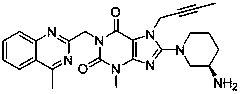
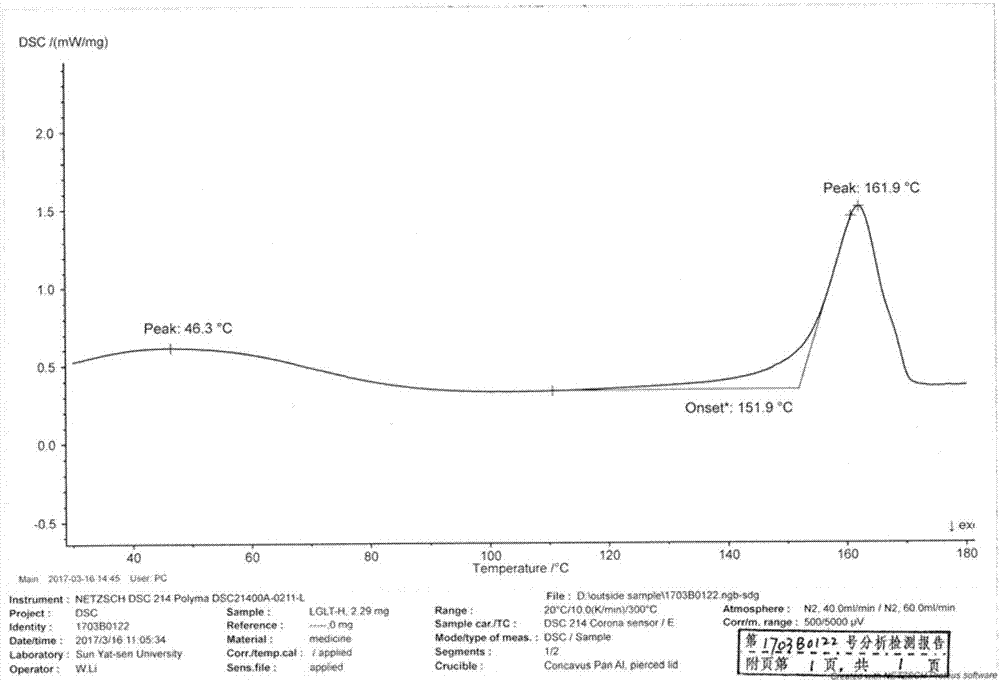
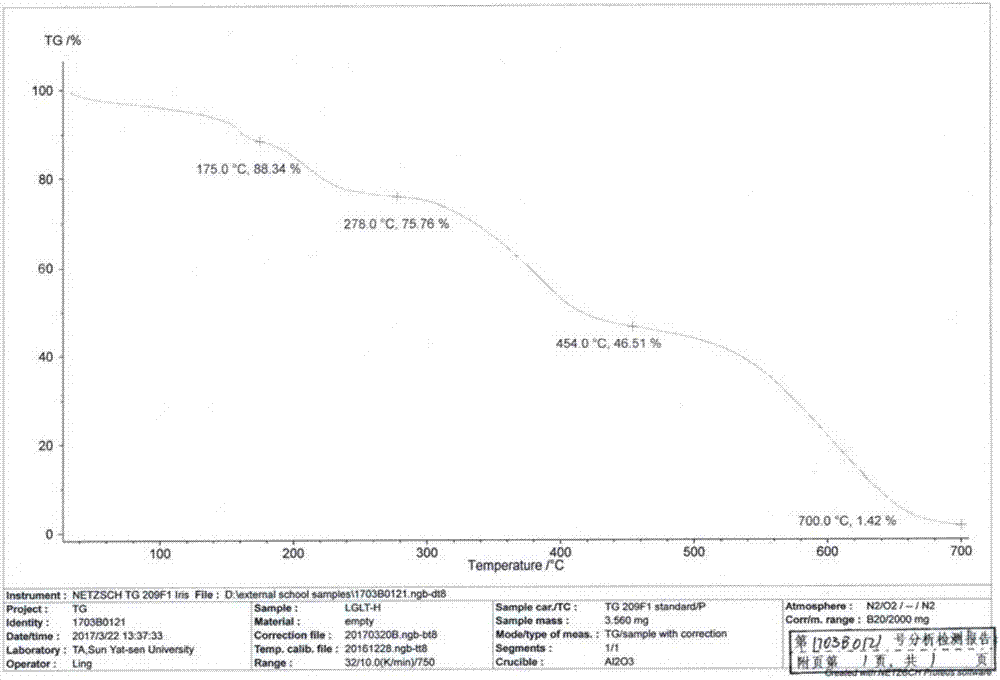
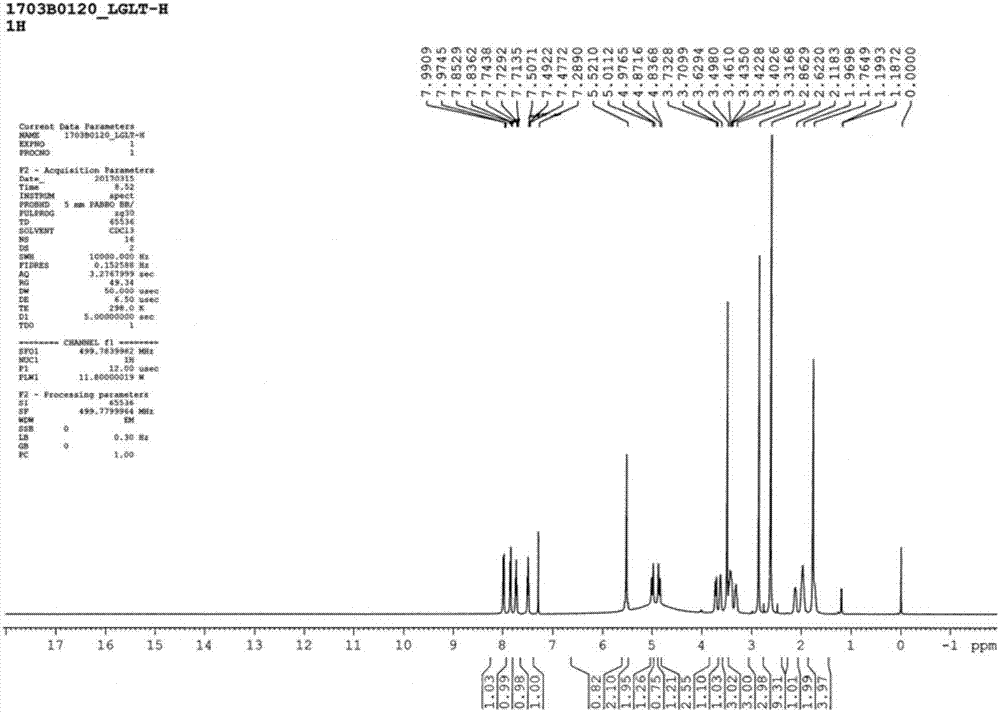
Patents
Literature
136 results about "Linagliptin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
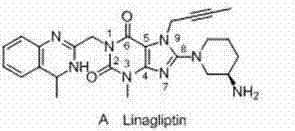
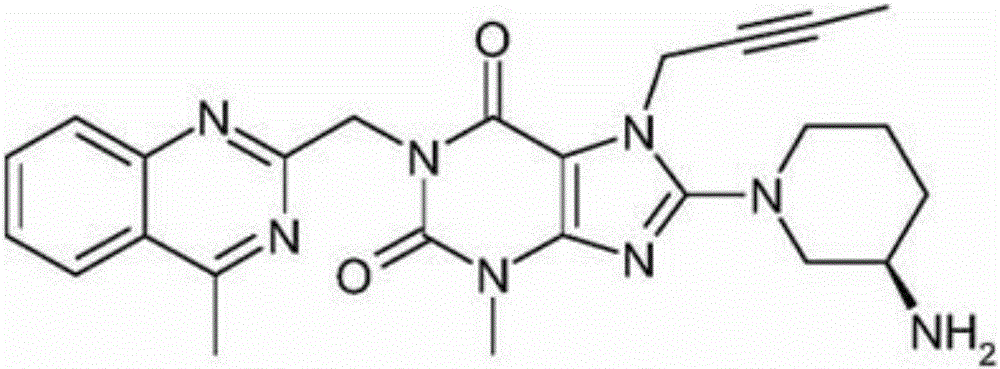
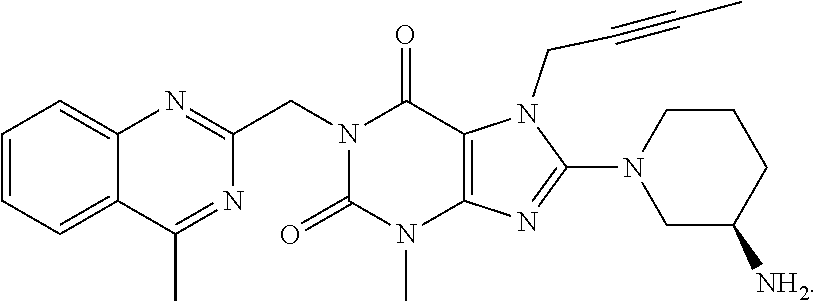
Linagliptin is used with a proper diet and exercise program and possibly with other medications to control high blood sugar. It is used by people with type 2 diabetes.
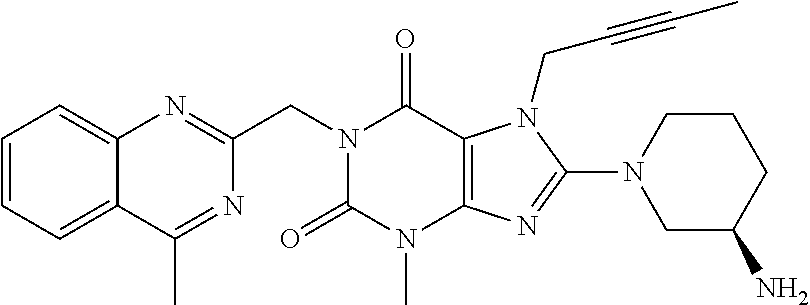
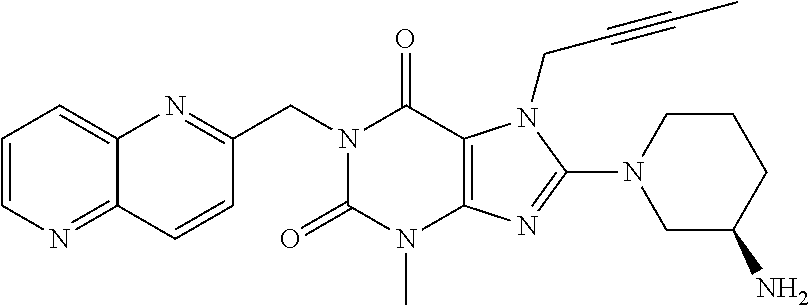
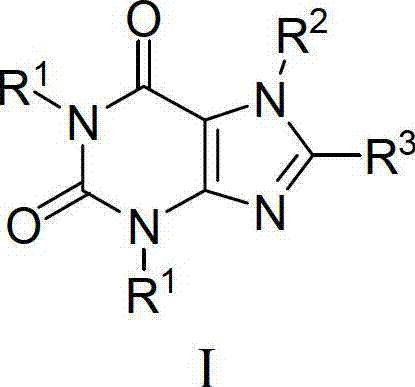
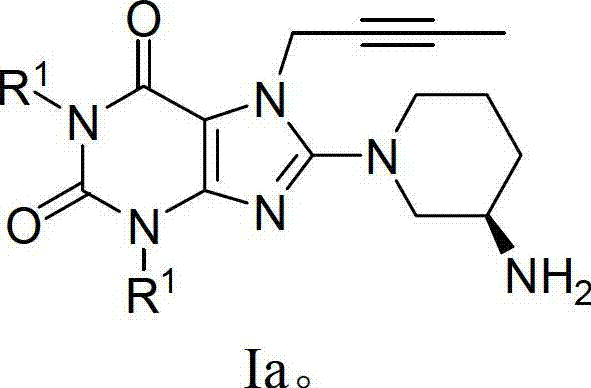
Pharmaceutical composition, pharmaceutical dosage form, process for their preparation, methods for treating and uses thereof
The present invention relates to pharmaceutical compositions of linagliptin, pharmaceutical dosage forms, their preparation, their use and methods for treating metabolic disorders.
Owner:BOEHRINGER INGELHEIM INT GMBH
Antidiabetic medications comprising a dpp-4 inhibitor (linagliptin) optionally in combination with other antidiabetics
InactiveUS20120094894A1Reduce weightAvoiding weight increaseAntibacterial agentsBiocideIGT - Impaired glucose toleranceAcute hyperglycaemia
The invention relates to antidiabetic medications which are suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia, inter alia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions. The medication is a mono treatment with a DPP-4 inhibitor <preferably linagliptin> or a combination treatment with a DPP-4 inhibitor and a second and / or third antidiabetic.
Owner:BOEHRINGER INGELHEIM INT GMBH
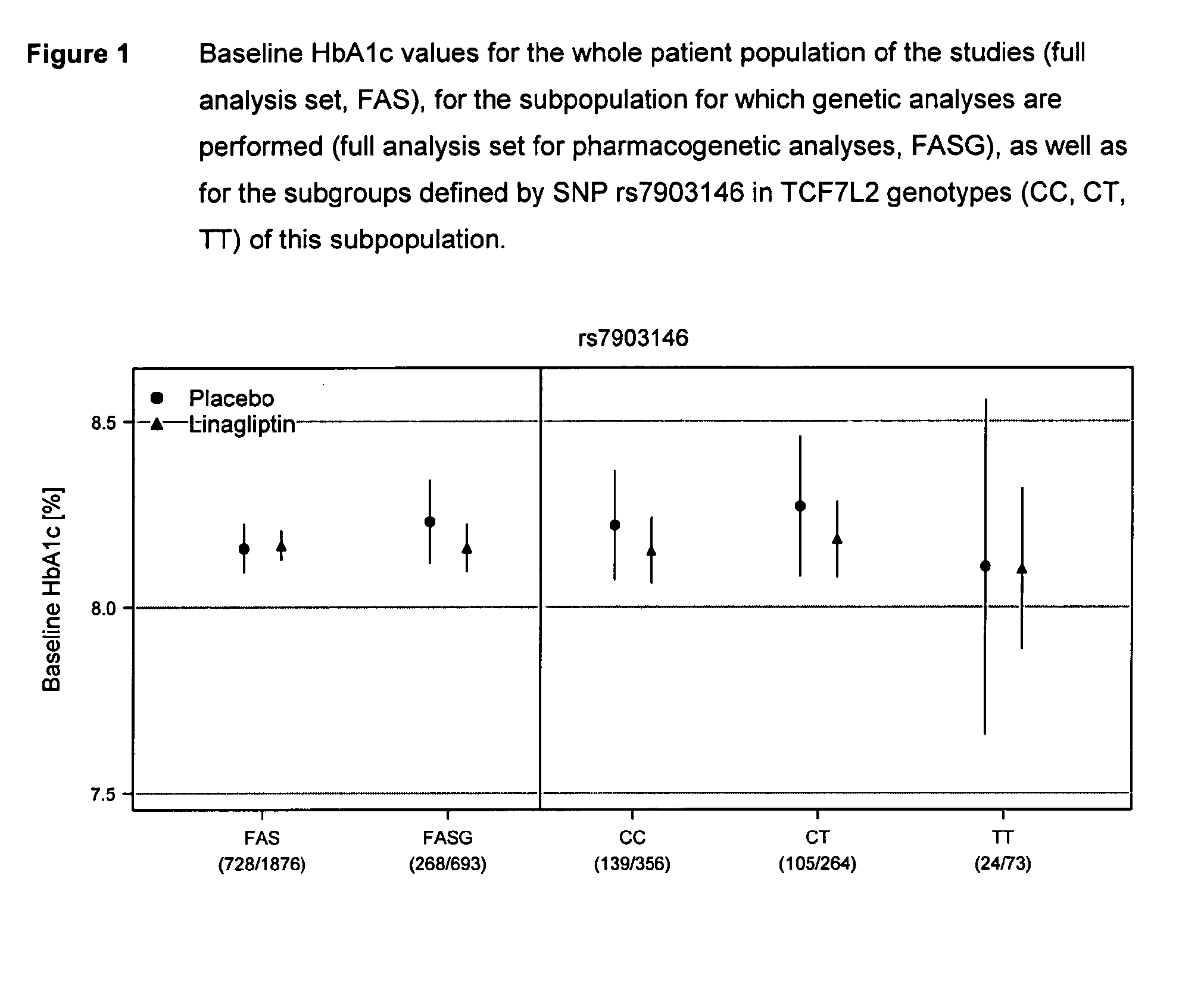
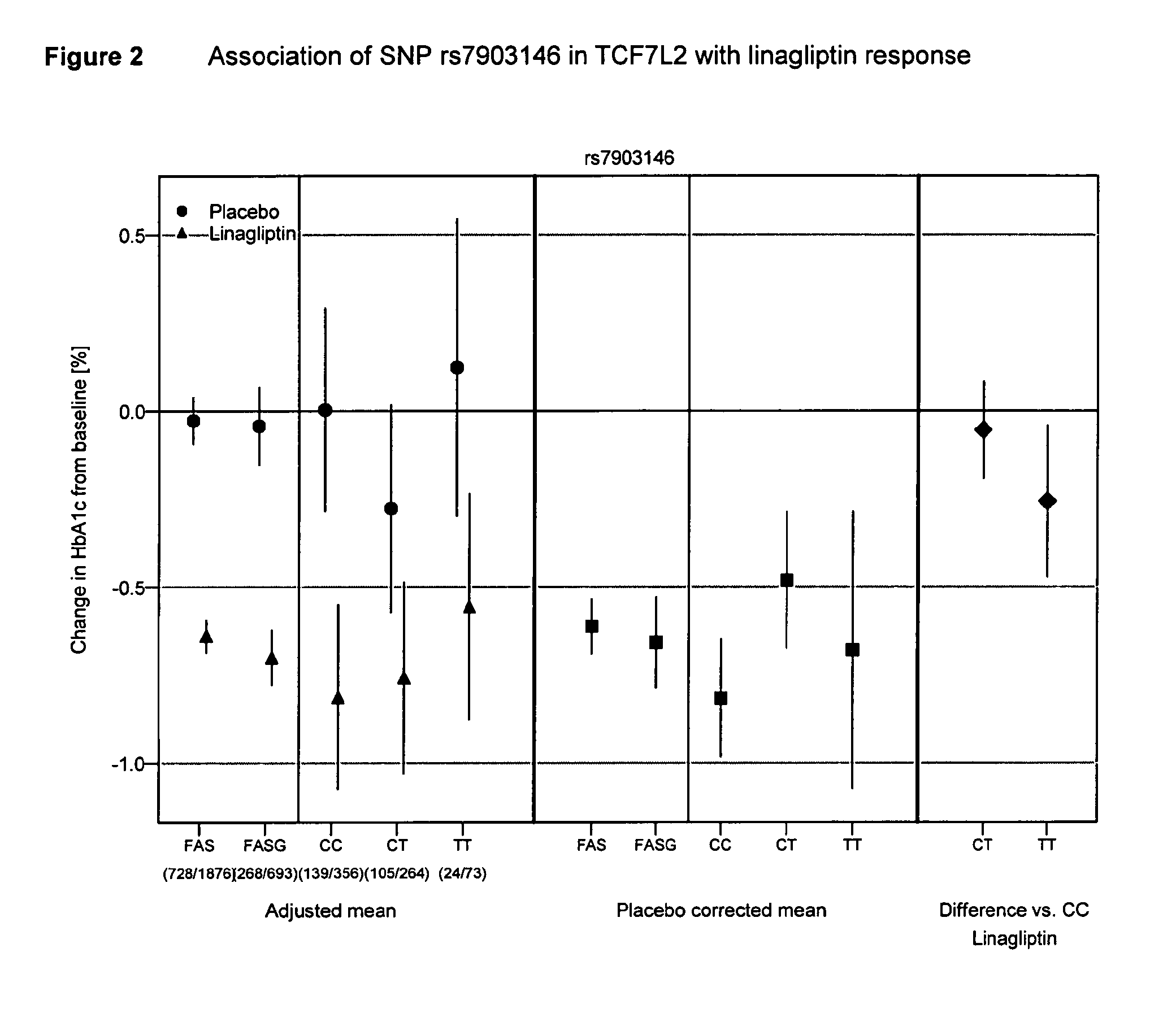
Treatment of genotyped diabetic patients with dpp-iv inhibitors such as linagliptin
ActiveUS20130196898A1Weight increaseLose weightBiocidePeptide/protein ingredientsPatient groupGenotype
Owner:BOEHRINGER INGELHEIM INT GMBH
Polymorph of linagliptin benzoate
The present invention relates to a novel polymorph of Linagliptin benzoate and to methods for its preparation. Furthermore the present invention relates to the use of the novel polymorph for the preparation of a medicament. In addition the present invention relates to pharmaceutical compositions comprising an effective amount of the novel polymorph of Linagliptin benzoate.
