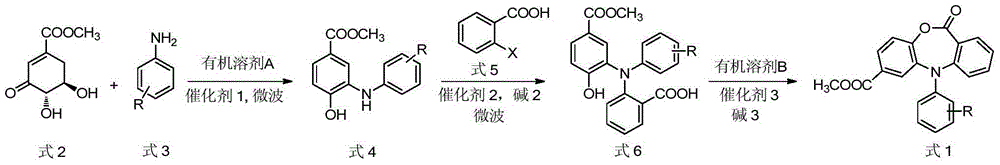
N-aryl oxazepine ketone compound and preparation method thereof
The invention discloses a 5-aryl-7-methoxycarbonyl dibenzo [b, e][1, 4] oxazepine-11(5H)-ketone compound and a preparation method thereof. The preparation method comprises the steps of carrying out condensation, isomerization and dehydration reaction on 3-dehydrogenated methyl shikimate and an aryl amine compound in the presence of an organic solvent and a catalyst in the microwave condition to obtain an aryl-substituted o-aminophenol intermediate; further adding o-halogen benzoic acid and an alkali neutralization catalyst in the microwave condition, cooling, pumping filtering, and carrying out recrystallization to obtain an N, N-diaryl-substituted o-aminophenol intermediate; and in the organic solvent, carrying out molecular lactonization cyclization reaction on the N, N-diaryl-substituted o-aminophenol intermediate in the presence of the catalyst and alkali to obtain the 5-aryl-7-methoxycarbonyl dibenzo [b, e][1, 4] oxazepine-11(5H)-ketone compound. The preparation method is simple, is short in the reaction time, is convenient for postprocessing and is high in the yield.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Benzoxazepine derivatives and use thereof
Owner:TAKEDA PHARMA CO LTD
5-amino-oxazepine and 5-amino-thiazepane compounds as beta secretase antagonists and methods of use
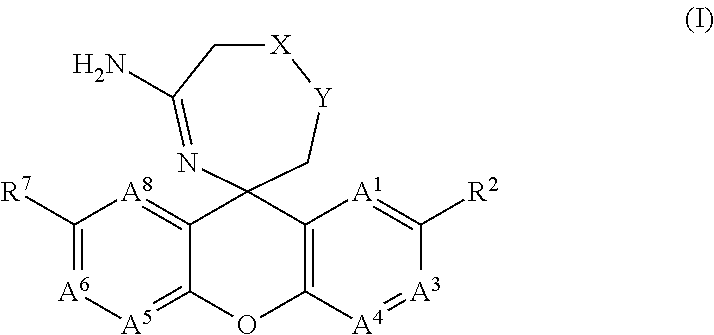
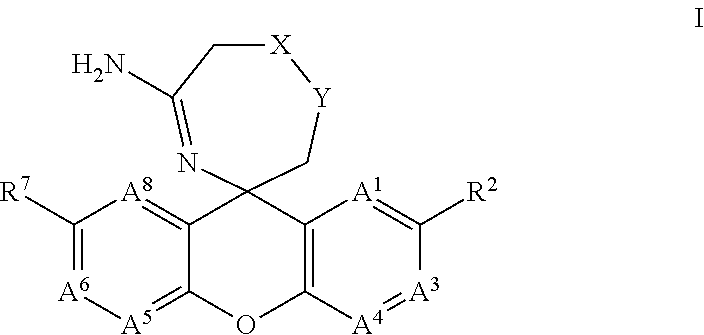
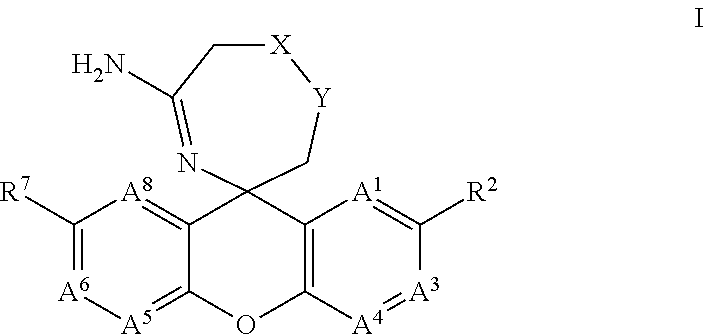
The present invention provides a new class of compounds useful for the modulation of beta-secretase enzyme (BACE) activity. The compounds have a general Formula (I); wherein variables A1, A3, A4, A5, A6, A8, R2, R7, X and Y of Formula (I) are defined herein. The invention also provides pharmaceutical compositions comprising the compounds, and corresponding uses of the compounds and compositions for treatment of disorders and / or conditions related to A-beta plaque formation and deposition, resulting from the biological activity of BACE. Such BACE mediated disorders include, for example, Alzheimer's Disease, cognitive deficits, cognitive impairments, schizophrenia and other central nervous system conditions. The invention further provides compounds of Formulas (II) and sub-formula embodiments of Formula (I) and (II), intermediates and processes and methods useful for the preparation of compounds of Formulas (I)-(II).
