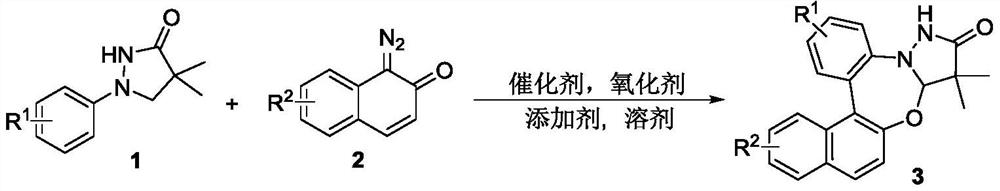
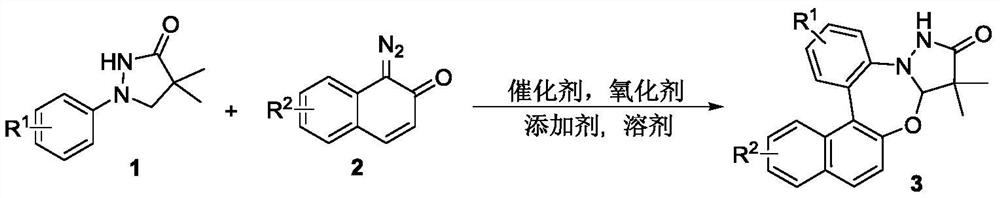
Synthetic method of pyrazolidinone-fused benzo 1,3-oxazepine compound
A technology of pyrazolidone and oxazepine, which is applied in the field of organic synthesis, can solve the problems of low atom economy, generation of by-products, and difficulty in obtaining, and achieves the effects of high atom economy, simple operation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under an argon atmosphere, 1a, 2a, an organic solvent, an oxidizing agent, an additive and a catalyst were sequentially added into a 15mL reaction bottle, the stopper was covered and sealed, and it was placed in an oil bath to raise the temperature and stir for reaction. After the reaction was completed, it was cooled to room temperature, extracted, dried, spin-dried, and separated through a silica gel column (petroleum ether / ethyl acetate=3 / 1) to obtain the white solid product 3a. The reaction equation is expressed as:
[0024]
[0025] By changing the reaction conditions such as reaction solvent, oxidant, additive, catalyst, reaction temperature and equivalent ratio between reactants, the experimental results are shown in Table 1.
[0026] Synthesis of 3a under different reaction conditions in table 1 a
[0027]
[0028]
Embodiment 2
[0030]
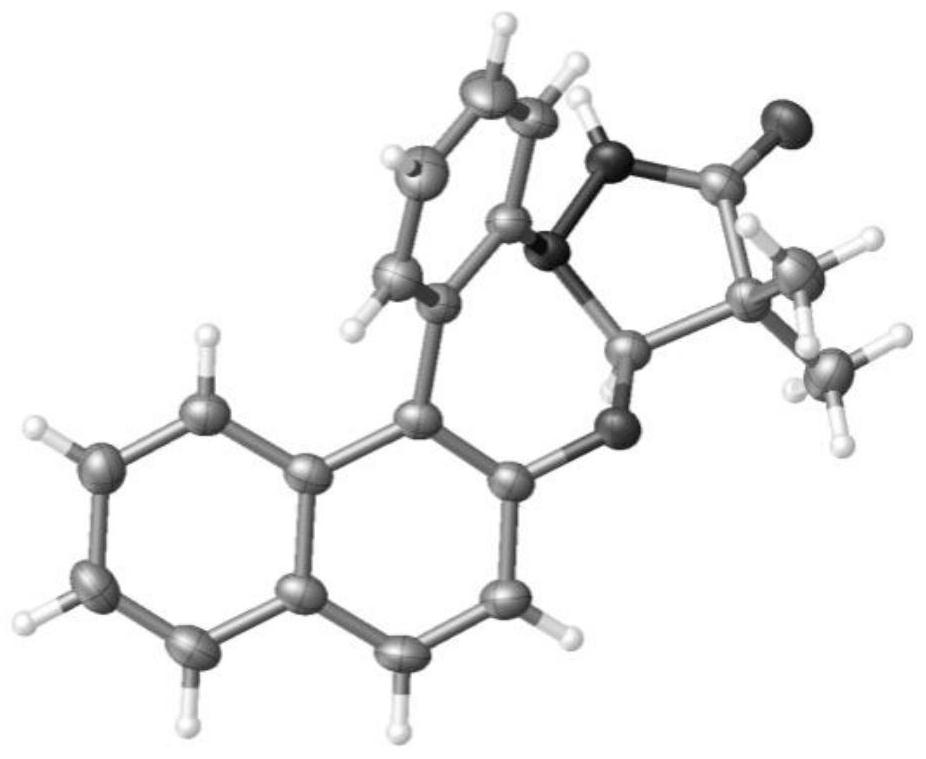
[0031] Under argon atmosphere, 1a (57.0mg, 0.3mmol), 2a (76.5mg, 0.45mmol), acetonitrile (3mL), [RhCp*Cl 2 ] 2 (4.7mg, 0.0075mmol), silver acetate (100.1mg, 0.6mmol) and 2,4,6-trimethylbenzoic acid (98.5mg, 0.6mmol), cover the stopper and place it in an oil bath at 100°C The reaction was stirred for 12 hours. After the