Owner:SANDOZ AG
Pharmaceutical composition for the prevention or the treatment of non-alcoholic fatty liver disease and the method for prevention or treatment of non-alcoholic fatty liver disease using the same
InactiveCN102883721AInhibit progressInhibitory activityOrganic active ingredientsMetabolism disorderSitagliptinBULK ACTIVE INGREDIENT
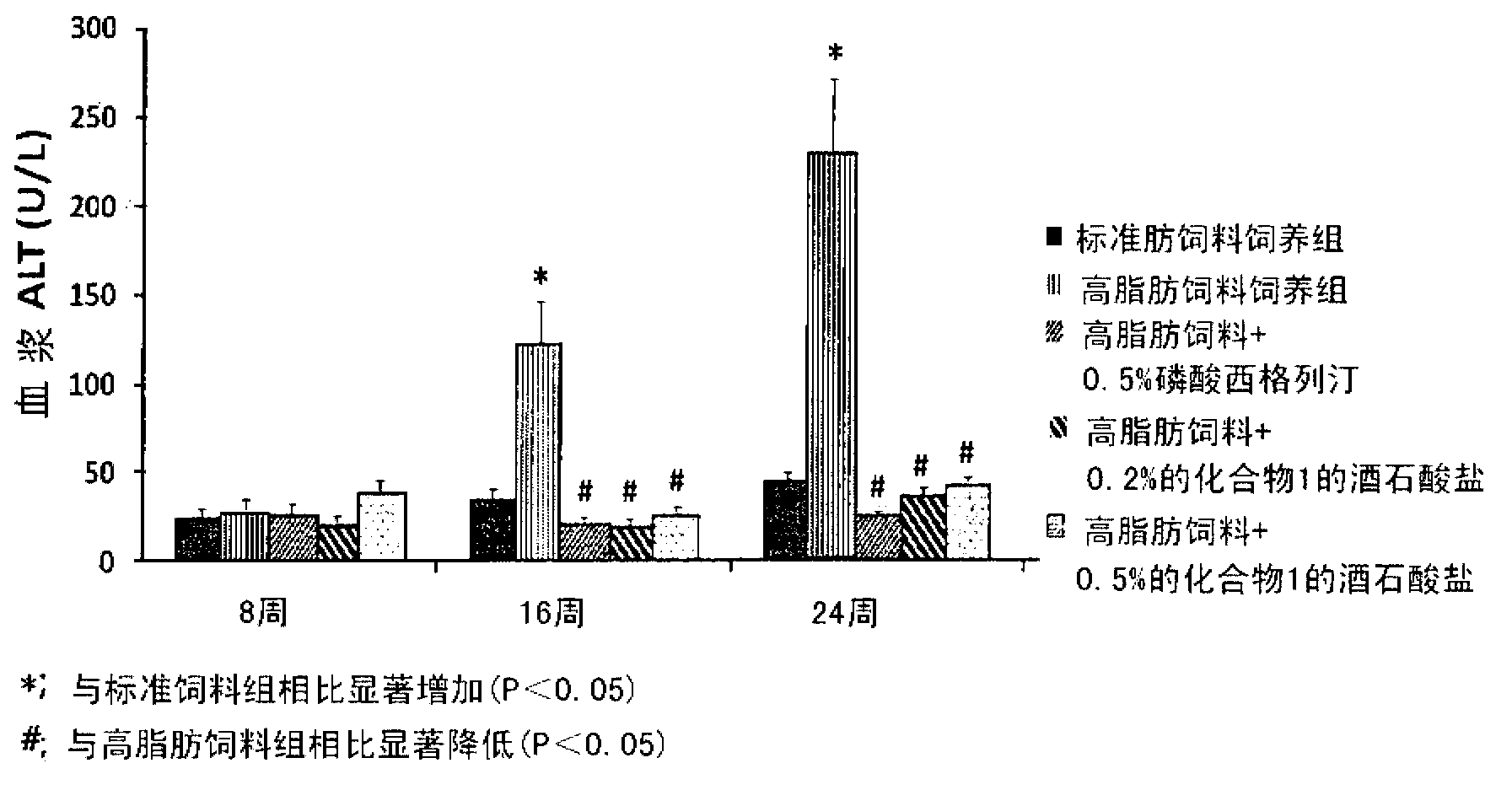
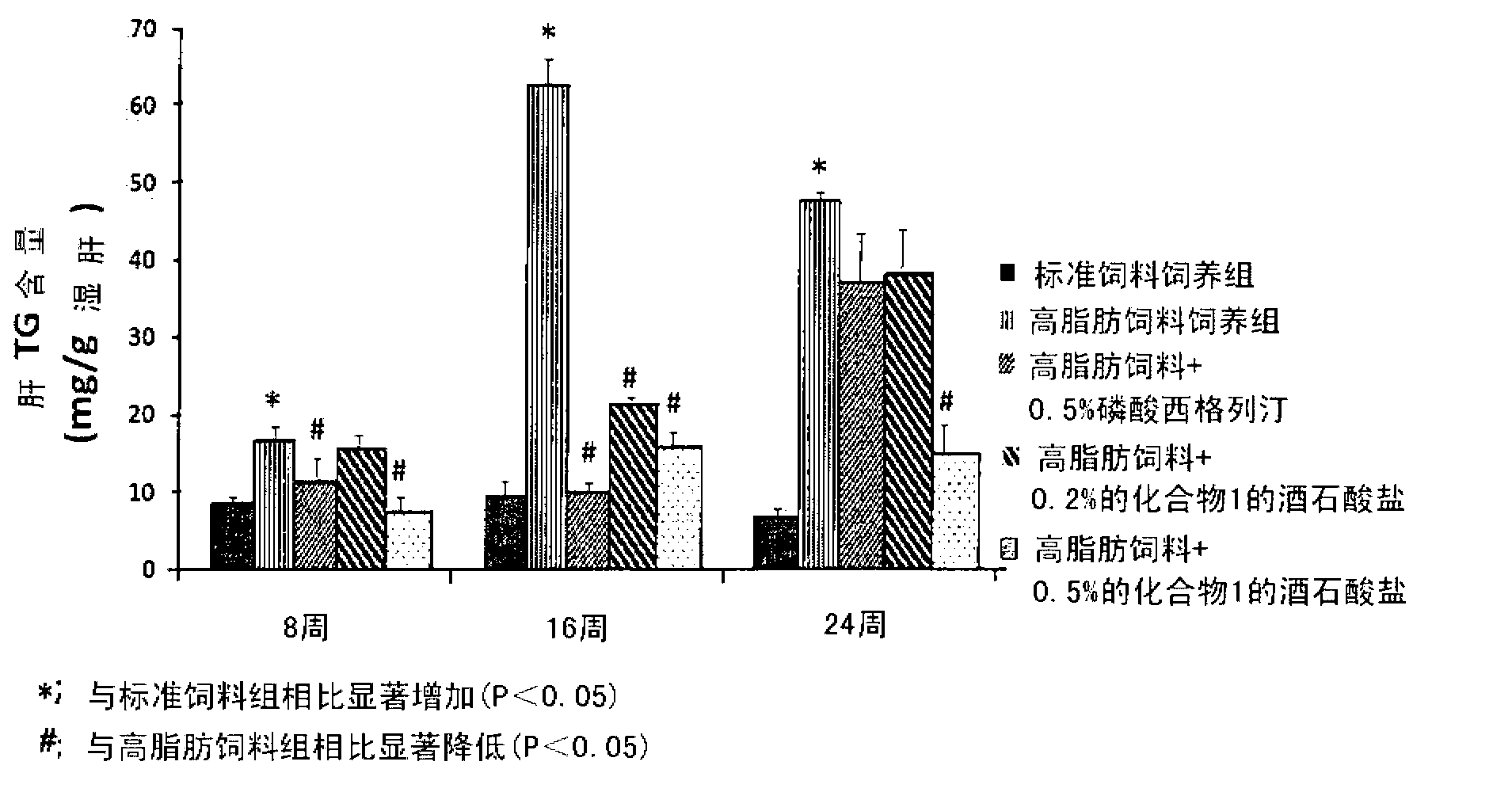
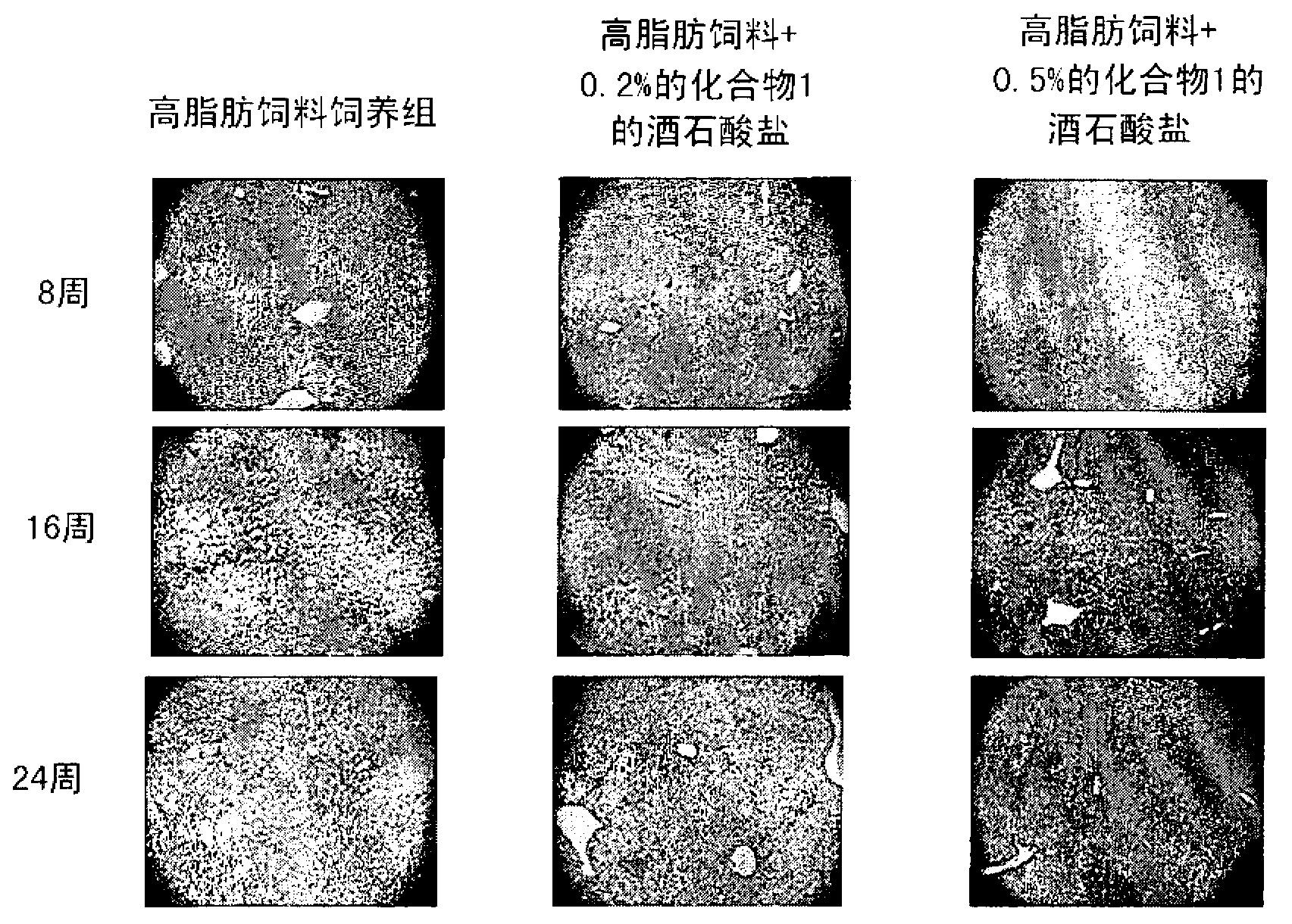
The present invention provides a pharmaceutical composition for the prevention and treatment of a non-alcoholic fatty liver disease (NAFLD), containing an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof. Further, the present invention provides a method for the prevention or treatment of a non-alcoholic fatty liver disease, including administering an effective amount of an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof to a mammal including a human in need thereof.; Further, the present invention provides use of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof, for manufacturing a pharmaceutical composition for the prevention or treatment of a non-alcoholic fatty liver disease.