Owner:AMGEN INC
Oxazepine [3, 2-b] indole compound and synthetic method thereof
InactiveCN111825686AImplement the buildWide range of derivatizationOrganic chemistryOrganic synthesisCycloaddition
The invention discloses a synthetic method of an oxazepine [3, 2-b] indole compound, and belongs to the technical field of organic synthesis. The synthetic method comprises the following steps: performing [4 + 3] cycloaddition reaction on a crotonate-derived sulfur ylide reagent shown as a formula (2) and a 3-oxindolone substrate shown as a formula (1) under an alkaline condition to obtain the oxazepine [3, 2-b] indole compound shown as a formula (3), the invention also provides the oxazepine [3, 2-b] indole compound. The synthetic method is simple, convenient and efficient, large instrumentsand expensive raw materials are not needed, the reaction conditions are mild, the derivatization range of the substrate is wide, and a foundation is laid for industrial production.
Owner:GUIZHOU MEDICAL UNIV
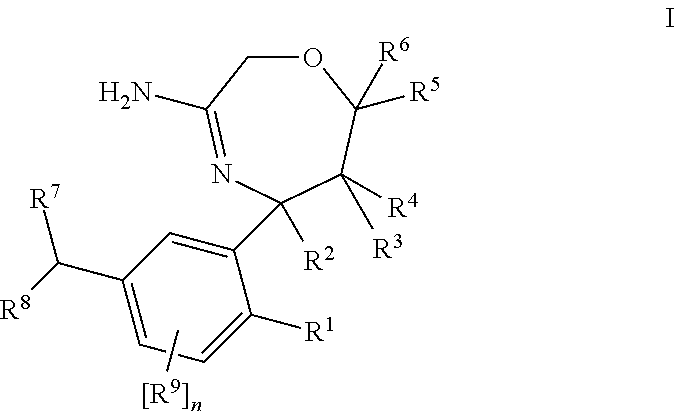
6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof
InactiveCN103965212ALess quantityOrganic active ingredientsOrganic chemistryProliferative diseaseOrganic acid
The invention relates to a 6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative with a structure shown as formula I, tautomers, stereisomers and pharmaceutically acceptable salts thereof, especially pharmaceutically acceptable salts formed by the derivative and inorganic or organic acids or alkalis, and also relates to a preparation method, and application of the receptor tyrosine kinase inhibition property of the compounds in preparation of drugs treating proliferative diseases like cancer. (formula I).
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
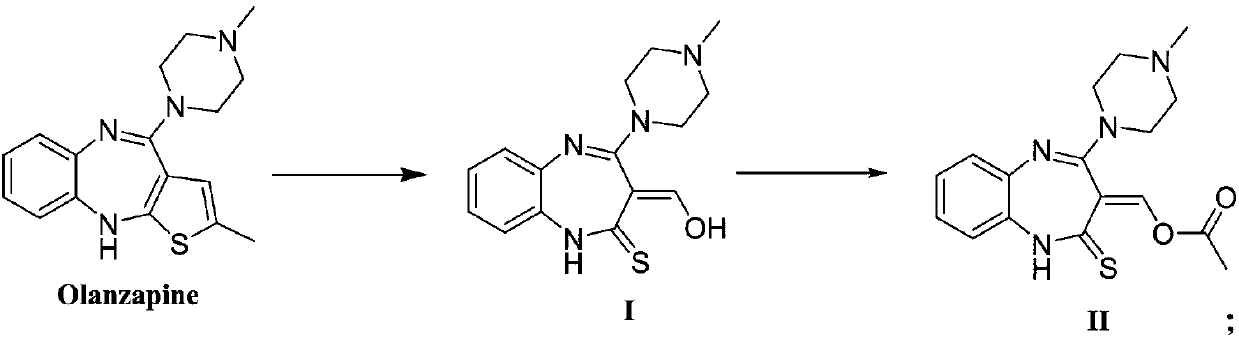
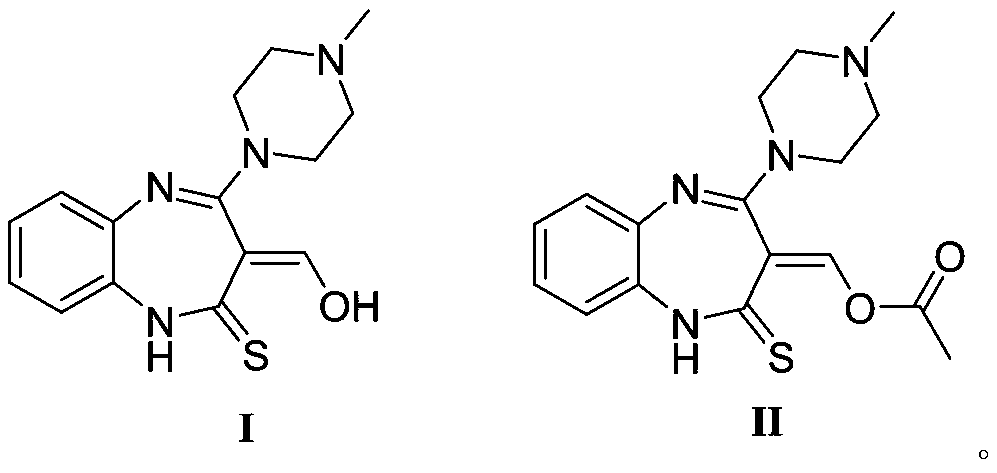