reaction was completed, it was cooled to room temperature, extracted, dried, spin-dried, and separated through a silica gel column (petroleum ether / ethyl acetate=3 / 1) to obtain a white solid product 3a (74.3mg, 75%) . The X-ray single crystal diffraction pattern is figure 1 . mp 244.8-245.7℃. 1 H NMR (600MHz, CDCl 3 ):δ8.13-8.12(m,1H),8.10(s,1H),7.91-7.89(m,1H),7.86(d,J=9.0Hz,1H),7.57(dd,J 1 =7.8Hz,J 2 =1.8Hz,1H),7.50-7.47(m,3H),7.42-7.39(m,1H),7.28(d,J=9.0Hz,1H),7.23(td,J 1 =7.8Hz,J 2 =1.2Hz,1H),5.52(s,1H),1.29(s,3H),1.13(s,3H). 13 C{ 1 H}NMR (150MHz, CDCl 3 ): δ178.3, 151.2, 146.5, 132.6, 132.0, 131.5, 130.2, 128.8, 128.6, 128.4, 126.9, ...
Embodiment 3
[0033] According to the method and steps of Example 2, by changing the reactants 1 and 2, various pyrazolidinone-benzo-1,3-oxazepine compounds 3 were synthesized, and the specific results are shown in Table 2.
[0034] Table 2 Synthesis of various pyrazolidinone-benzo-1,3-oxazepine compounds 3 a,b
[0035]
[0036] Representative product characterization data are as follows:
[0037] 8,8,14-Trimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10H)-one(3b)
[0038] White solid (68.1mg, 66%), mp 241.2-242.2℃. 1 H NMR (400MHz, CDCl 3 ):δ8.14-8.12(m,1H),8.08(s,1H),7.89-7.86(m,1H),7.82(d,J=8.8Hz,1H),7.51-7.45(m,2H), 7.37(d,J=1.2Hz,1H),7.34(d,J=8.4Hz,1H),7.25(s,1H),7.19(dd,J 1 =8.4Hz,J 2 =1.6Hz,1H),5.45(s,1H),2.39(s,3H),1.27(s,3H),1.12(s,3H). 13 C{ 1 H}NMR (100MHz, CDCl 3): δ178.3, 151.2, 144.1, 132.6, 132.5, 132.2, 131.5, 130.1, 129.4, 128.6, 128.5, 126.8, 125.7, 125.4, 124.4, 119.8, 115.9, 108.9, 43.5, 21.5, 208.8 m / z:[M+Na] + Calcd for C 22 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com