Owner:DONG A PHARMA
Treatment of genotyped diabetic patients with DPP-IV inhibitors such as linagliptin
ActiveUS9457029B2Good effectFew adverse effectPeptide/protein ingredientsMetabolism disorderPatient groupDiabetic patient
Owner:BOEHRINGER INGELHEIM INT GMBH
Preparation method of Linagliptin
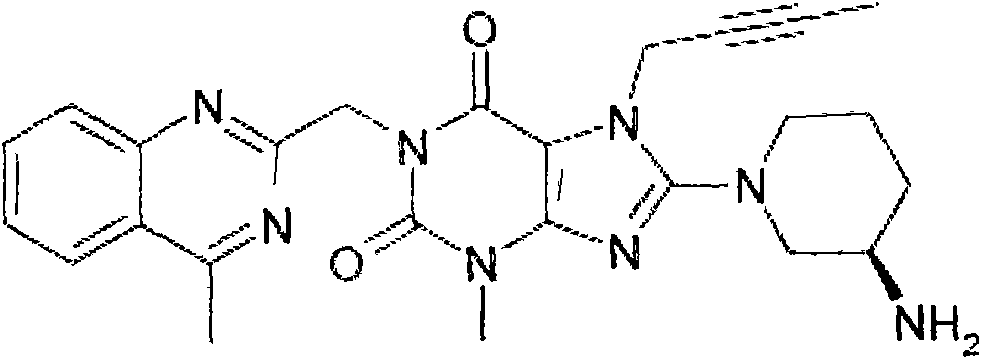
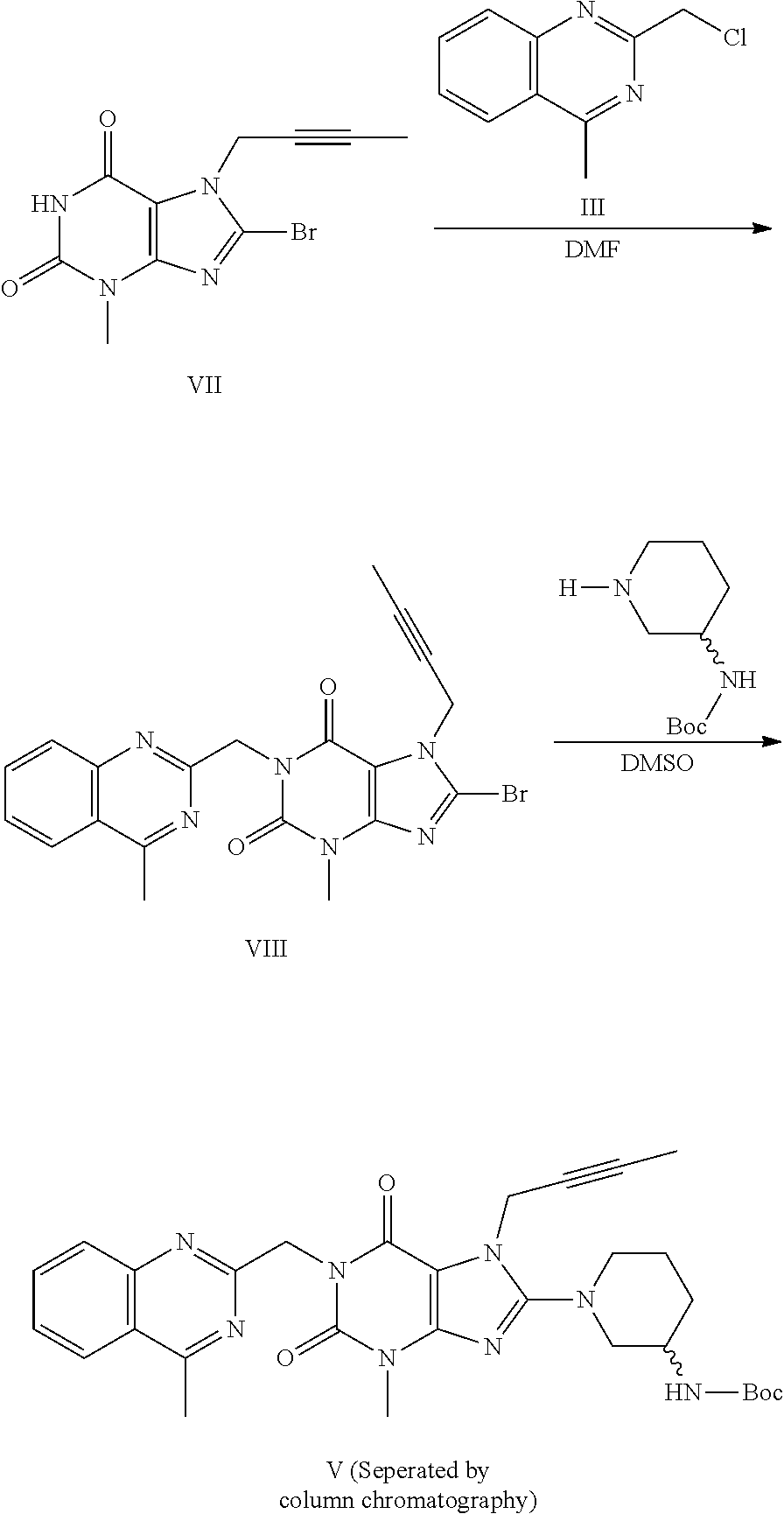
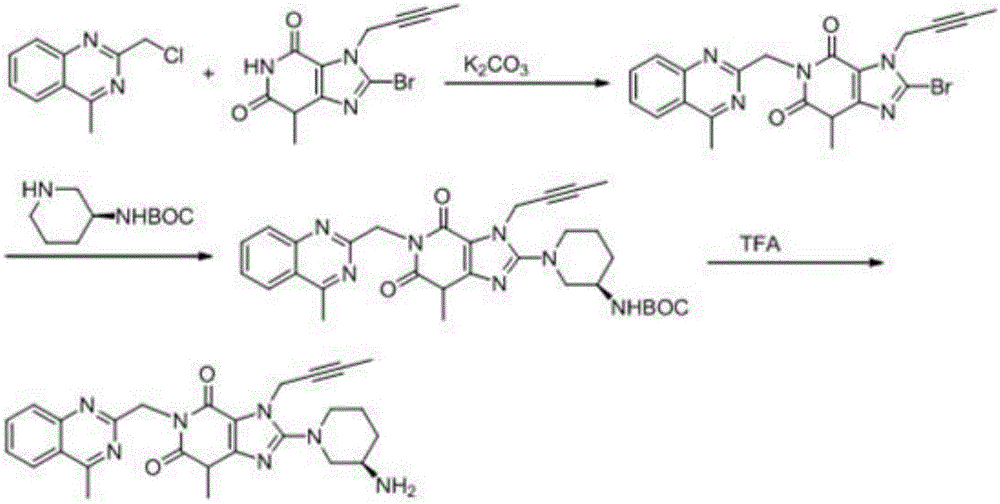
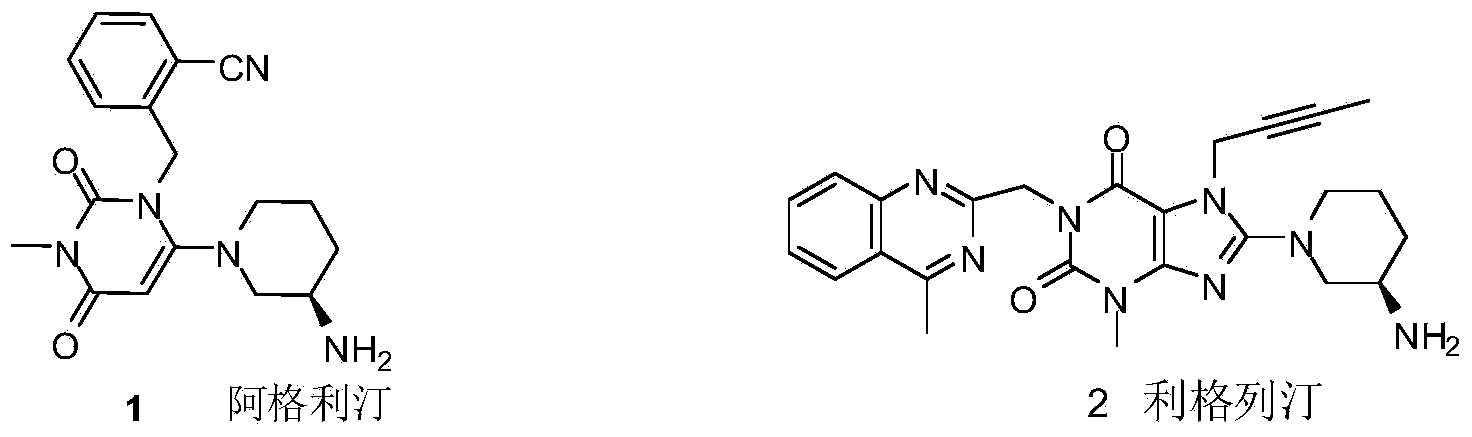
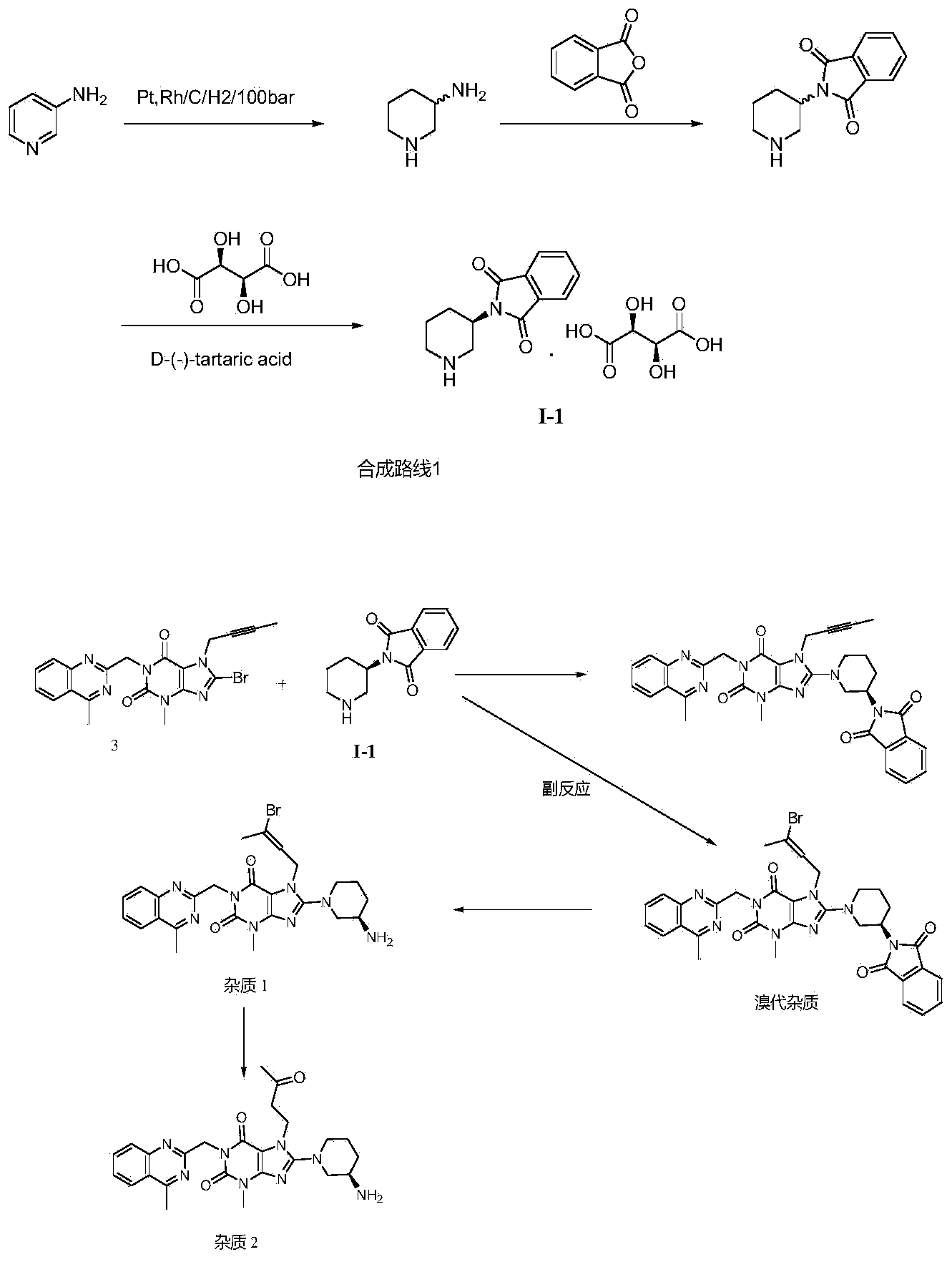
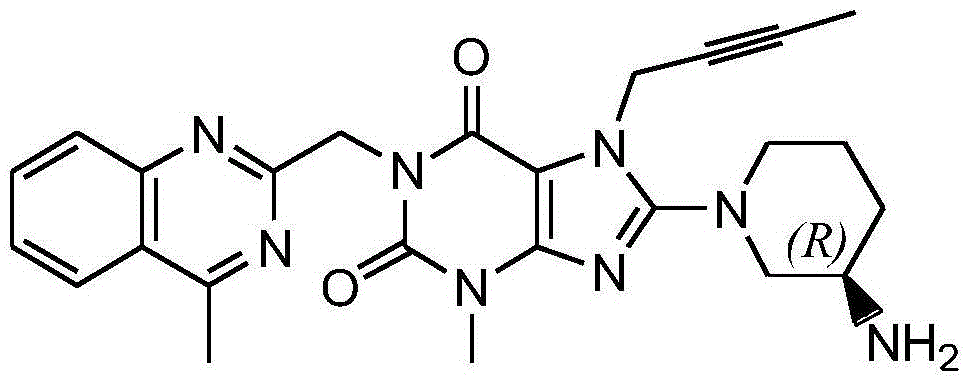
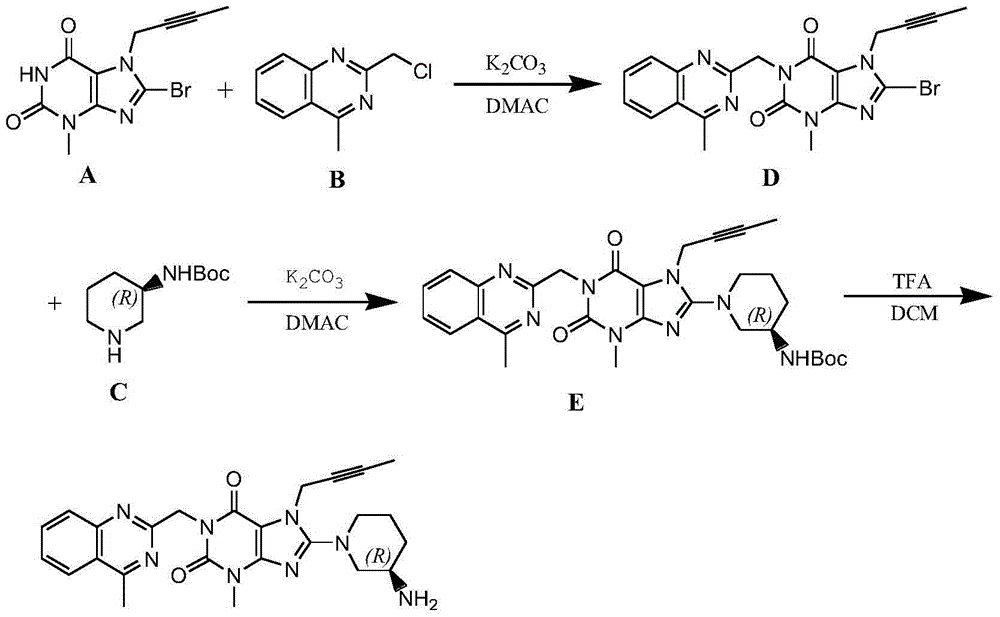
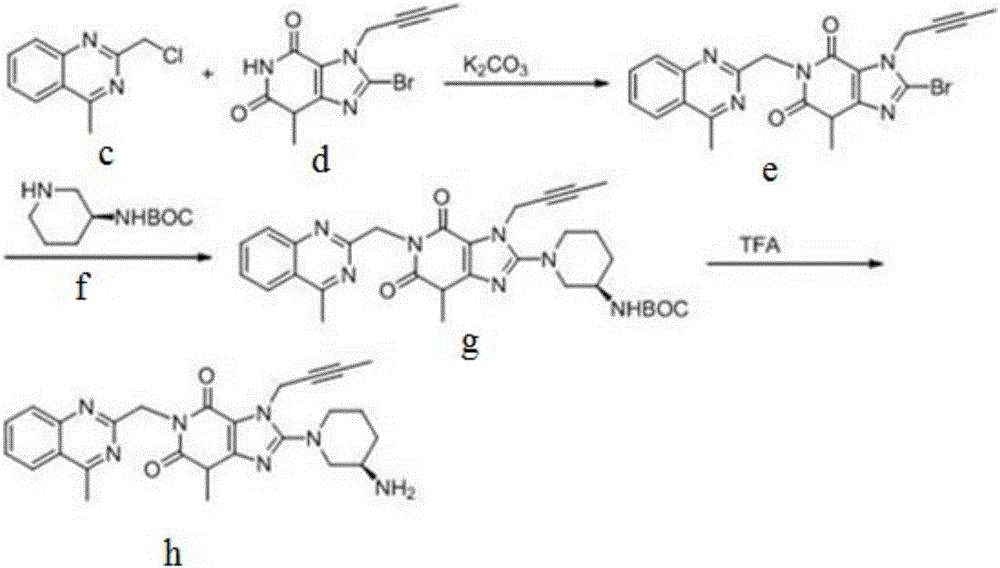
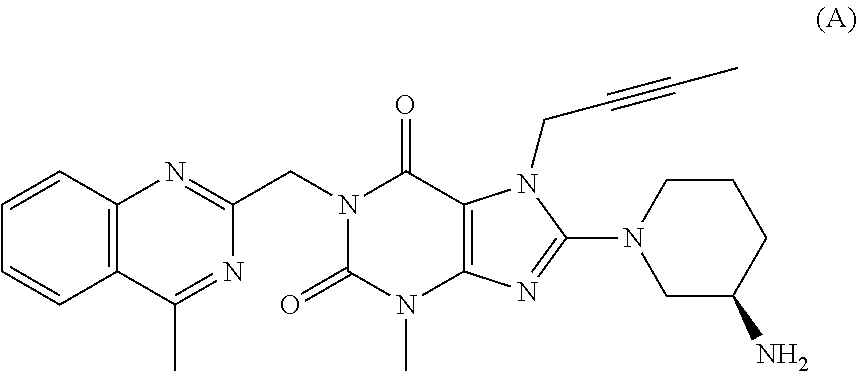
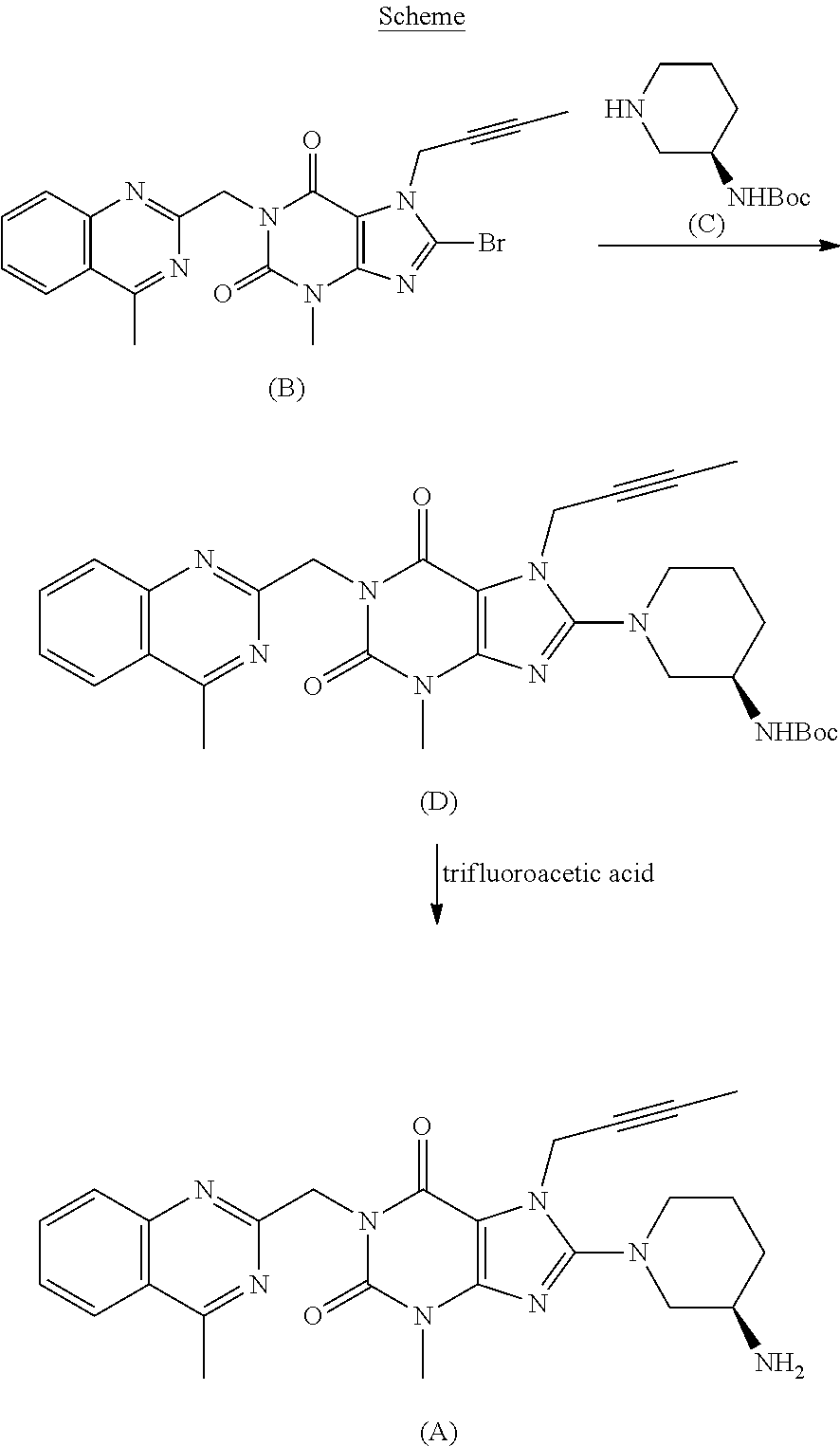
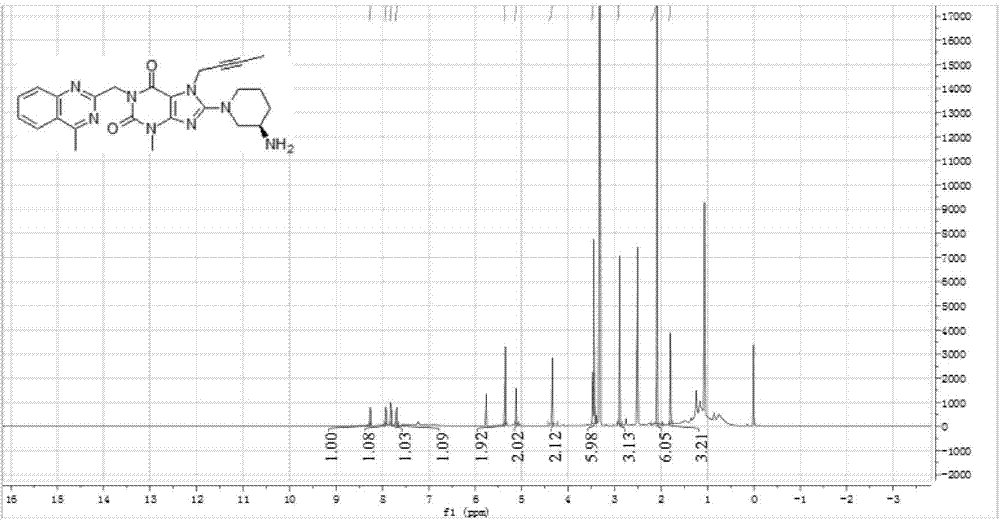
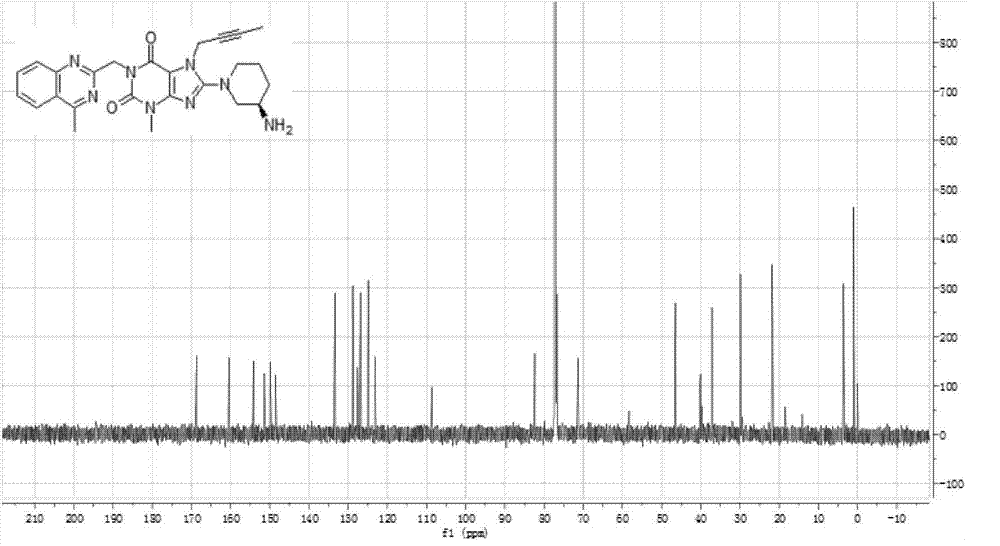
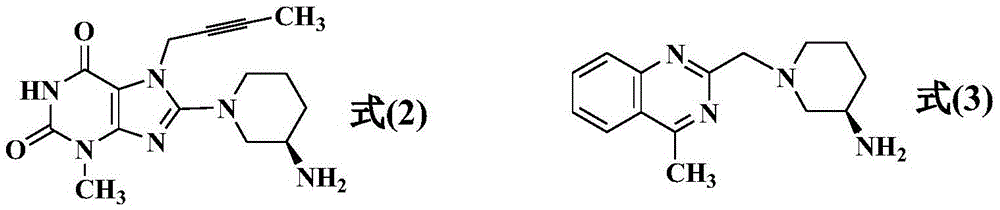
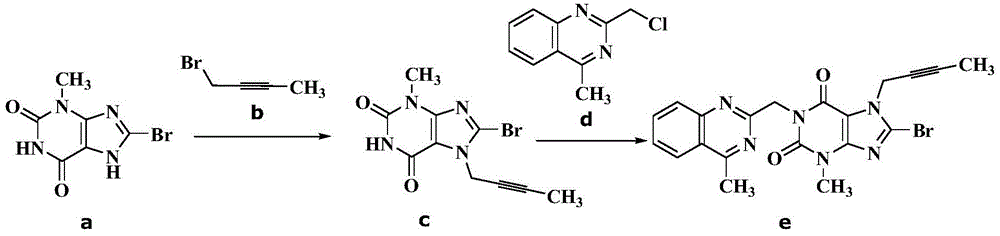
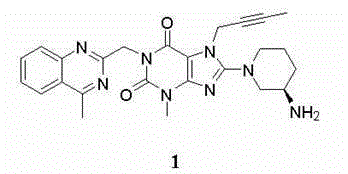
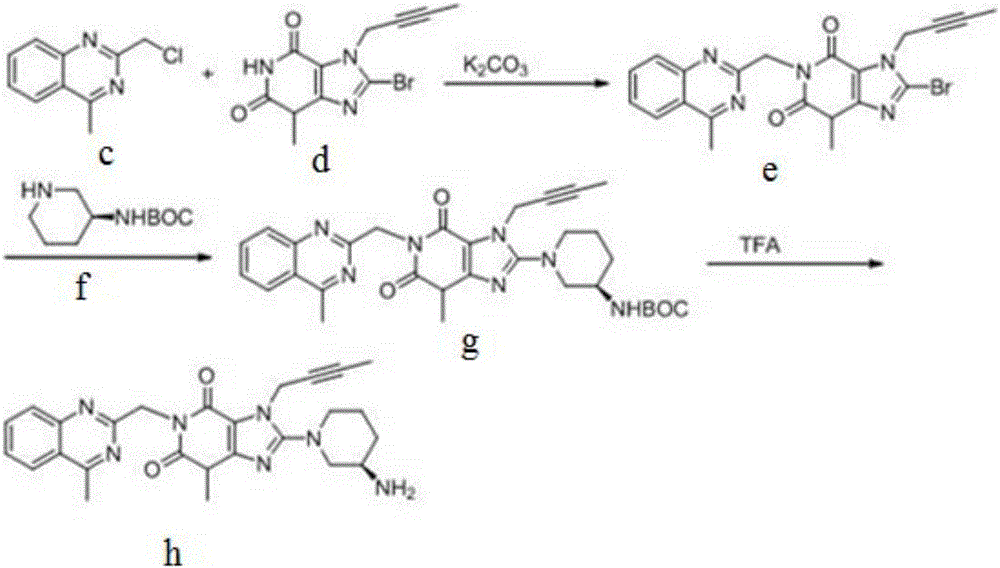
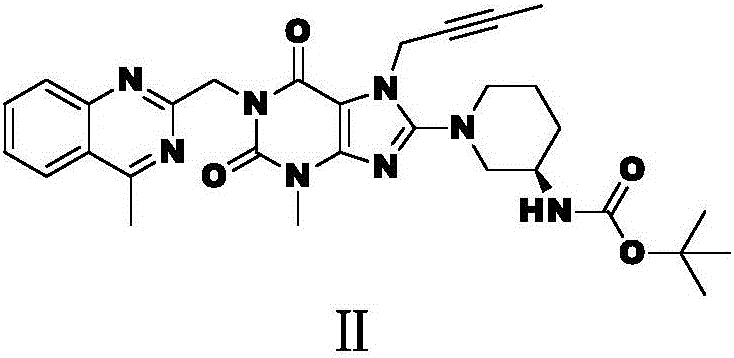
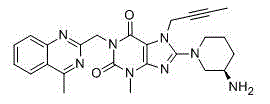
The invention relates to a preparation method of Linagliptin. According to the preparation method, potassium carbonate or sodium carbonate is taken as an alkali; an iodine-containing inorganic salt is taken as a catalyst; N-methyl-2-pyrrolidone (NMP) or N,N-Dimethylformamide (DMF) is taken as a solvent; 8-bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1H-purine-2,6-dione