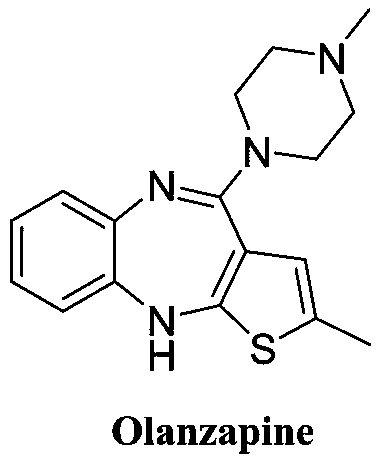
Synthesis method of olanzapine related substances like compound I and compound II
The invention belongs to the technical field of medicine synthesis, and discloses a synthesis method of olanzapine related substances like namely a compound I and a compound II. The preparation methodcomprises the following steps: S1, synthesis of a compound I: adding olanzapine, an organic solvent and water into a reaction flask, sequentially adding Oxone and sodium hydroxide, continuously reacting until olanzapine spots disappear, then adjusting the pH of the system to 6-7, and carrying out extraction and recrystallization to obtain the compound I; S2, synthesis of a compound II: dissolvingthe compound I in an organic solvent, adding inorganic alkali liquor at 0 DEG C, then adding acetyl chloride, continuing to stir and react at 0 DEG C until the compound I disappears, carrying out liquid separation extraction, and carrying out silica gel column chromatography purification to obtain the compound II. The preparation method provided by the invention has the advantages of short reaction route, mild conditions, low overall cost and high yield and purity, and provides reliable material guarantee for subsequent quality control research and safety property research in the olanzapine preparation process.
Owner:湖北扬信医药科技有限公司 +1
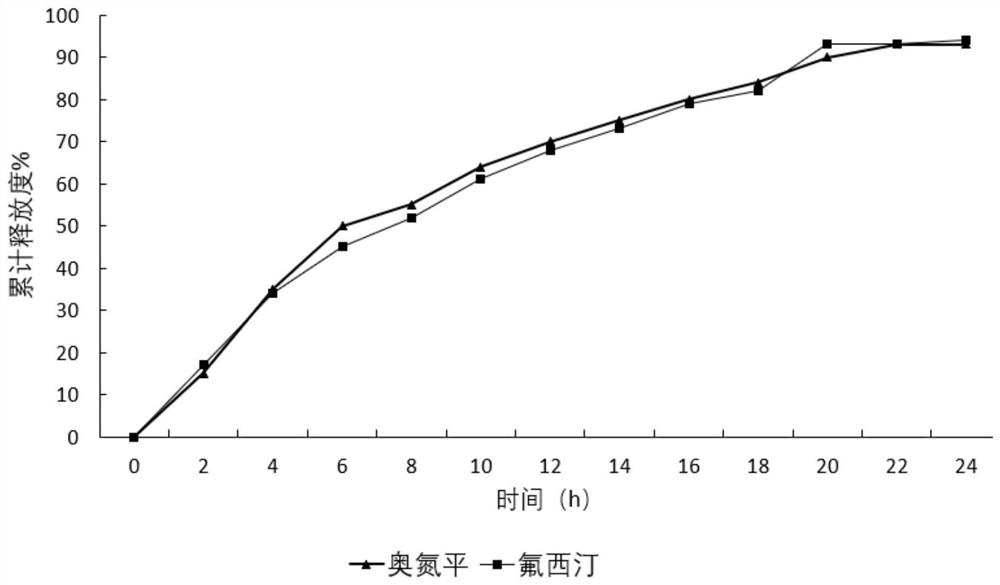
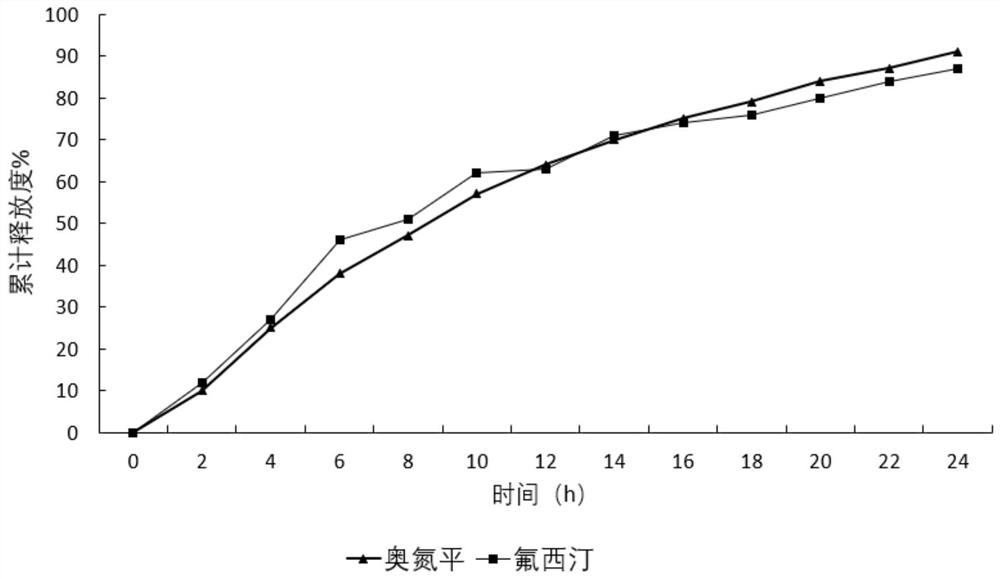
A kind of olanzapine-fluoxetine compound capsule preparation and preparation method thereof
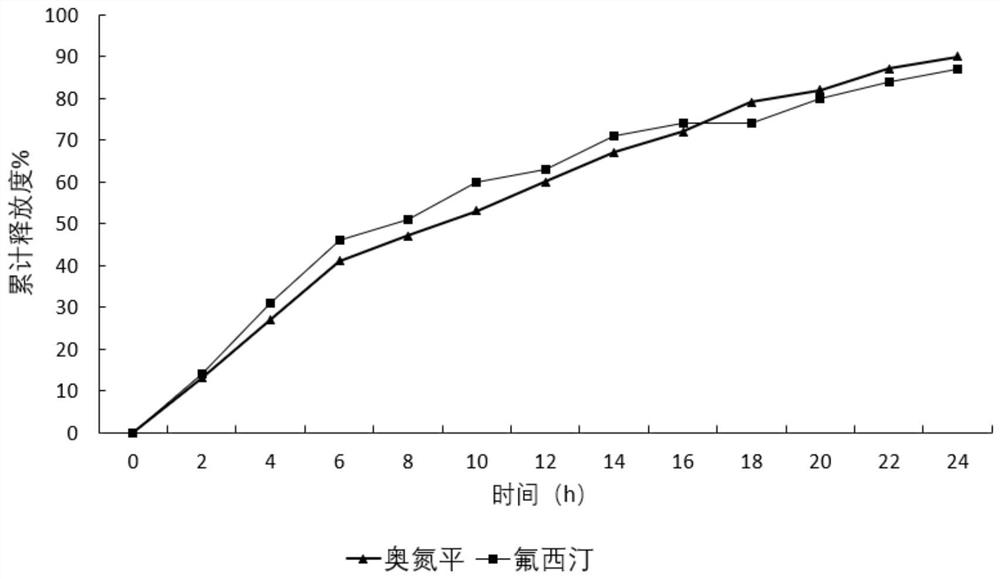
ActiveCN111643506BIncrease contentImprove uniformityOrganic active ingredientsNervous disorderSustained release pelletsPharmaceutical drug
