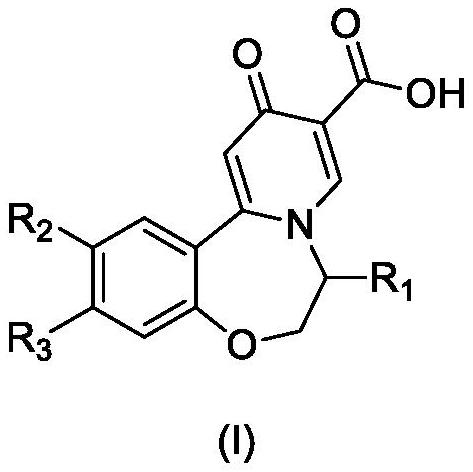
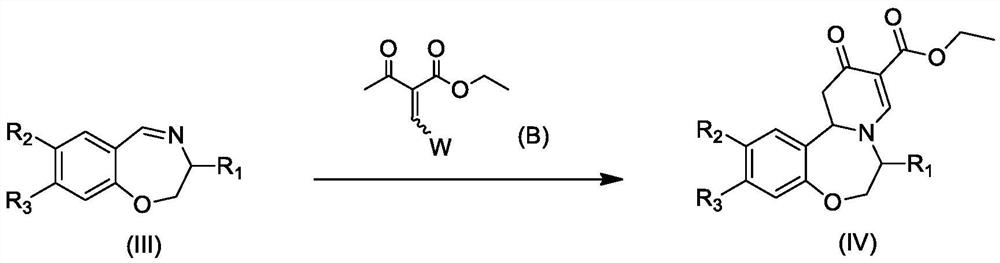
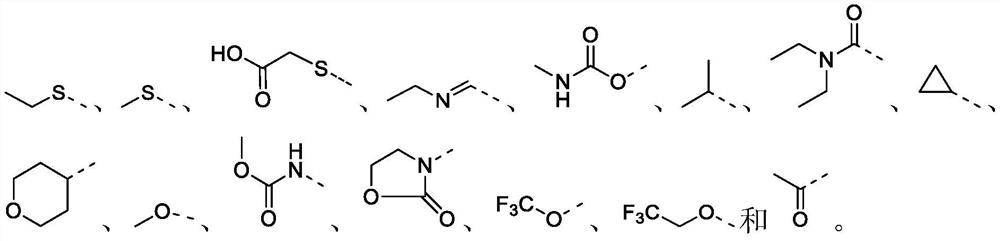
Preparation method of oxazepine compound
A compound and alkoxy technology, which is applied in the field of preparation of oxazepine compounds, can solve the problems of poor curative effect of HBsAg and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the preparation of compound 4
[0079]
[0080] Step 1: Compound 2
[0081] In a 50L reactor, add ethanol (6.0L) and water (6.0L) at room temperature, and then add compound 1 (3000.00g, 22.871mol), stir evenly, and insoluble. Sodium hydroxide (1006.20 g, 25.155 moles) was dissolved in water (6.0 liters), and the temperature was controlled at 20-30° C., and slowly added dropwise to the reaction kettle, and the dripping was completed in about 0.5 hours. Will Boc 2 O (5989.80 g, 27.445 mol) was dissolved in ethanol (3.0 liters), and the temperature was controlled at 20-30°C, and slowly added dropwise to the reaction kettle, and the drop was completed in about 1 hour. Stir and react at a temperature of 25-30°C for 16 hours. TLC central control detects that the reaction is complete. The reaction solution is depressurized at -0.095Mpa and rotated at 50°C to evaporate a mixture of ethanol and water (9.2 liters). Add ethyl acetate to the concentrate Esters (...
Embodiment 2
[0089] Embodiment 2: the preparation of compound 6
[0090]
[0091] In a 50L reaction kettle, add water (20.0 liters) at room temperature, add compound 5 (4025.94 g, 21.634 moles), and control the temperature at 26-35 ° C to stir and react for 4 hours. The HPLC central control detects that the reaction is complete, and the reaction solution is allowed to stand , separate the lower organic phase, extract the upper aqueous phase with methyl tert-butyl ether (4.0 liters * 2), separate the layers, combine the organic phases, wash with saturated brine (4.0 liters), and wash the organic phase with anhydrous sodium sulfate ( 1500.00 g) was dried, filtered, and the mother liquor was collected, and decompressed at -0.095Mpa. Rotary evaporation was performed at 45°C, the solvent was removed, and the mixture was concentrated to obtain light yellow liquid compound 6 (3230.13 g, yield 93.89%, purity 99.397%).
[0092] 1H NMR (400MHz, CHLOROFORM-d) δ=9.21(d, J=6.0Hz, 1H), 4.27(q, J=7.2...
Embodiment 3
[0093] Embodiment 3: the preparation of compound 13
[0094]
[0095] Step 1: Compound 8
[0096] In a 50L reactor, add absolute ethanol (20.0 liters) and water (10.0 liters) at room temperature, then add compound 7 (5000.00 grams, 32.226 moles), potassium carbonate (4946.65 grams, 35.450 moles) and 1-bromo- 3-Methoxypropane (5601.50 g, 35.450 mol), stirred well. The temperature was raised to 75-85°C for reflux reaction for 16 hours, and the reaction was completed by the HPLC control detection. The reaction liquid was depressurized at -0.095Mpa, and 50°C was rotary evaporated to distill off the mixture of ethanol and water (22.0 L). Add water (15.0 L) to the concentrated solution to dilute, extract with ethyl acetate (15.0 L), separate the layers, wash the ethyl acetate layer with saturated brine (10.0 L), separate the layers, and use anhydrous sodium sulfate for the organic phase (2.0 kg) was dried, filtered, and the mother liquor was collected, decompressed at -0.095Mp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com