is reacted with a (R)-3-aminopiperidine compound firstly at 40 to 50 DEG C; after reaction, 2-(chloromethyl)-4-methylquinazoline is directly added for reaction so as obtain a compound D; and the compound D is subjected to step 1-2 to prepare Linagliptin. The preparation method is capable of shortening reaction time, and increasing yield.
Owner:迪嘉药业集团股份有限公司
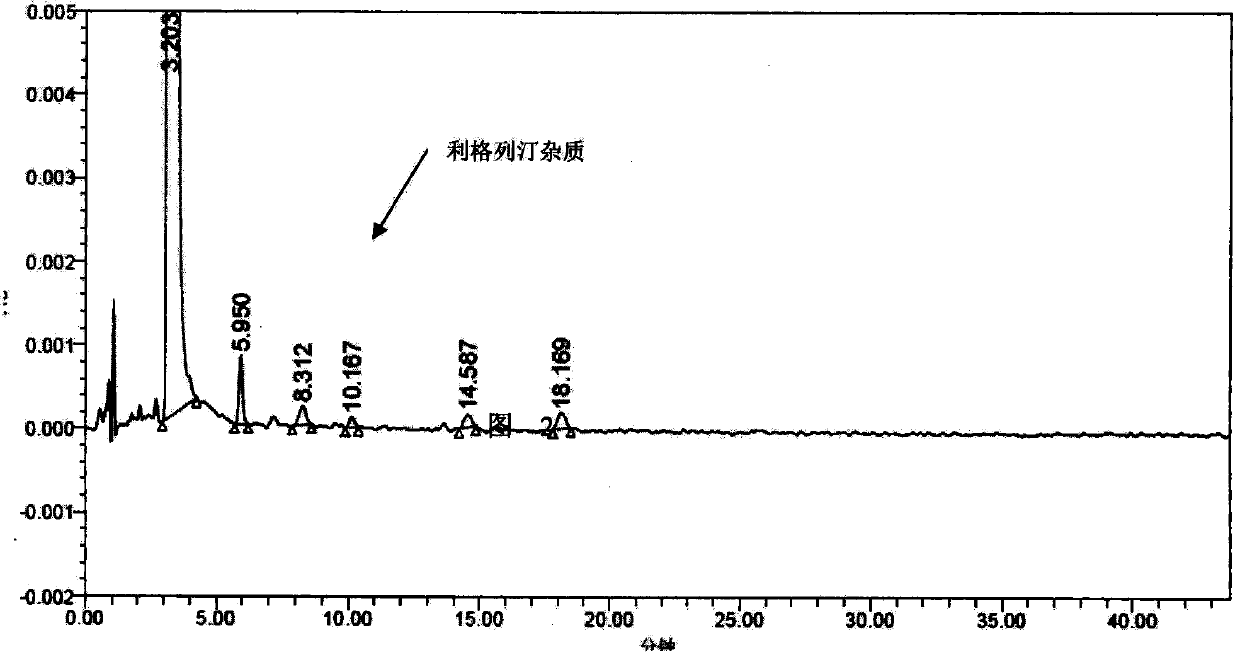
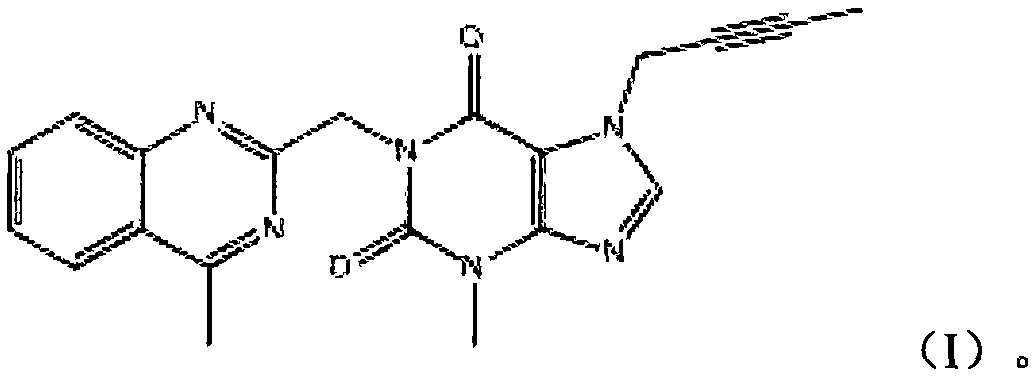
Linagliptin impurity, and preparation method and application thereof
ActiveCN105503872AQuality improvementEffective monitoringOrganic chemistryComponent separationMedicineDrug product
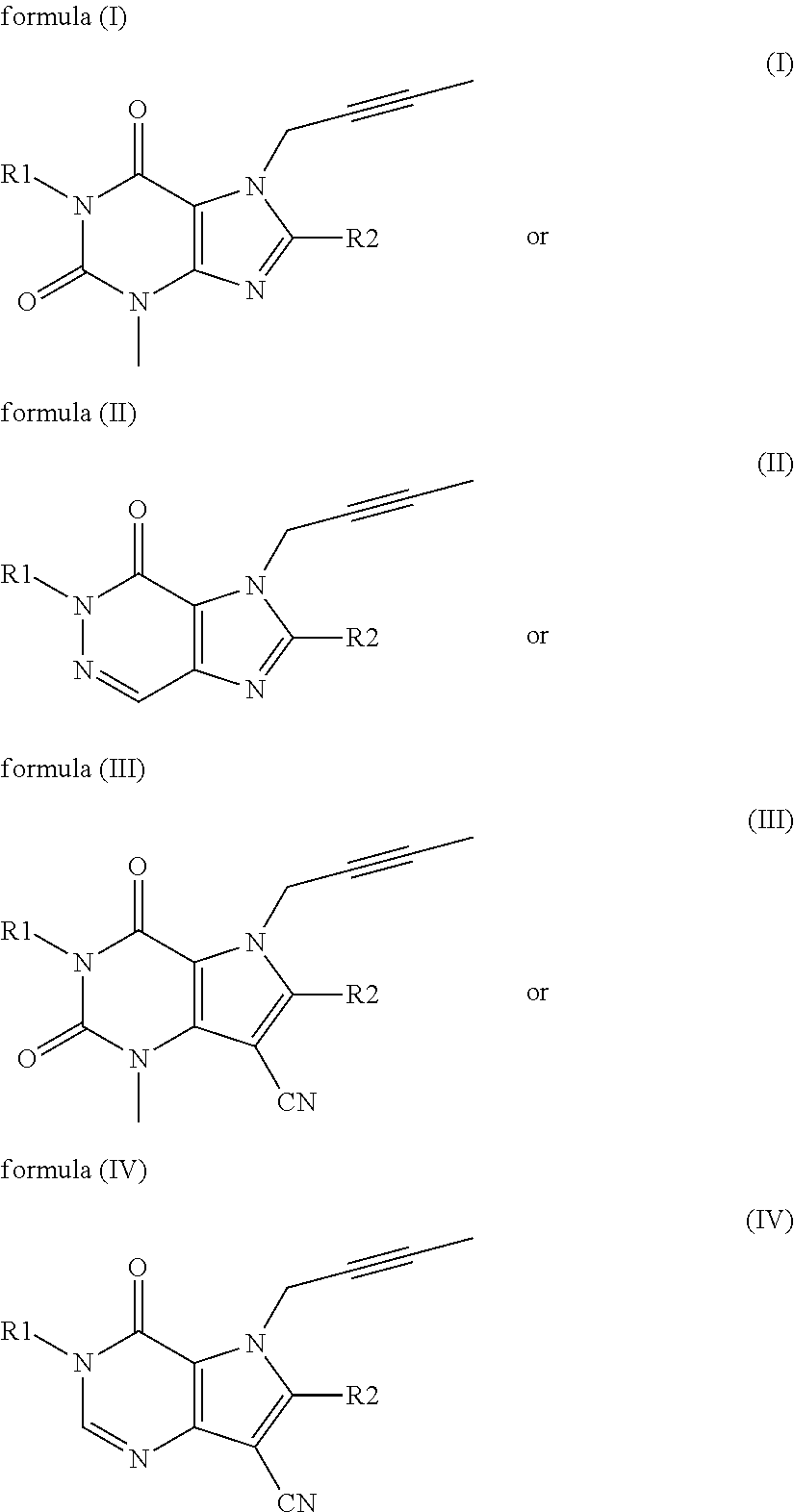
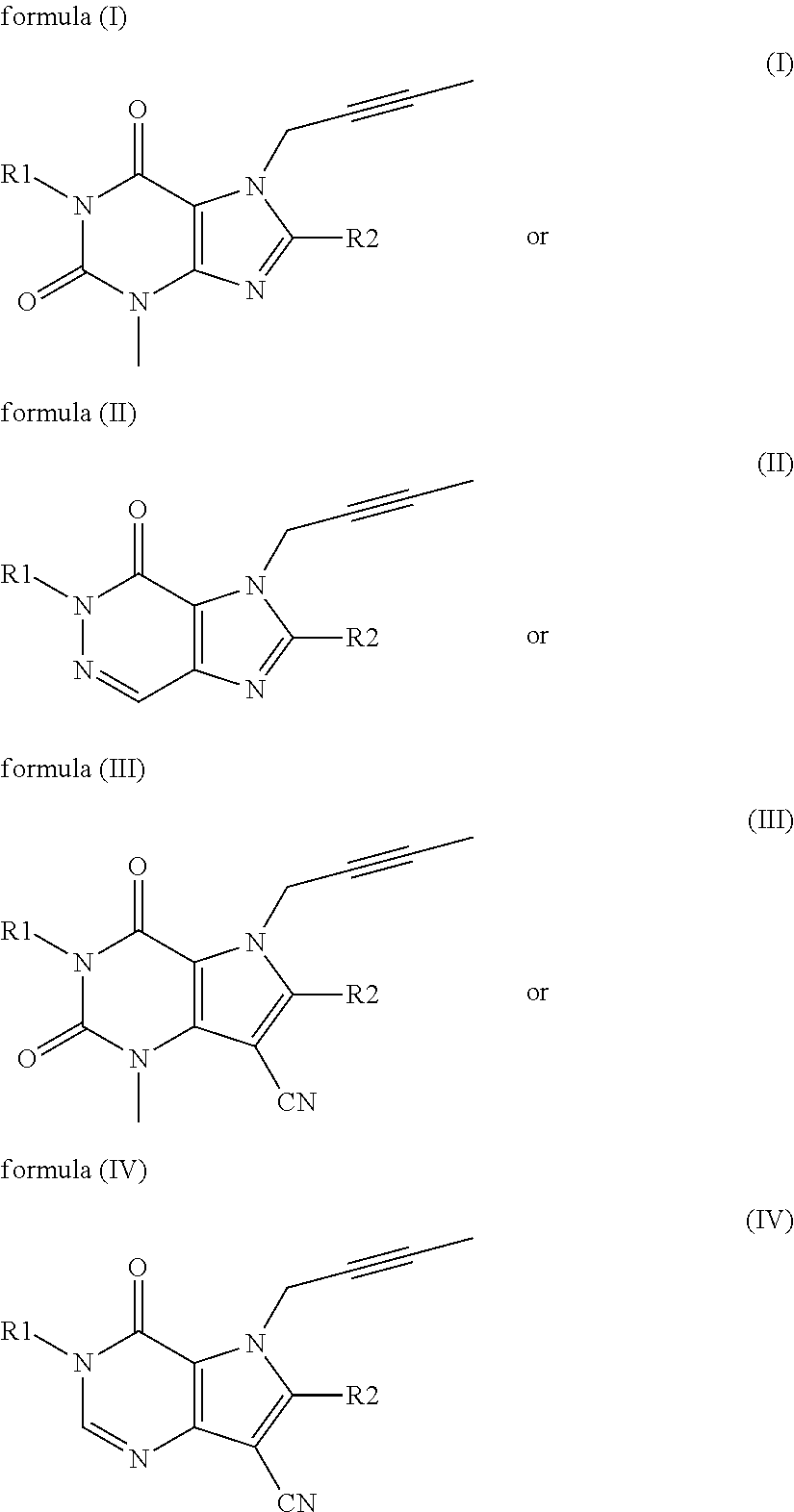
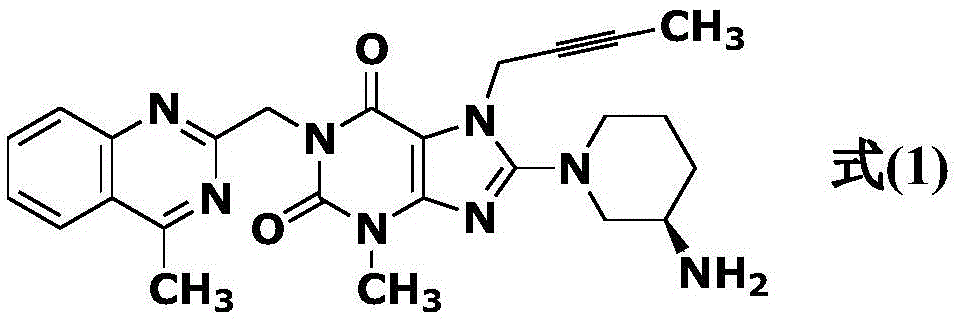
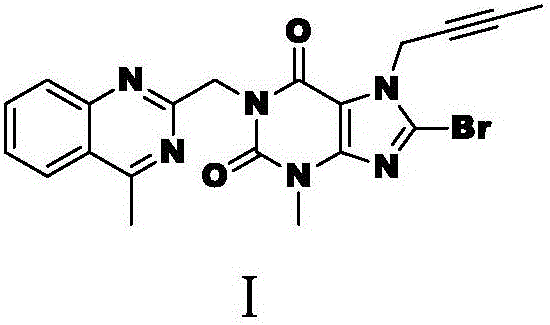
The invention discloses a linagliptin impurity as shown in a formula (I) which is described in the specification, a preparation method for the linagliptin impurity and application of the linagliptin impurity as an impurity reference substance for inspection of substance related to linagliptin. As the linagliptin impurity is applied as a standard reference substance, linagliptin quality can be effectively controlled, and security risks of a drug can be reduced, so security and validity of the linagliptin preparation in clinical application can be guaranteed.