The invention discloses an olanzapine-fluoxetine compound capsule preparation and a preparation method thereof, which are used for the drug treatment of bipolar affective disorder. The compound preparation contains two different pellets, which are respectively enteric-coated olanzapine-containing Flat sustained-release pellets and gastric-soluble fluoxetine-containing sustained-release pellets; the olanzapine-fluoxetine compound capsule preparation of the present invention is combined according to the different in vivo pharmacokinetic laws of olanzapine and fluoxetine Two types of pellets with different ratios and different release parts complete the preparation of products of different specifications; achieve rapid onset of effect, synergistic effect, and reduce the number of times of taking medicine.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
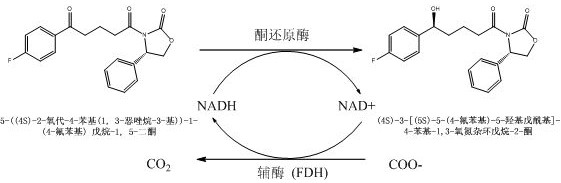
An improved ketoreductase and its application
ActiveCN109055324BChange structureIncrease enzyme activityOxidoreductasesGenetic engineeringKetoneMutant
The invention relates to an improved ketoreductase and belongs to the technical field of biocatalytic synthesis. The ketoreductase mutant of the present invention, the amino acid sequence of the ketoreductase mutant is an amino acid sequence in which the amino acid sequence shown in SEQ ID NO: 1 is mutated, and the mutation sites of the mutated amino acid sequence are respectively: position 140 I is mutated to S, and the optional mutation points include at least one of the following points: the 205th D mutation to E, the 125th H mutation to Q, the 329th N mutation to S, and the 294th L mutation to I. The 150th D is mutated to Q, and the 36th P is mutated to S. The invention provides a ketoreductase and application thereof, which can effectively reduce (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1 , 3-oxazolane-2-one and the cost of industrial production of ezetimibe.
