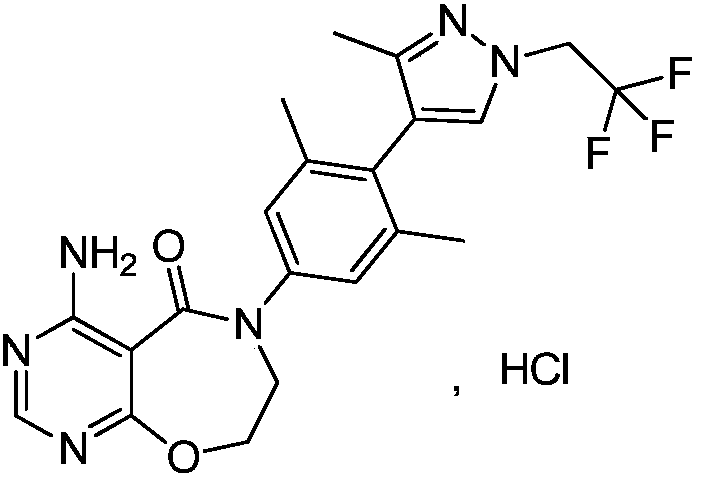
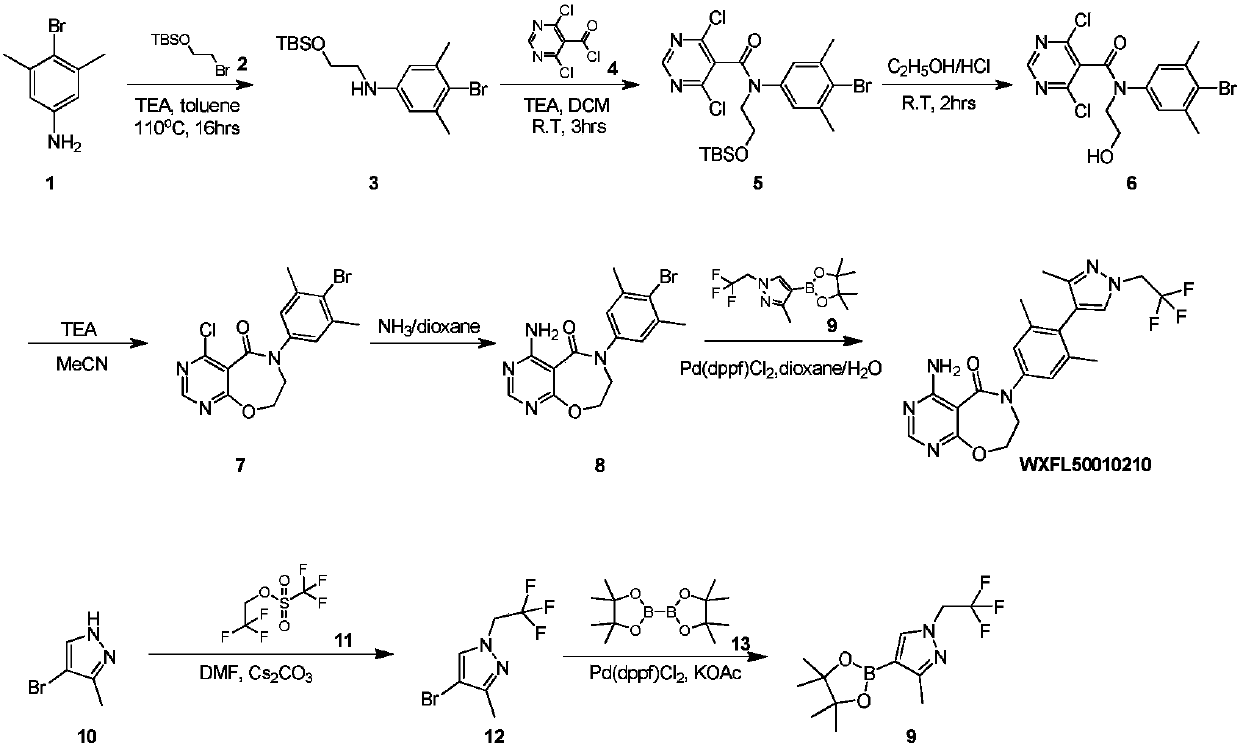
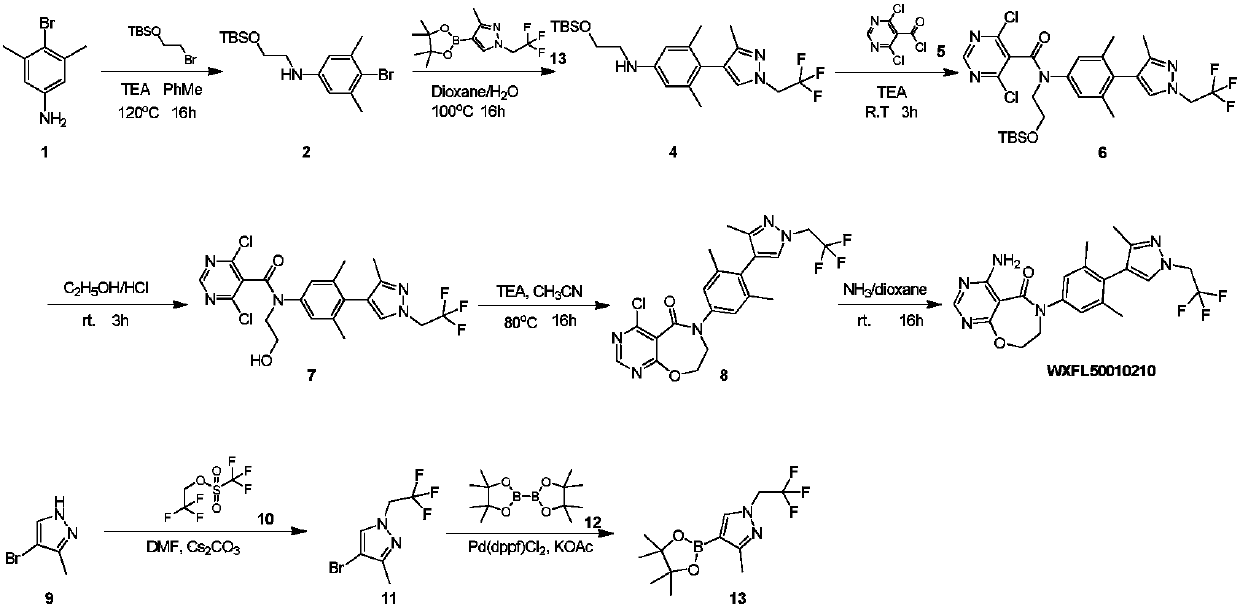
Preparation method of DGAT1 inhibitor
A technology of inhibitor and acid binding agent, applied in the field of medicine, can solve the problems of unsuitable purification, inability to remove, unfavorable process amplification, etc., and achieves the effect of avoiding post-treatment process, suitable process amplification, and reducing residual amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1: Condensation procedure, synthesis of intermediate 1
[0036] 950 g of starting material 3,5-dimethyl-4-(3-methyl-1-(2,2,2 trifluoroethyl)-1 hydrogen-pyrazol-4-yl)aniline-hydrochloride and 9.5L of dichloromethane were added to a 50L reactor, 630g of pyridine was added, the temperature of the reactor was adjusted to -5°C, 472g of chloroethyl chloroformate was added dropwise into the reactor, and stirred for 2 hours.
[0037] After the reaction was complete, water was added for extraction, and the organic phase was concentrated in vacuo to obtain 1.09 kg of Intermediate 1 with a yield of 98%.
[0038] Step 2: Cyclization, hydrolysis process, synthesis of intermediate 2
