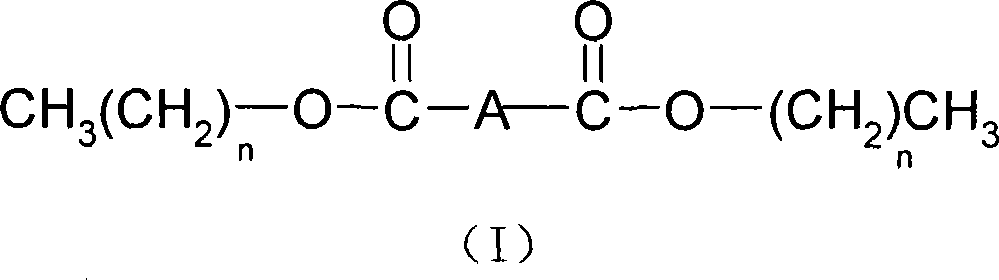
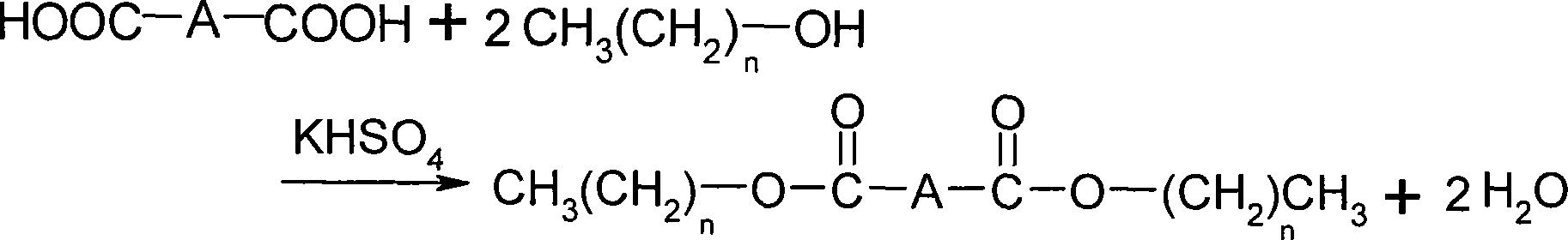Method for catalyzing and synthesizing bibasic carboxylic ester by potassium hydrogen sulfate
A technology of potassium bisulfate catalysis and dibasic carboxylate, which is applied in the preparation of carboxylate, chemical instruments and methods, and the preparation of organic compounds. Problems such as complicated regeneration and complicated post-treatment process can be achieved to facilitate long-term operation, easy storage and transshipment, and reduce the loss of chemical raw materials and products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of dimethyl adipate
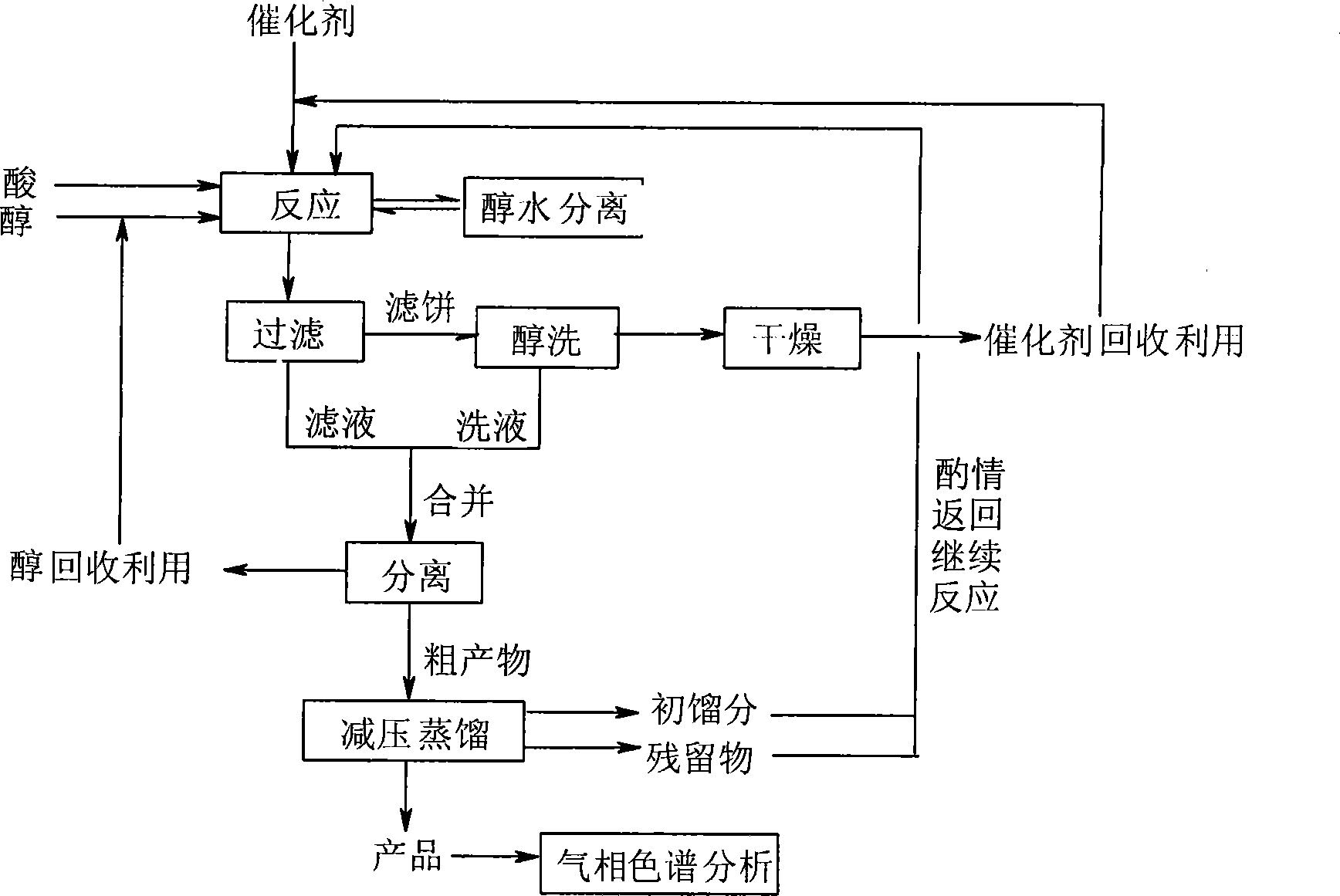
[0025] In a three-necked flask equipped with a thermometer and a reflux condenser, add 14.61g of adipic acid (0.1mol), 20mL of anhydrous methanol (0.5mol), 4g (0.3mol) of potassium hydrogensulfate, and heat the oil bath to 100°C. The reaction was carried out under reflux, the progress of the reaction was monitored by TLC, and the reaction was completed after 2 hours. After cooling, the reactant is filtered, the filter cake is washed with methanol, and the recovered potassium bisulfate can be reused after being dried. Combine the filtrate and washing liquid, evaporate unreacted methanol in the filtrate, methanol can be recycled, and the residue is the crude product of dimethyl adipate. The crude product is distilled under reduced pressure and collected at 115-117°C / 1.73 The fraction of KPa can obtain a colorless transparent liquid product with a yield of 98.55%, and a purity of 98.99% through gas chromatography detection...