Owner:天津迪嘉医药技术开发有限公司 +1
Preparation method of DGAT1 inhibitor
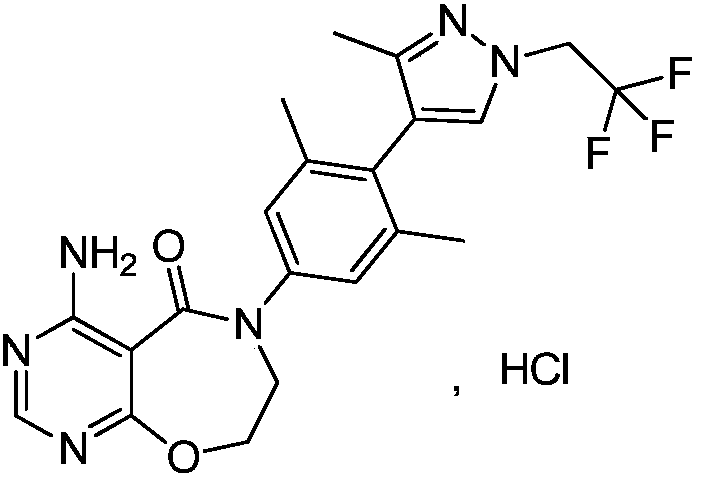
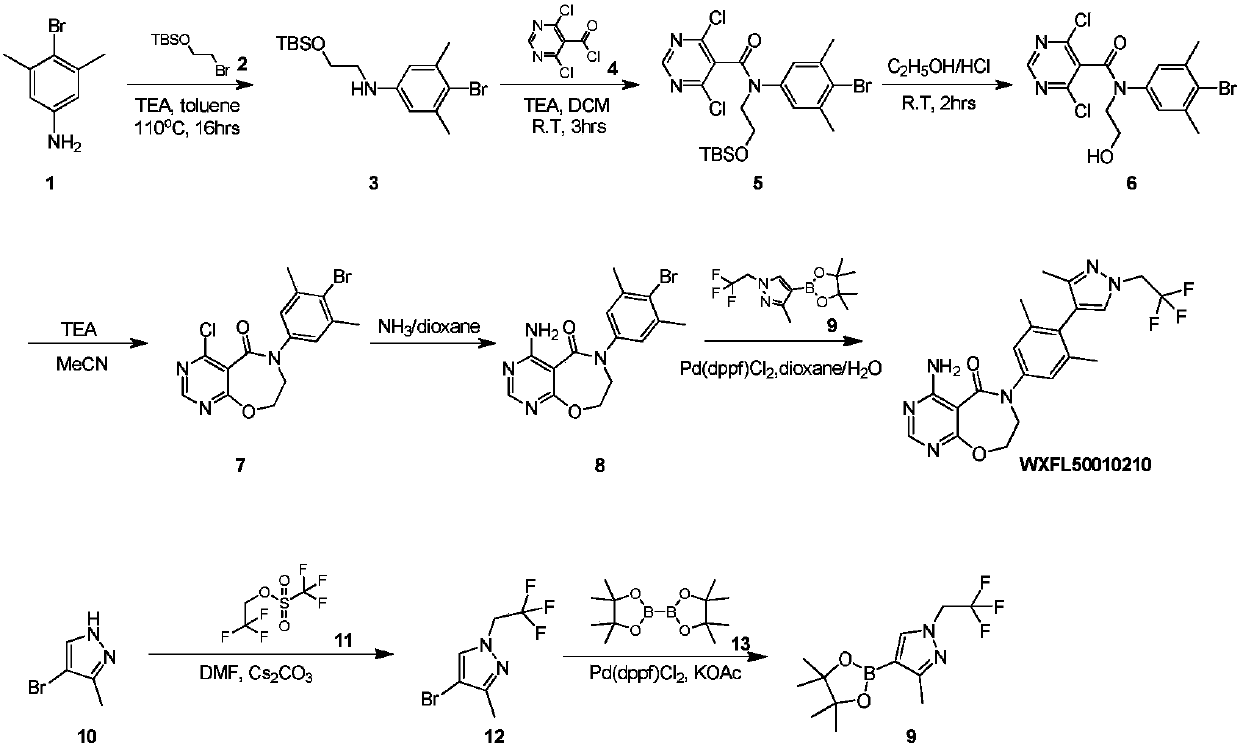
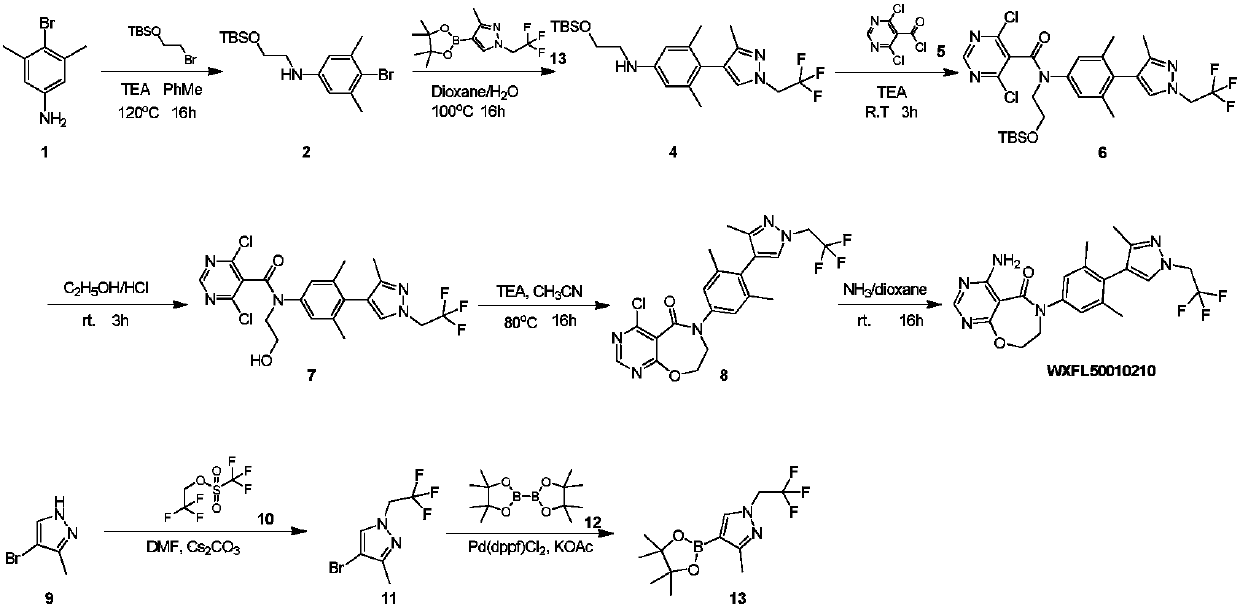
ActiveCN109970763AAvoid postprocessingResidue reductionOrganic chemistryReaction intermediateAniline hydrochloride
Belonging to the field of medical technology, the invention specifically relates to a new preparation method of a DGAT1 inhibitor 4-amino-6-(3, 5-dimethyl-4-(3-methyl-1-(2, 2, 2-trifluoroethyl)-1H-pyrazole-4-yl)phenyl)-7, 8-dihydropyrimidine[5, 4-f][1, 4]oxazepine-5(6H)-one-hydrochloride. The method includes: taking 3, 5-dimethyl-4-(3-methyl-1-(2, 2, 2-trifluoroethyl)-1hydro-pyrazole-4-yl)aniline-hydrochloride as the starting raw material for condensation reaction with chloroethyl chloroformate to generate an intermediate 1; alkylating the intermediate 1 in an alcohol and alkali solution and then performing hydrolysis to generate an intermediate 2; subjecting the intermediate 2 to condensation reaction with 4, 6-dichloropyrimidine-5-acyl chloride to generate an intermediate 3; alkylating the intermediate 3, then carrying out intramolecular ring closure reaction to generate an intermediate 4; subjecting the intermediate 4 to ammoniation in a dioxane solution of ammonia to generate an intermediate 5; and subjecting the intermediate 5 to salt formation in an acetone solution to obtain a finished product. All the reaction intermediates involved in the invention are all solids, thus being easy for purification.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Preparation method of oxazepine compound
ActiveCN112390816AExcellent response resultHigh yieldOrganic active ingredientsOrganic chemistryCombinatorial chemistryOxazepine
The invention discloses a preparation method of an oxazepine compound, and particularly discloses a preparation method and an intermediate of a compound shown as a formula (I) in the specification.