[0039] 1.09kg of Intermediate 1 and 11L of methanol were added to a 30L reactor, a solution of sodium hydroxide (408.9g) in water (2.2L) was added, the temperature of the reactor was adjusted to 80°C, and stirred at this temperature for 16 hours.
[0040] After the reaction was complete, the syst...
Embodiment 2
[0054] Step 1: Condensation procedure, synthesis of intermediate 1
[0055] 1.5 kg of starting material 3,5-dimethyl-4-(3-methyl-1-(2,2,2 trifluoroethyl)-1 hydrogen-pyrazol-4-yl)aniline-hydrochloric acid Add salt and 15L of dichloromethane into a 50L reactor, add 667g of pyridine, adjust the temperature of the reactor to -8°C, add 1.2kg of chloroethyl chloroformate dropwise into the reactor, and stir for 2 hours.
[0056] After the reaction was complete, water was added for extraction, and the organic phase was concentrated in vacuo to obtain 1.63 kg of Intermediate 1 with a yield of 99%.
[0057] Step 2: Cyclization, hydrolysis process, synthesis of intermediate 2
[0058] 1.6kg of Intermediate 1 and 16L of methanol were added to a 30L reactor, a solution of sodium hydroxide (985g) in water (3.2L) was added, the temperature of the reactor was adjusted to 80°C, and stirred at this temperature for 16 hours.
[0059] After the reaction was complete, the system was concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com