Embodiment 2
[0033] Embodiment 2: the synthesis of diethyl adipate
[0034] In a three-necked flask equipped with a thermometer and a reflux condenser, add 14.61g of adipic acid (0.1mol), 30mL of absolute ethanol (0.5mol), and 4g of potassium bisulfate, heat the oil bath to 80°C, and reflux for 2 hours Finish. After cooling, the reactant is filtered, the filter cake is washed with ethanol, and the recovered potassium bisulfate can be reused after being dried. Combine the filtrate and washing liquid, evaporate the unreacted ethanol in the filtrate, the ethanol can be recycled, and the residue is the crude product of diethyl adipate. The crude product is then distilled under reduced pressure to obtain a colorless transparent liquid product 19.6 g, yield 97.13%.
Embodiment 3
[0035] Embodiment 3: the synthesis of n-butyl adipate
[0036] In a three-necked flask equipped with a thermometer and a reflux condenser, add 7.31g of adipic acid (0.05mol), 23mL of n-butanol (0.25mol), and 2g of potassium bisulfate, heat the oil bath to 120°C, and reflux for 4 hours Finish. After cooling, the reactant is filtered, the filter cake is washed with n-butanol alcohol, and the recovered potassium bisulfate can be reused after being dried. Combine the filtrate and washing liquid, steam the unreacted n-butanol alcohol in the filtrate, the n-butanol alcohol can be recycled, and the residue is the crude product of dimethyl adipate, and the crude product is then distilled under reduced pressure, finally 12.47 g of a colorless transparent liquid product was obtained with a yield of 95.90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com