Owner:BEIJING PUDEKANGLI PHARMA TECH DEV CO LTD
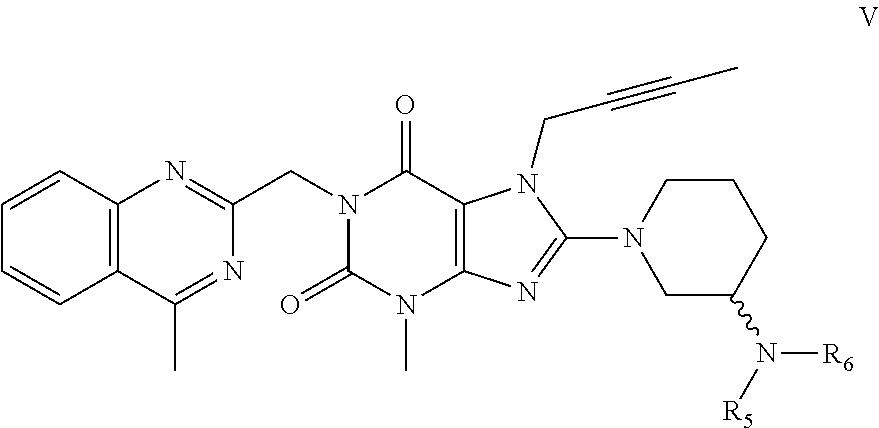
Method for preparing an important intermediate of linagliptin
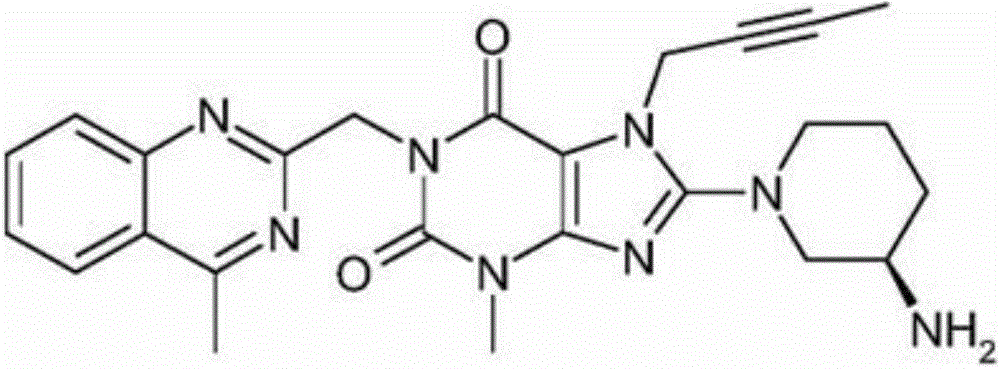
The present invention discloses an improved process for preparing an important intermediate of linagliptin. In particular, disclosed are a process for preparing a compound V which is an important intermediate of linagliptin and has the structure V, and an industrial process of preparing linagliptin having excellent chemical and optical purities, which is an inhibitor of dipeptidyl peptidase-4 (DPP-IV), from the compound V. The process employs a phase-transfer catalyst, is high in yield, easy and simple to handle, environmentally friendly, suitable for industrial mass production, and can be implemented by a “one-pot process”.
Owner:2Y CHEM
Synthetic method of linagliptin
ActiveCN105936634AHigh purityPurity does not affectOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupXanthine
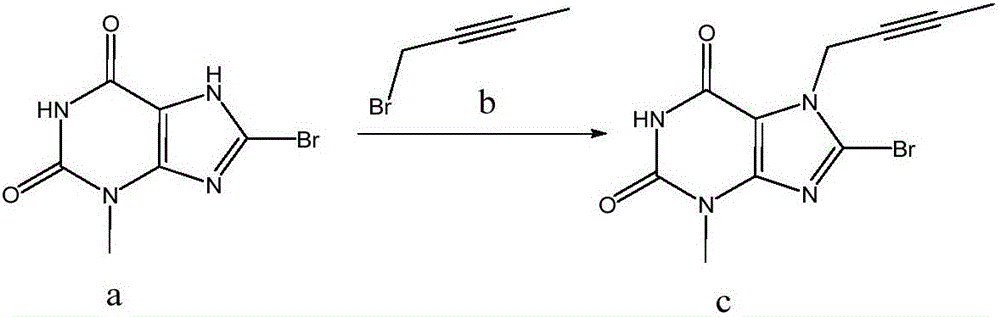
The invention discloses a synthetic method of linagliptin, wherein the method includes the following steps: (1) carrying out a reaction of 8-bromo-3-methylxanthine (a) and 1-bromo-2-butyne (b), to obtain 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine (c); (2) carrying out a reaction of 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine (c) and 2-chloromethyl-4-methylquinazoline (d), to obtain 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e); (3) adding 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e), (R)-3-Boc-aminopiperidine (f), potassium carbonate and acetonitrile into a reactor and mixing evenly, and carrying out a reaction under a state of heating reflux, to obtain t-butyloxycarboryl-linagliptin (g); and (4) removing a Boc protective group of t-butyloxycarboryl-linagliptin (g) in a methanol aqueous solution, to obtain linagliptin. The synthetic method has the advantages of environmental protection, no pollution, high production rate, and no impurities.
Owner:赤峰赛林泰药业有限公司
Industrial preparation process of linagliptin
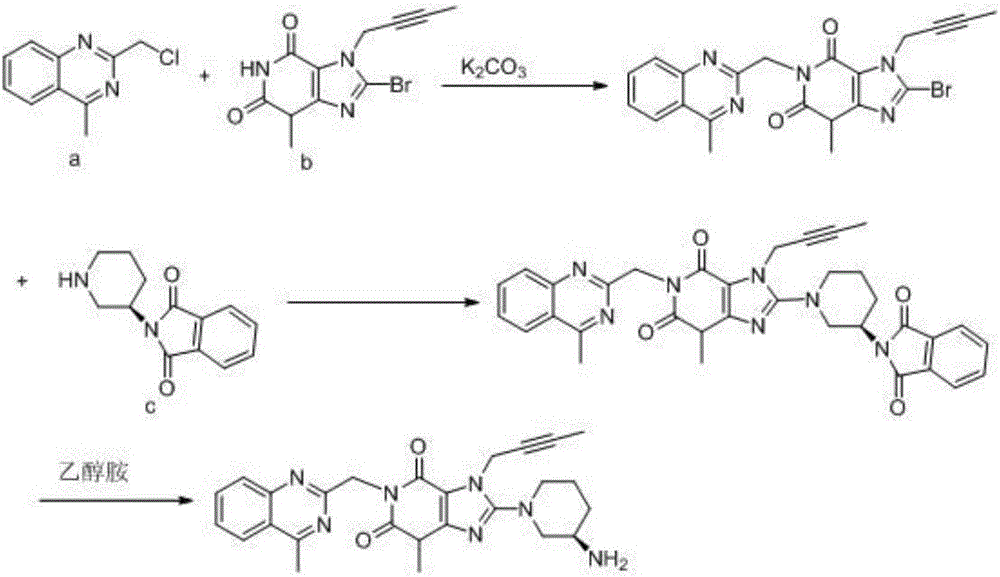
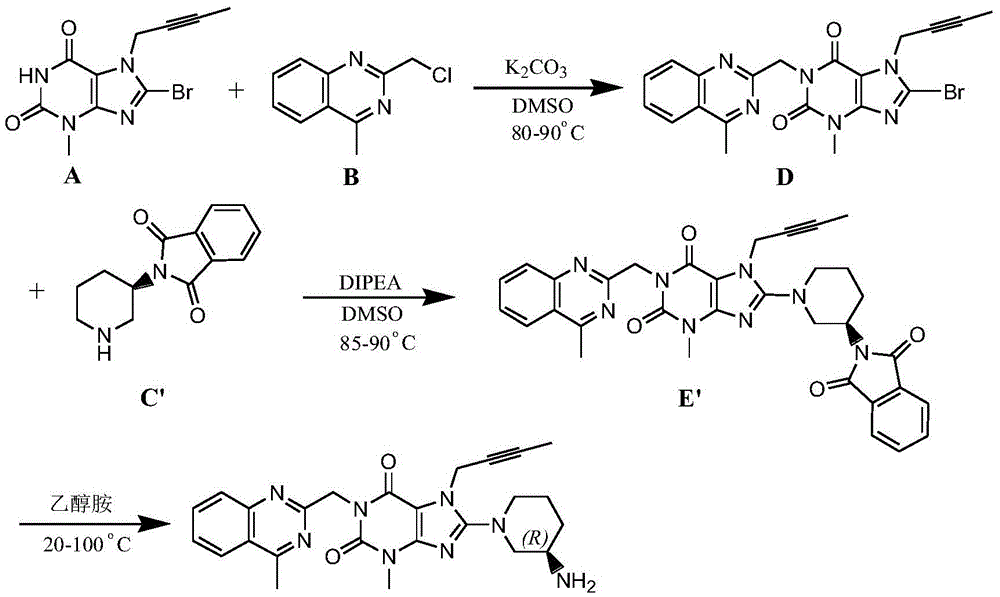
The invention relates to the technical field of medicines and particularly relates to an industrial preparation process of linagliptin. The industrial preparation process comprises the steps of adding a reactant a (2-chloromethyl-4-methyl-quinazoline), an equal molar ratio of reactant b (8-bromo-7-(2-butynyl)-3-methylxanthine), an acid-binding agent and a proper amount of solvent into a reaction kettle to react at 0-140 DEG C for 3-8 hours, after TLC detection reaction is finished, directly adding a reactant c ((R)-3-phthalimide piperidine-tartaric acid) and an acid-binding agent, namely N,N-diisopropylethylamine without processing a reaction mother liquid to react at 0-125 DEG C for 3-10 hours, after the TLC detection reaction is finished, adding ethanolamine without processing the reaction mother liquid to react for 2-10 hours, after the TLC detection reaction is finished, dropwise adding purified water, carrying out suction filtration to obtain a linagliptin rough product, and refining by virtue of a refining method disclosed in a patent CN101437823A, so as to obtain a linagliptin refined product. According to the industrial preparation process, linagliptin is synthesized by virtue of a one-pot continuous feeding method, the consumption of the solvent is low, and the operation is easy; and the industrial preparation process is suitable for industrial production.