Owner:푸지엔에이키링크바이오테크놀로지컴퍼니리미티드
Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine
ActiveCN108774192BRaw materials are easy to getMild reaction conditionsOrganic chemistry methodsMethylanilinePhenacyl
The invention discloses a method for synthesizing (E)-2-benzal-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine in multiple steps, and belongs to the technical field of organic synthesis. Aethrization of o-nitrobenzyl alcohol and propargyl bromide or propargyl alcohol is performed to obtain 1-nitro-2-(propynyloxymethyl)-benzene, the 1-nitro-2-(propynyloxymethyl)-benzene is reduced by iron powder / aceticacid or NiCl2 (dppp) / tetrahydroxy diborane / organic base to obtain 2-(propynyloxymethyl)-aniline, the 2-(propynyloxymethyl)-aniline is coupled with iodobenzene by Sonogashira to obtain 2-[(3-phenyl-2-alkynyloxy)methyl]anilide, and the (E)-2-benzal-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine is obtained by benzoyl protection of amino group, ring closure by cuprous bromide / cesium carbonate and deprotection under basic conditions.
Owner:山东三牧新材料科技有限公司
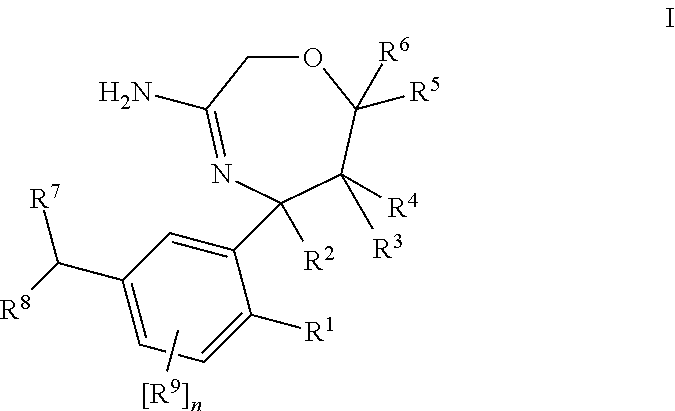
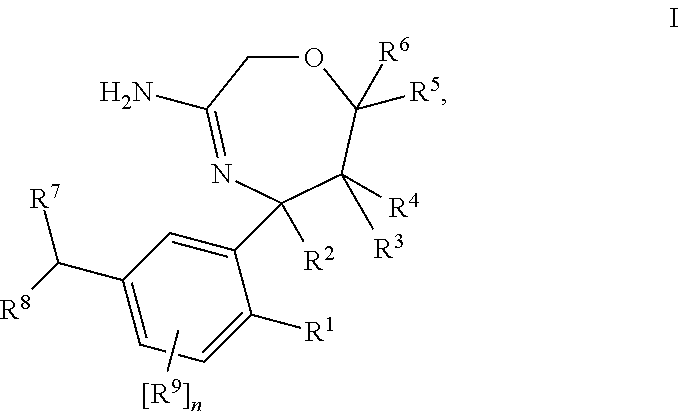
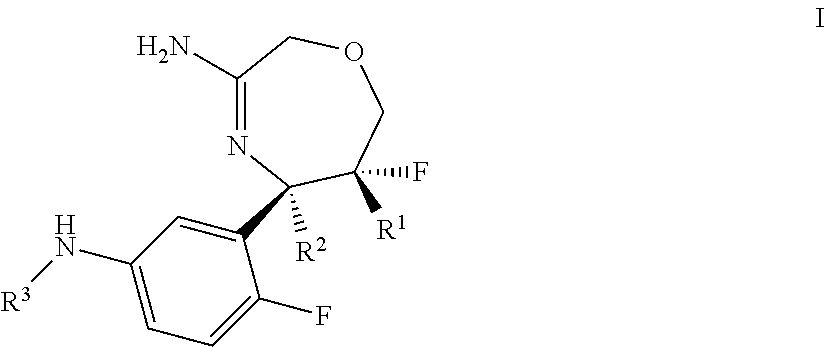
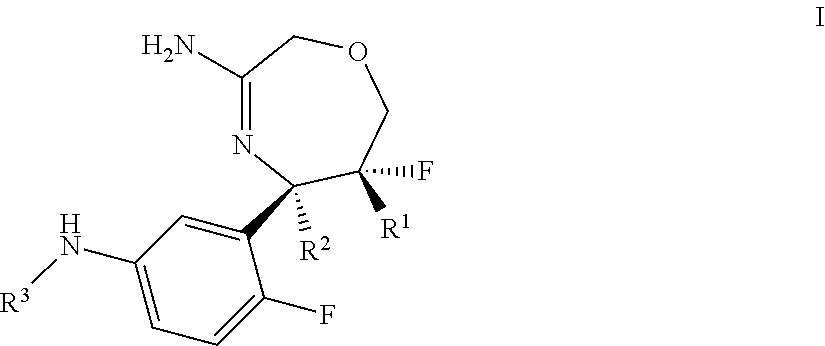
Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors
The present invention provides spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines of formula I having BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections](https://images-eureka.patsnap.com/patent_img/6c3f089e-e4cf-4756-aced-265f1c834861/US09216996-20151222-C00001.PNG)
![Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections](https://images-eureka.patsnap.com/patent_img/6c3f089e-e4cf-4756-aced-265f1c834861/US09216996-20151222-C00002.PNG)
![Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections](https://images-eureka.patsnap.com/patent_img/6c3f089e-e4cf-4756-aced-265f1c834861/US09216996-20151222-C00003.PNG)
![Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/934a1904-9305-41a6-ae9f-59ca54622ec6/BDA0000838768520000011.PNG)
![Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/934a1904-9305-41a6-ae9f-59ca54622ec6/BDA0000838768520000021.PNG)
![Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof Polysubstituted benzo[b][1,4] oxazepine derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/934a1904-9305-41a6-ae9f-59ca54622ec6/BDA0000838768520000031.PNG)
![Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof](https://images-eureka.patsnap.com/patent_img/a2213ed9-4a5f-410f-9242-64a5d97591bc/HDA0000871310800000011.PNG)
![Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof](https://images-eureka.patsnap.com/patent_img/a2213ed9-4a5f-410f-9242-64a5d97591bc/HDA0000871310800000012.PNG)
![Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof Optical pure 7-amino-2,3,4,5-tetrahydrobenzo[b] [1,4]oxazepine-3-ol and applications thereof](https://images-eureka.patsnap.com/patent_img/a2213ed9-4a5f-410f-9242-64a5d97591bc/BDA0000871310790000021.PNG)
![Tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives and preparation method and application thereof Tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b0a97f89-d403-4c8b-baa9-54071c6e057c/150901091621.PNG)
![Tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives and preparation method and application thereof Tricyclo-pyrazole [1,5-d] [1,4] benzo oxazepine-one derivatives and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b0a97f89-d403-4c8b-baa9-54071c6e057c/540051DEST_PATH_IMAGE002.PNG)
![Oxazepine [3, 2-b] indole compound and synthetic method thereof Oxazepine [3, 2-b] indole compound and synthetic method thereof](https://images-eureka.patsnap.com/patent_img/714c3922-2a25-49f2-b547-cc83968fd220/FDA0002595095280000011.png)
![Oxazepine [3, 2-b] indole compound and synthetic method thereof Oxazepine [3, 2-b] indole compound and synthetic method thereof](https://images-eureka.patsnap.com/patent_img/714c3922-2a25-49f2-b547-cc83968fd220/FDA0002595095280000012.png)
![Oxazepine [3, 2-b] indole compound and synthetic method thereof Oxazepine [3, 2-b] indole compound and synthetic method thereof](https://images-eureka.patsnap.com/patent_img/714c3922-2a25-49f2-b547-cc83968fd220/FDA0002595095280000021.png)
![6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof 6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/95abdf9a-d306-4e72-9dd8-ef8a9edb4e9b/FDA00002811194000011.png)
![6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof 6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/95abdf9a-d306-4e72-9dd8-ef8a9edb4e9b/FDA00002811194000031.png)
![6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof 6, 7, 8, 9-tetrahydro-[1, 4]oxazepine[3, 2-g] quinazoline derivative, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/95abdf9a-d306-4e72-9dd8-ef8a9edb4e9b/FDA00002811194000041.png)
![Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/1d023286-0450-4a13-9bae-9fd0a913e828/BDA0001705505380000021.png)
![Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/1d023286-0450-4a13-9bae-9fd0a913e828/BDA0001705505380000061.png)
![Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/1d023286-0450-4a13-9bae-9fd0a913e828/BDA0001705505380000091.png)
![Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors](https://images-eureka.patsnap.com/patent_img/1f199daf-bf26-44d0-a1ec-026f354a4227/FPA0000181659680000011.png)
![Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors](https://images-eureka.patsnap.com/patent_img/1f199daf-bf26-44d0-a1ec-026f354a4227/FPA0000181659680000091.png)
![Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors](https://images-eureka.patsnap.com/patent_img/1f199daf-bf26-44d0-a1ec-026f354a4227/BPA0000181659690000011.png)