Owner:SHOUGUANG FUKANG PHARMA
Stimulators of incretin hormones secretion, method for preparation and use thereof
InactiveUS20140100216A1Improve treatment efficiencySimplifies direct careOrganic active ingredientsBiocideDiseaseTreatment effect
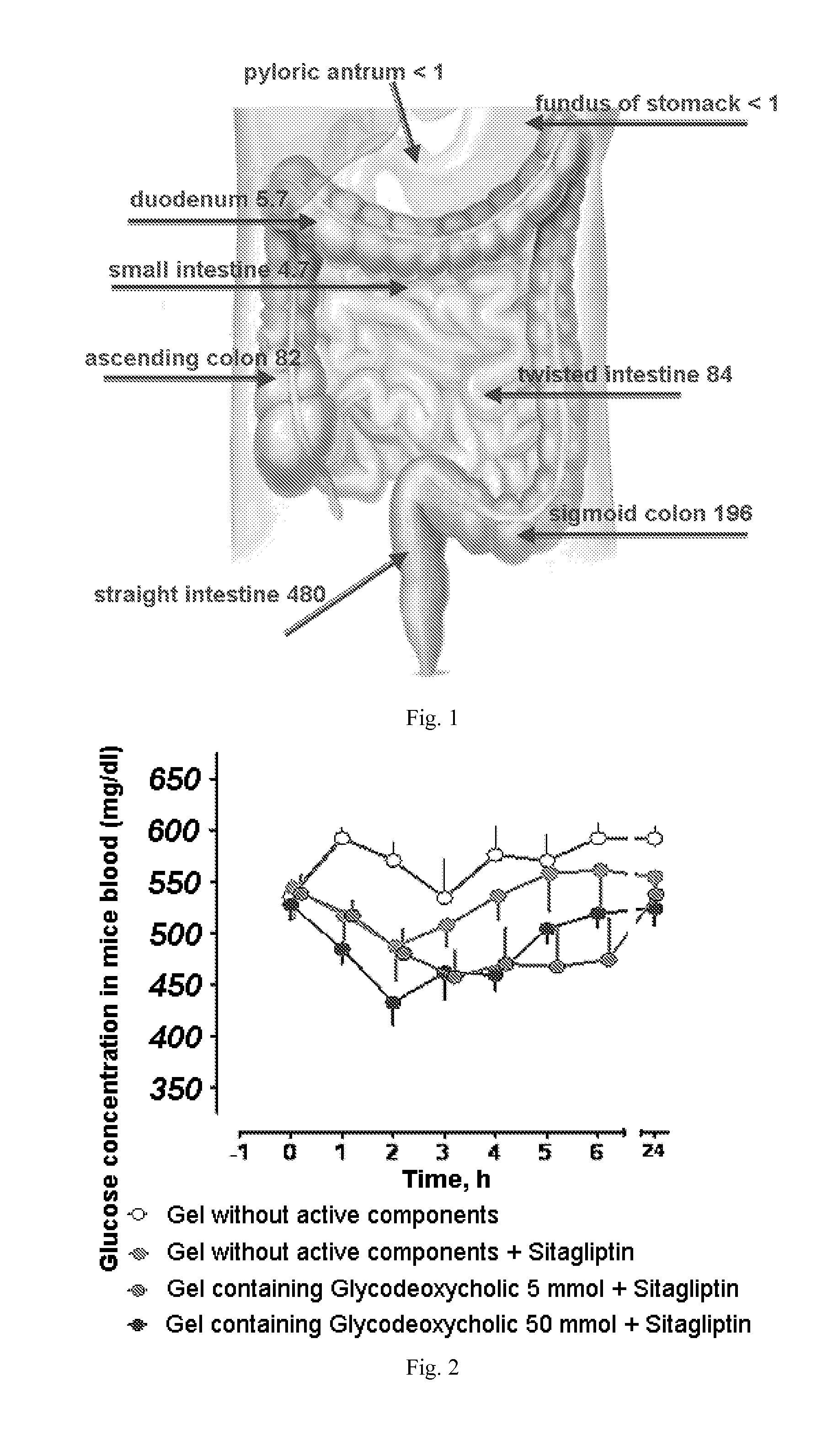
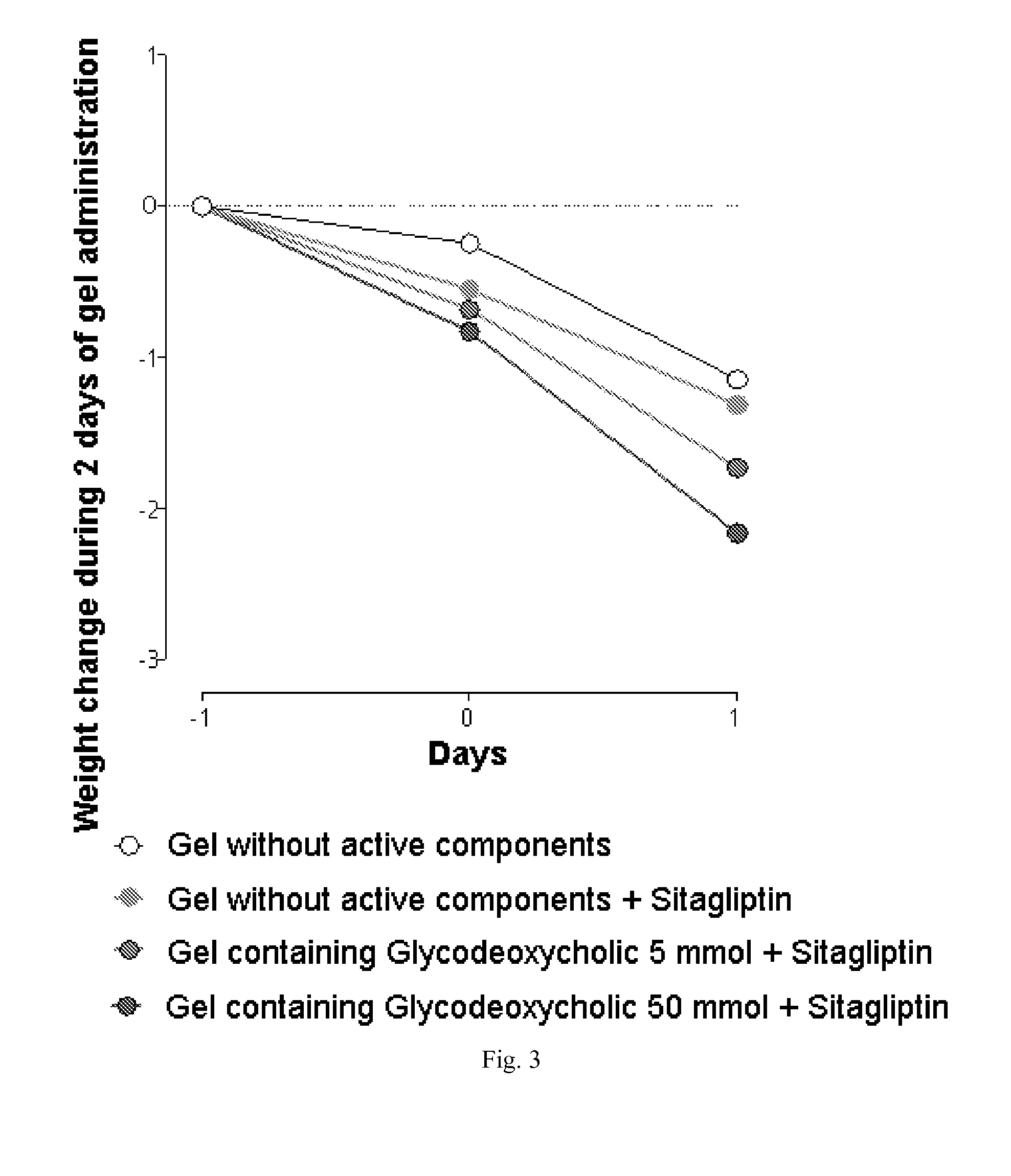
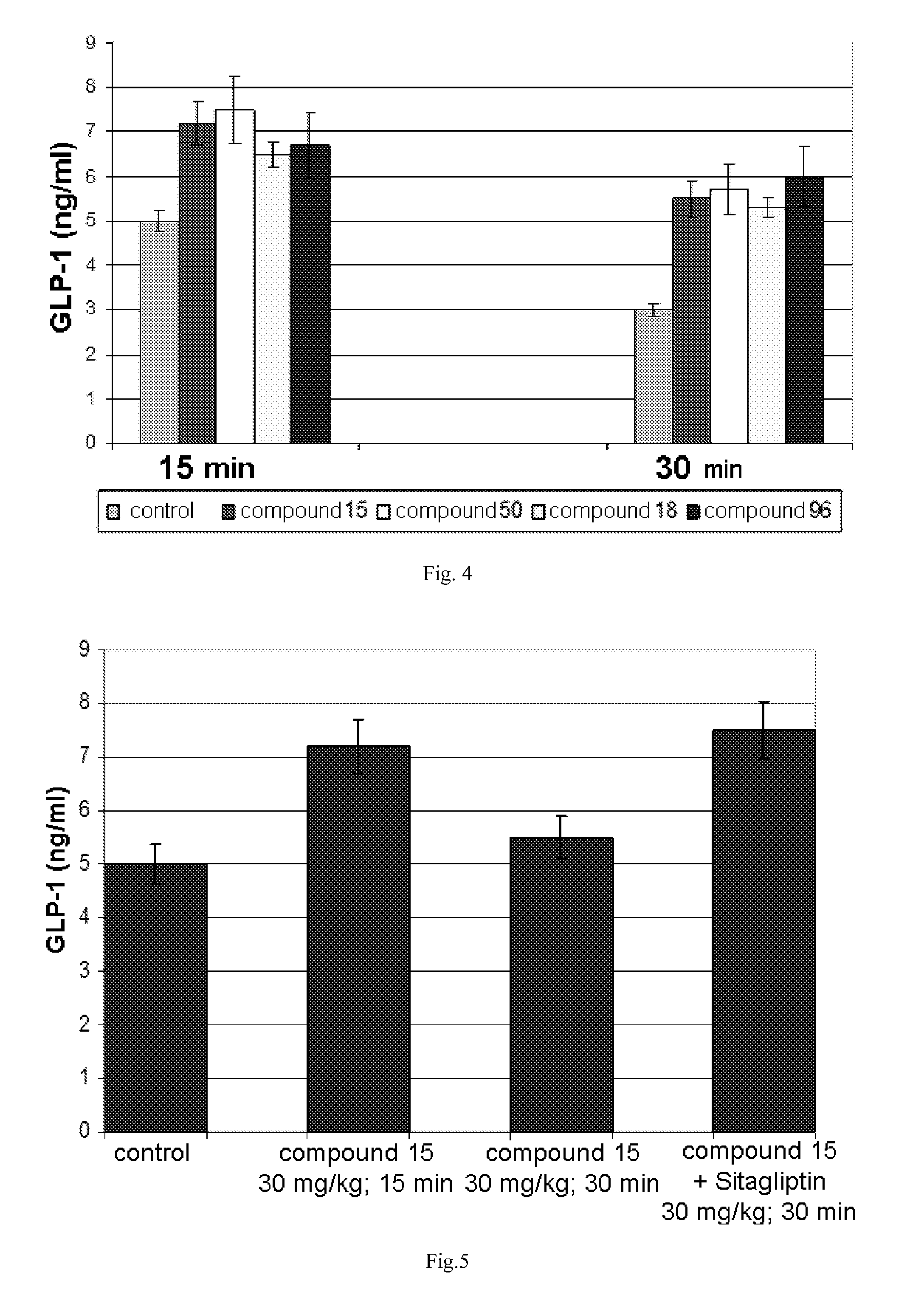
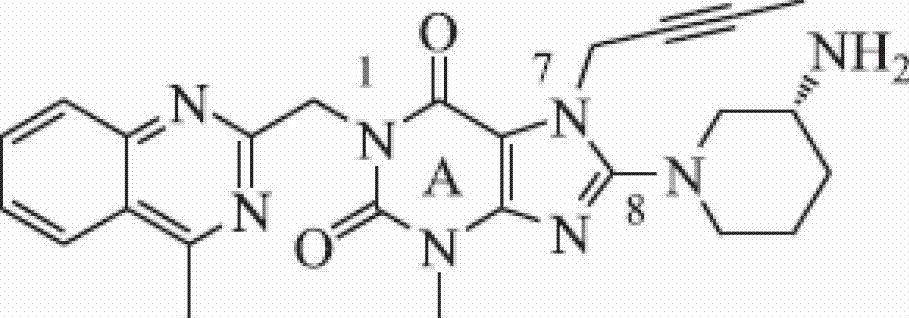
The invention relates to the area of medicinal chemistry, pharmacology and medicine and includes description of pharmaceutical compositions and combined medicaments on the base of secretion stimulators and protectors of incretin hormones for treatment of metabolic diseases (among them, diabetes, obesity, metabolic syndrome and the like). The invention consists in that that pharmaceutical composition or combined medicament comprises a derivative of tetrahydrobenzo[f][1,4]oxazepine—either nonsteroidal agonist of bile aids receptor TGR5, or one of endogenous bile acids which stimulate incretin hormones secretion, and also one of the known inhibitors of DPP-IV proteinase. In this case administration of TGR5 agonists is carried out peroral, and administration of endogenous bile acids is exercised rectal in the form of suppository or gel. As proteinase DPP-IV inhibitors could be used Vildagliptin, Saxagliptin, Sitagliptin, Teneligliptin, Linagliptin, Dutogliptin, Alogliptin, Gemigliptin, Carmegliptin and the like. Besides, the invention includes description of novel tetrahydrobenzo[f][1,4]oxazepine derivatives—nonsteroidal agonist of bile aids receptors TGR5, and also methods for their preparation. The invention provides enhancement of therapy effectiveness owing to synergetic action of the components, thus making possible simultaneous treatment of diabetes, and obesity, other metabolic diseases and their cardiovascular and renal complications.
Owner:SAVCHUK NIKOLAY FILIPPOVICH +2
Compound, and preparation method and application thereof
ActiveCN103172633AInhibitory activityHigh selectivityOrganic chemistryMetabolism disorderDiseaseUse medication
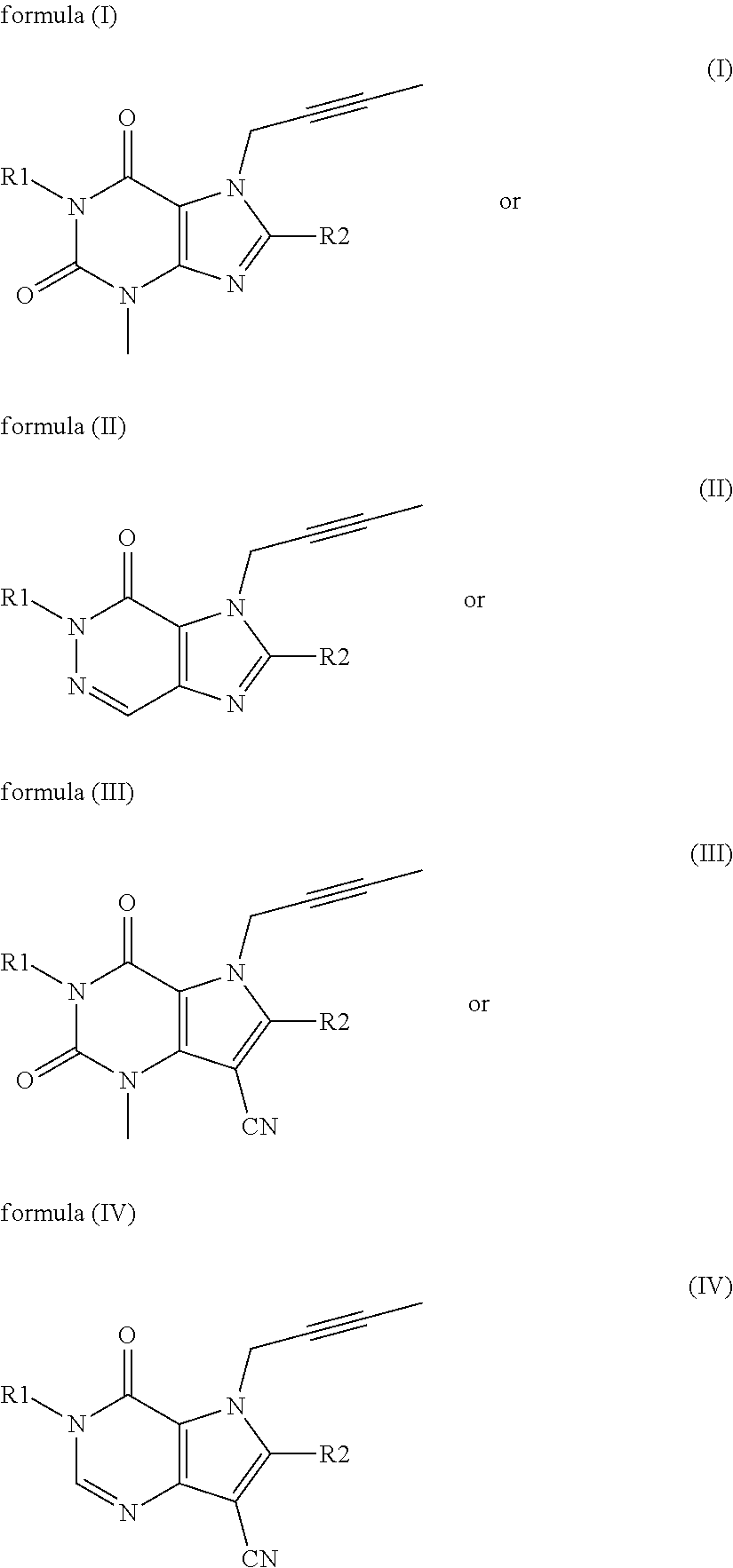
The invention provides a compound disclosed as Formula I or a pharmaceutically acceptable salt thereof, and a preparation method and application thereof. The new compound provided by the invention can effectively inhibit dipeptidyl peptidase-IV (DPP-IV) activity with better effects than the existing DPP-IV enzyme inhibitor Linagliptin; and meanwhile, the compound has the advantages of higher selectivity for DPP-IV, higher safety and lower toxic or side effect on the DPP inhibitor, and thus, provides a new medicine option for treating or / and preventing diseases related to DPP-IV enzyme activity.
Owner:CHENGDU DIAO PHARMA GROUP
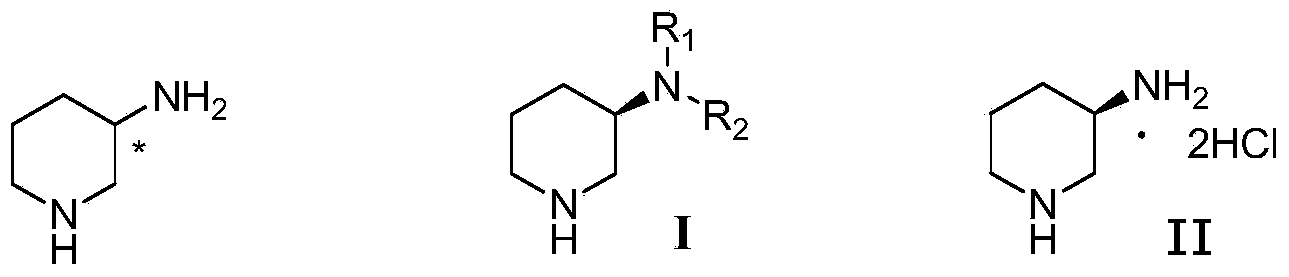
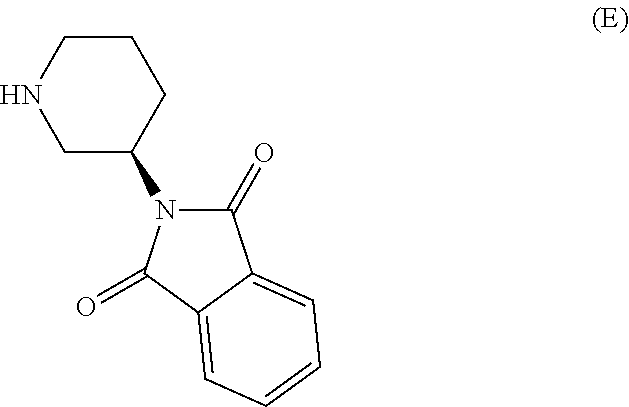
Compound I and (R)-3-aminopiperidine hydrochloride II, preparation method and application in Linagliptin synthesis
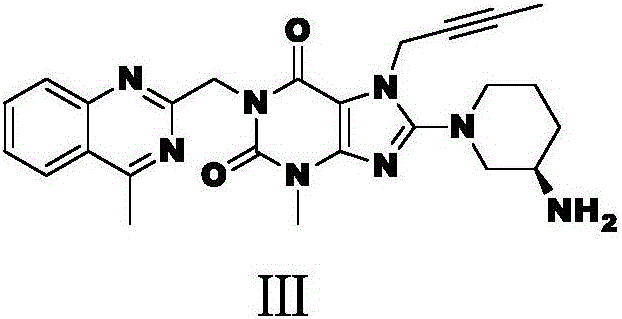
ActiveCN104387315AHigh optical purityHigh process reproducibilityOptically-active compound separationOrganic racemisationDipeptidyl peptidase3-Aminopyridine
The invention discloses a preparation method of 3-aminopiperidine and its derivative with optical activity and an application of the compound and its derivative in synthesis of a dipeptidyl peptidase-IV inhibitor Linagliptin. According to the preparation method, 3-aminopyridine is used as a raw material to prepare a compound I by 3- amino protection, catalytic hydrogenation reduction of pyridine ring and chiral reagent resolution, and deprotection is then carried out to obtain (R)-3-aminopiperidine hydrochloride II. In the application, a compound III prepared from the compound I is an important intermediate for synthesis of Linagliptin, and (R)-3-aminopiperidine hydrochloride II also can directly be used for synthesis of high-purity Linagliptin. The raw material used in the invention is low-cost and is easily-available in the market; each step is simple to operate; requirements on equipment are low; safety is high; and the preparation method is easy for industrial production.
Owner:2Y CHEM
Pharmaceutical composition, pharmaceutical dosage form, process for their preparation, methods for treating and uses thereof
InactiveUS20180104249A1Few complianceFew convenienceSenses disorderNervous disorderMetabolic disorderDosage form
The present invention relates to pharmaceutical compositions of linagliptin, pharmaceutical dosage forms, their preparation, their use and methods for treating metabolic disorders.
Owner:BOEHRINGER INGELHEIM INT GMBH
Preparation method for linagliptin
The invention belongs to the technical field of bulk drug preparation and particularly relates to an improvement in a preparation method for linagliptin. According to the preparation method for linagliptin, the particle diameter of an acid-binding agent anhydrous sodium carbonate used in a linagliptin two-step condensation reaction is controlled on a micron order, an iodide catalyst is not needed during the reaction, similarly, the reaction temperature is decreased, reaction time is shortened, the two-step reaction is changed into a one-pot reaction, and a key intermediate E compound with high purity and yield is prepared. The preparation method is suitable for industrial mass production of linagliptin, and finally linagliptin with high purity and yield is obtained.
Owner:SHANGHAI WANXIANG PHARMA
Synthesis method of linagliptin intermediate
ActiveCN105906627AHigh purityPurity does not affectOrganic chemistrySynthesis methods8-bromoxanthine
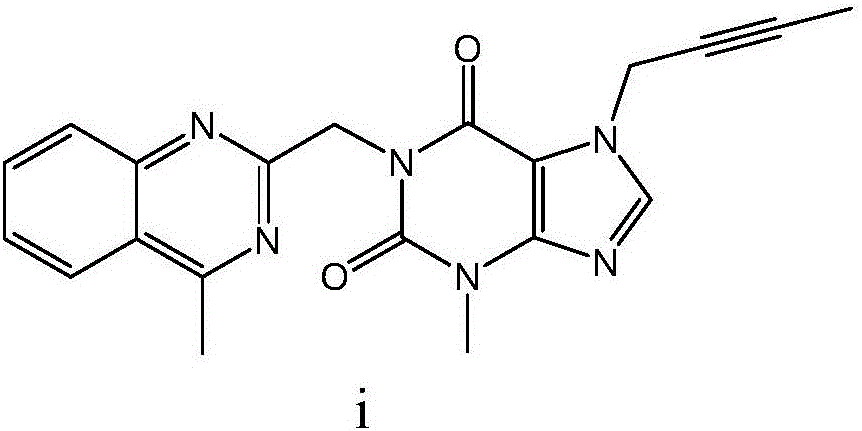
The invention discloses a synthesis method of a linagliptin intermediate. The synthesis method comprises adding 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e), (R)-3-Boc-aminopiperidine (f), potassium carbonate and acetonitrile into a reactor, carrying out uniform mixing and carrying out a reaction process under the conditions of heating reflux, a reaction temperature of 80-85 DEG C and reaction time of 24-48h, wherein a mole ratio of 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e), (R)-3-Boc-aminopiperidine (f) to potassium carbonate is 1: (1.2-1.6): (3-7) and a ratio of 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e) to acetonitrile is 100: (400-1000)g / ml. The synthesis method of the linagliptin intermediate (g) prevents generation of debromination impurities (i), is simple and easy in a post-treatment step and solves the problem that the intermediate is not easily separated from a solvent and is difficultly purified and separated.
Owner:赤峰赛林泰药业有限公司
Process for the preparation of linagliptin
Owner:DIPHARMA FRANCIS
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20180344647A1Reduce riskImprove blood sugar controlOrganic active ingredientsMetabolism disorderImmediate releaseMetformin Hydrochloride
The invention relates to solid pharmaceutical dosage forms comprising an extended release core comprising metformin hydrochloride and one or two immediate release coatings comprising linagliptin and / or empagliflozin.
Owner:BOEHRINGER INGELHEIM INT GMBH
Simple preparation method of II-type antidiabetic drug linagliptin
InactiveCN104844603AEasy to operateShort reaction stepsOrganic chemistryMetabolism disorderFiltrationXanthine
The invention discloses a simple preparation method of II-type antidiabetic drug linagliptin which comprises the following steps: adding 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine into a DMSO (dimethylsulfoxide) solution of 2-chloromethyl-4-methyl-quinazoline and potassium carbonate A, and under the catalysis of potassium iodide, reacting for 7-8 hours at a temperature of 70-80 DEG C; adding potassium carbonate B and (R)-3-aminopiperidine, and reacting for 7-8 hours at a temperature of 70-80 DEG C; after the reaction is completed, adding saturated salt water, separating out solids, and carrying out suction filtration to obtain a crude product linagliptin; and recrystallizing the crude product linagliptin with methyl alcohol, carrying out suction filtration and drying, thus obtaining a target product. The method disclosed by the invention is implemented by using a one-pot method, so that the method is simple and easy to operate and short in reaction steps, and raw materials are cheap and easily available, therefore, the method is applicable to industrial mass production; and the method overcomes the problems in the conventional method that impurities are difficult to remove, line operations are complicated and time-consuming, and too many raw materials are required.
Owner:WUHAN UNIV OF TECH
Simple preparation method of high-purity linagliptin
The invention relates to a simple preparation method of high-purity linagliptin. The method includes the steps of making 8-bromine-3-methyl xanthine and 1-bromo-2-butyne react, directly adding 2-chloromethyl-4-methylquinazoline without processing after reaction is completed, preparing a key intermediate 8-bromine-7-(2-butyne-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-diketone of linagliptin through a one-pot method, making the intermediate react with (R)-3-piperidinamine dihydrochloride after being filtered and separated to obtain a linagliptin solution, and obtaining a linagliptin pure product after processing the linagliptin solution. The key intermediate is prepared through the one-pot method, operation is convenient, and yield is increased; the key intermediate reacts with (R)-3-piperidinamine dihydrochloride after being separated, and therefore high-purity linagliptin is obtained, and the requirements for production and declaration of pharmaceutical enterprises are met to the maximum extent.
Owner:VALIANT CO LTD
Linagliptin composition and preparation method thereof
InactiveCN104644563ADisintegrates quicklyMask bitternessMetabolism disorderPharmaceutical non-active ingredientsMedicineDrug compliance
The invention discloses linagliptin particles and a preparation method thereof. Linagliptin is used for treating type 2 diebetes. At present, only linagliptin tablets are sold on the market. Aiming at dysphagia patients, compared with the above two dosage forms, the linagliptin particles have obvious advantages and can improve drug compliance of patients.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Dipeptidyl peptidase IV inhibitor pharmaceutical composition, use and preparation method thereof
ActiveCN105456270AReduce typesLow costMetabolism disorderPharmaceutical non-active ingredientsStarch cornMagnesium stearate
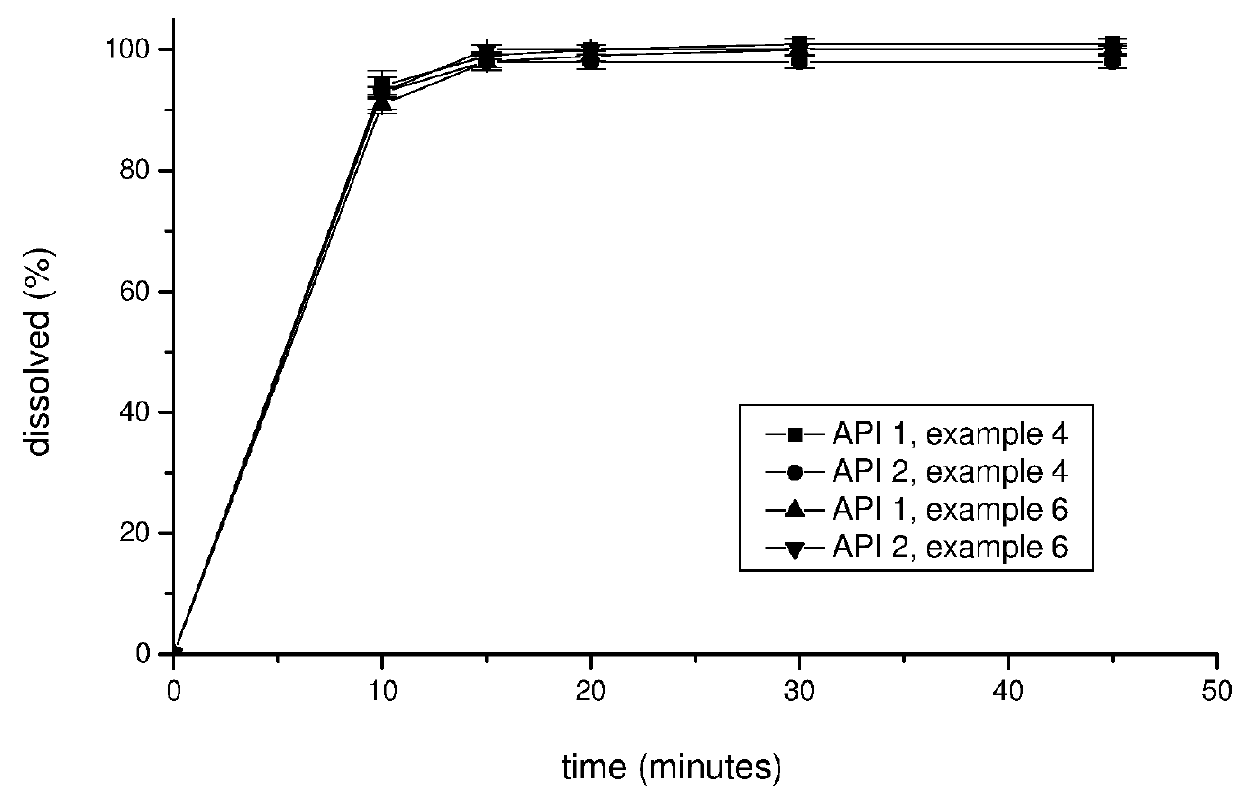
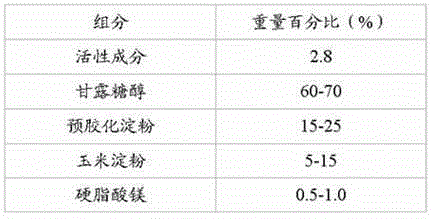
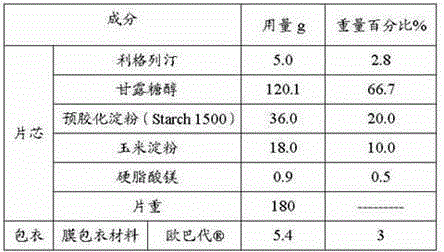
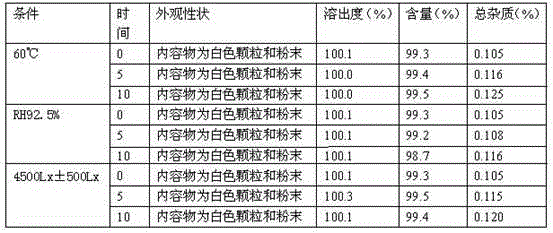
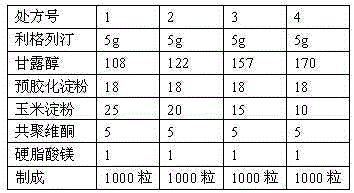
The invention discloses a pharmaceutical composition containing a dipeptidyl peptidase IV inhibitor linagliptin, use and preparation method thereof. The linagliptin containing pharmaceutical composition provided by the invention consists of linagliptin or a salt thereof serving as the active ingredient, and pharmaceutical excipients mannitol, pregelatinized starch, corn starch and magnesium stearate. The linagliptin containing pharmaceutical composition provided by the invention reduces the types of excipients, increases the stability of the preparation, reduces the cost of raw materials, and solves the hardness and friability problems of linagliptin tablets by controlling the particle size of the key excipient mannitol. The obtained table has all indicators especially the dissolution rate in line with the drug quality standards, and the process is simple, thus being more suitable for large scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method for crystal form of linagliptin
The invention specifically relates to a preparation method for a crystal form of linagliptin, belonging to the field of medicine. The preparation method has the advantages of simple operation, good reproducibility, good purification effect, suitability for industrial production, etc.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Linagliptin new crystal form and preparation method thereof
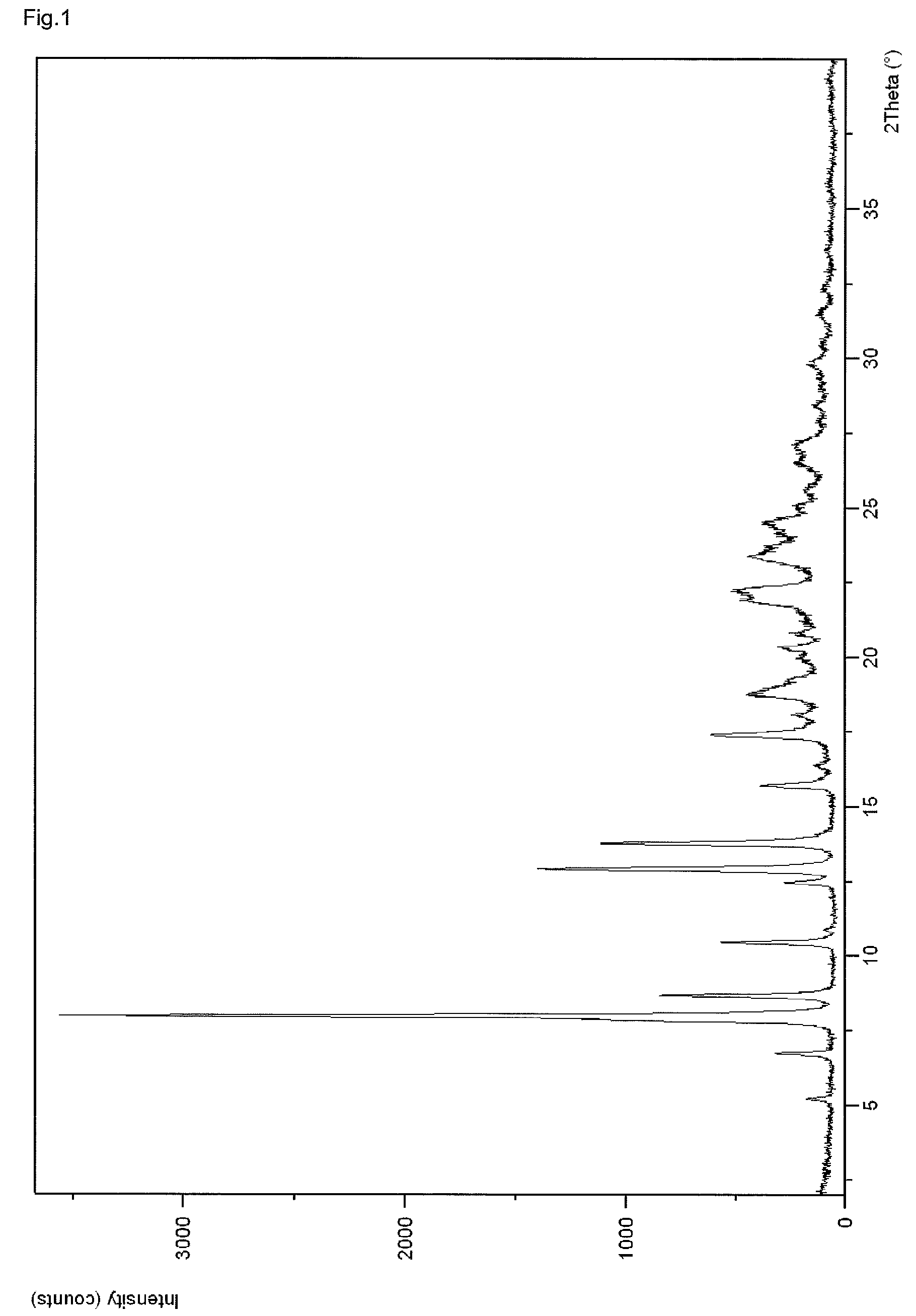
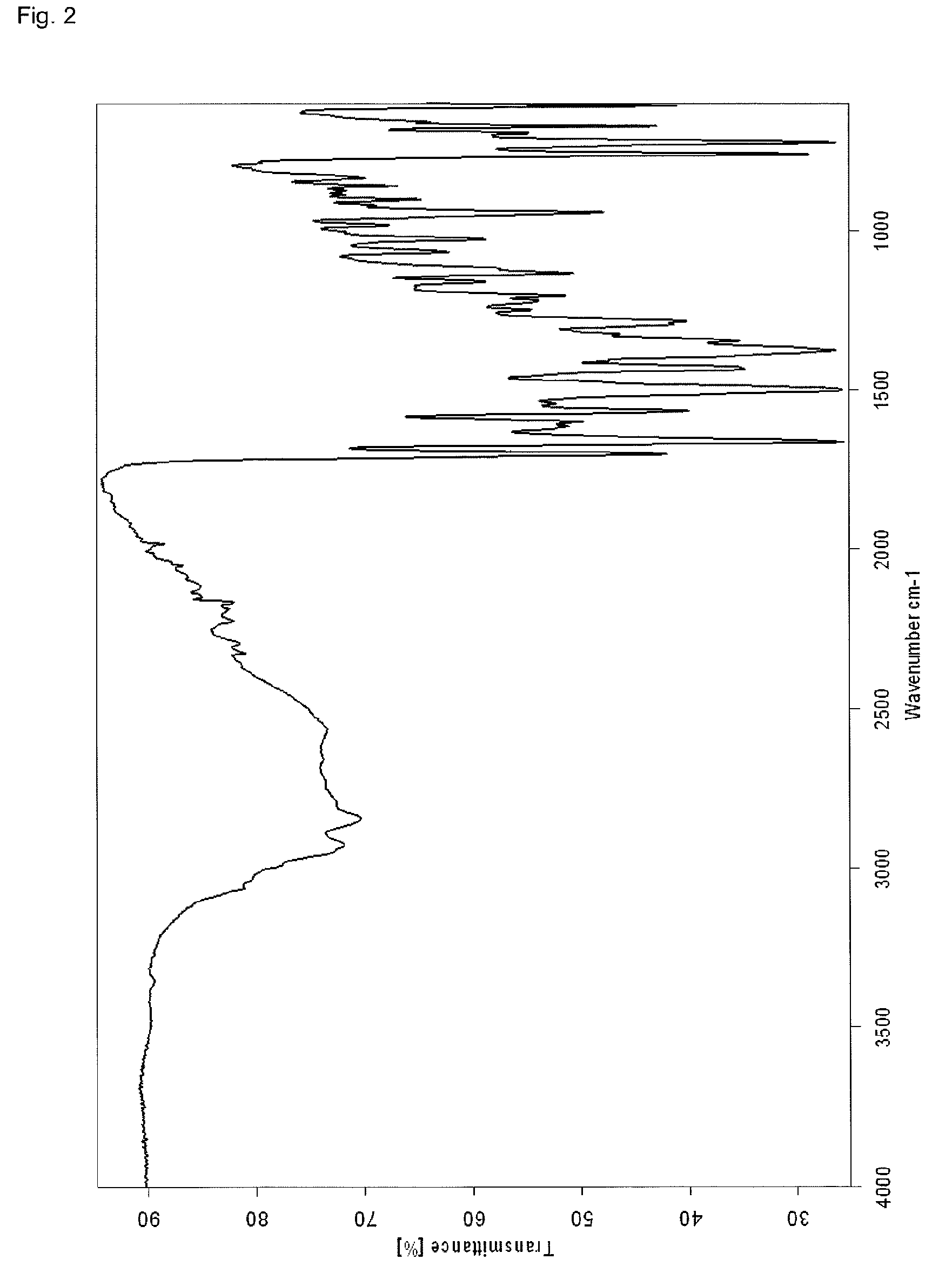
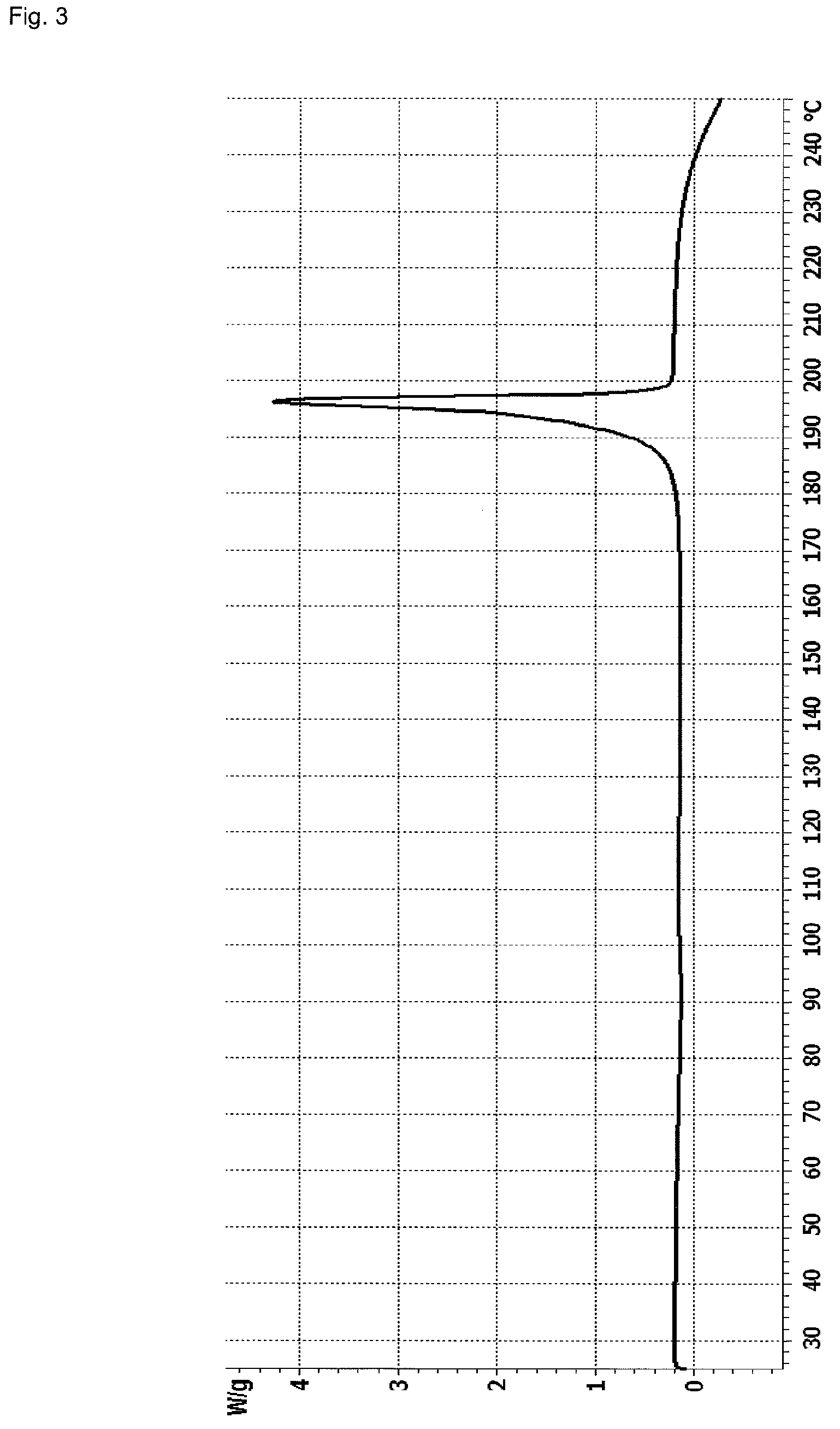
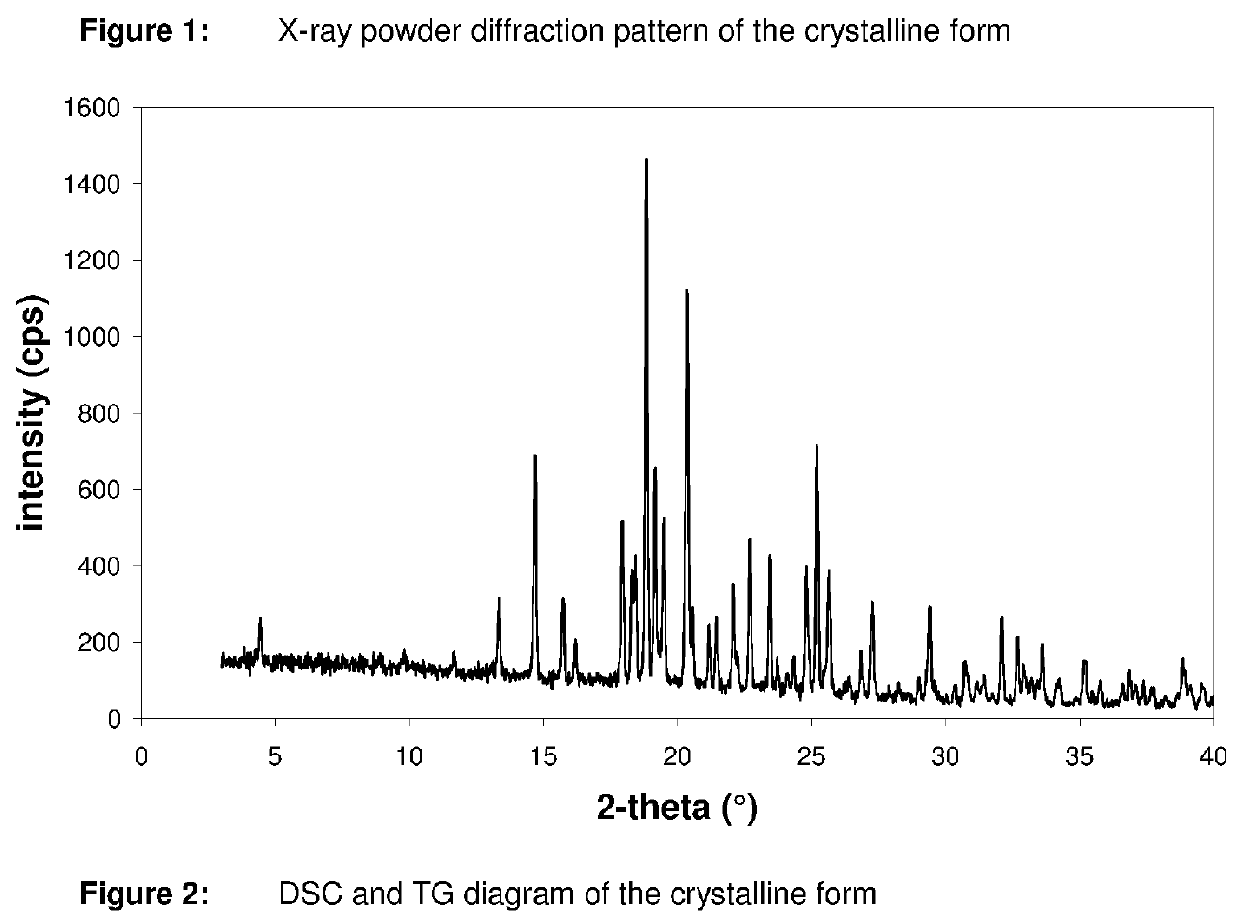
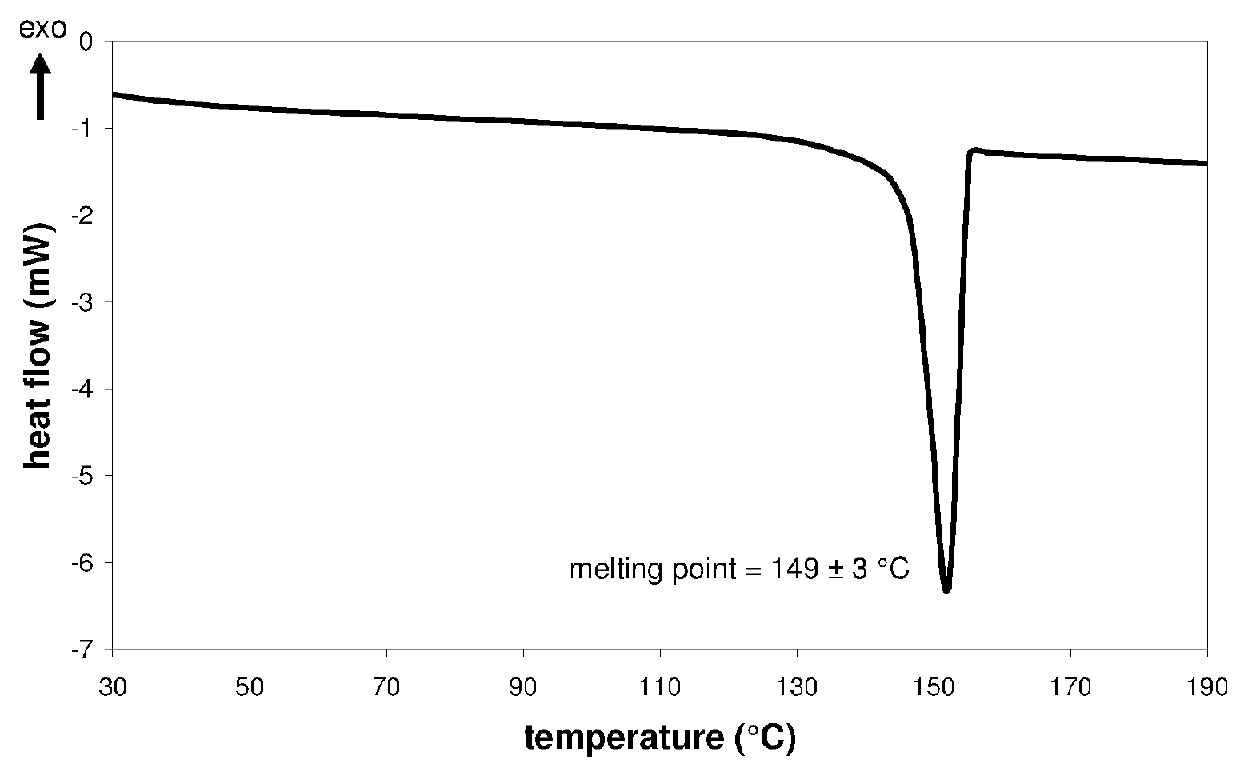
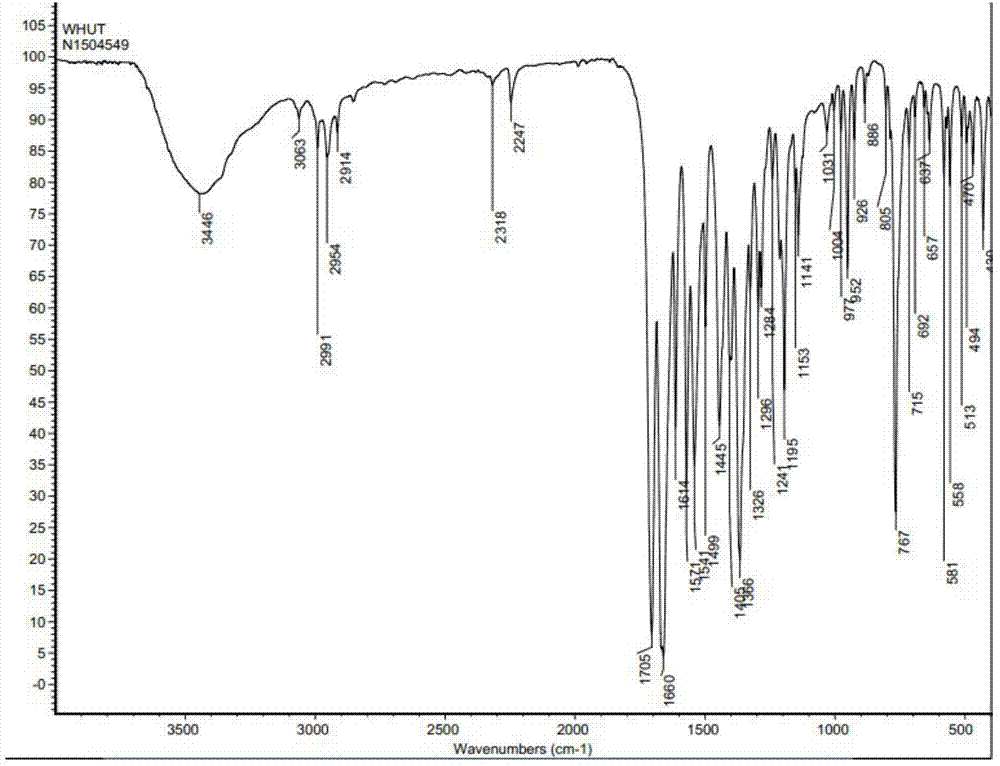
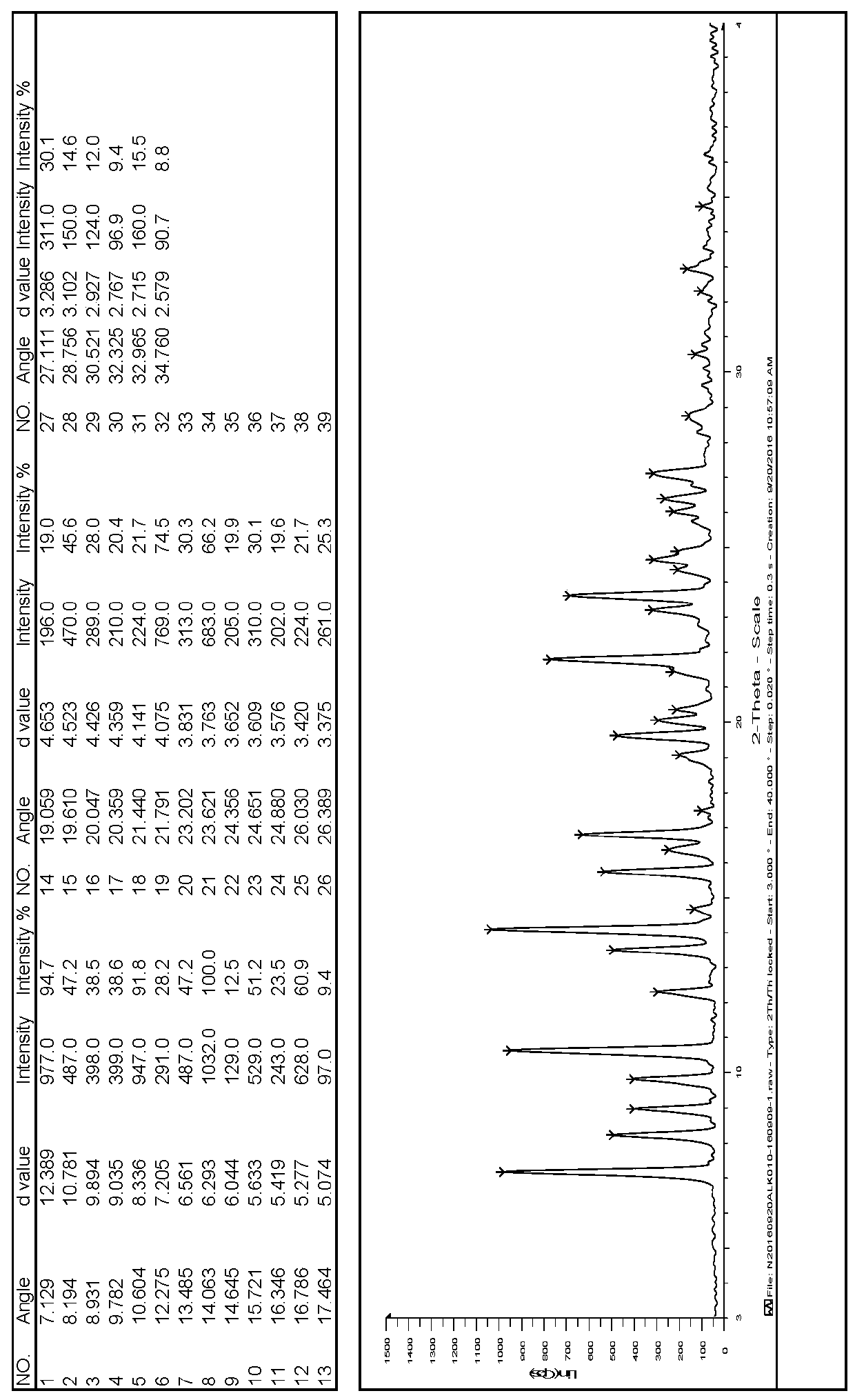
InactiveCN107043376AGood chemical stabilityEasy to operateOrganic chemistry methodsNMR - Nuclear magnetic resonanceX-ray
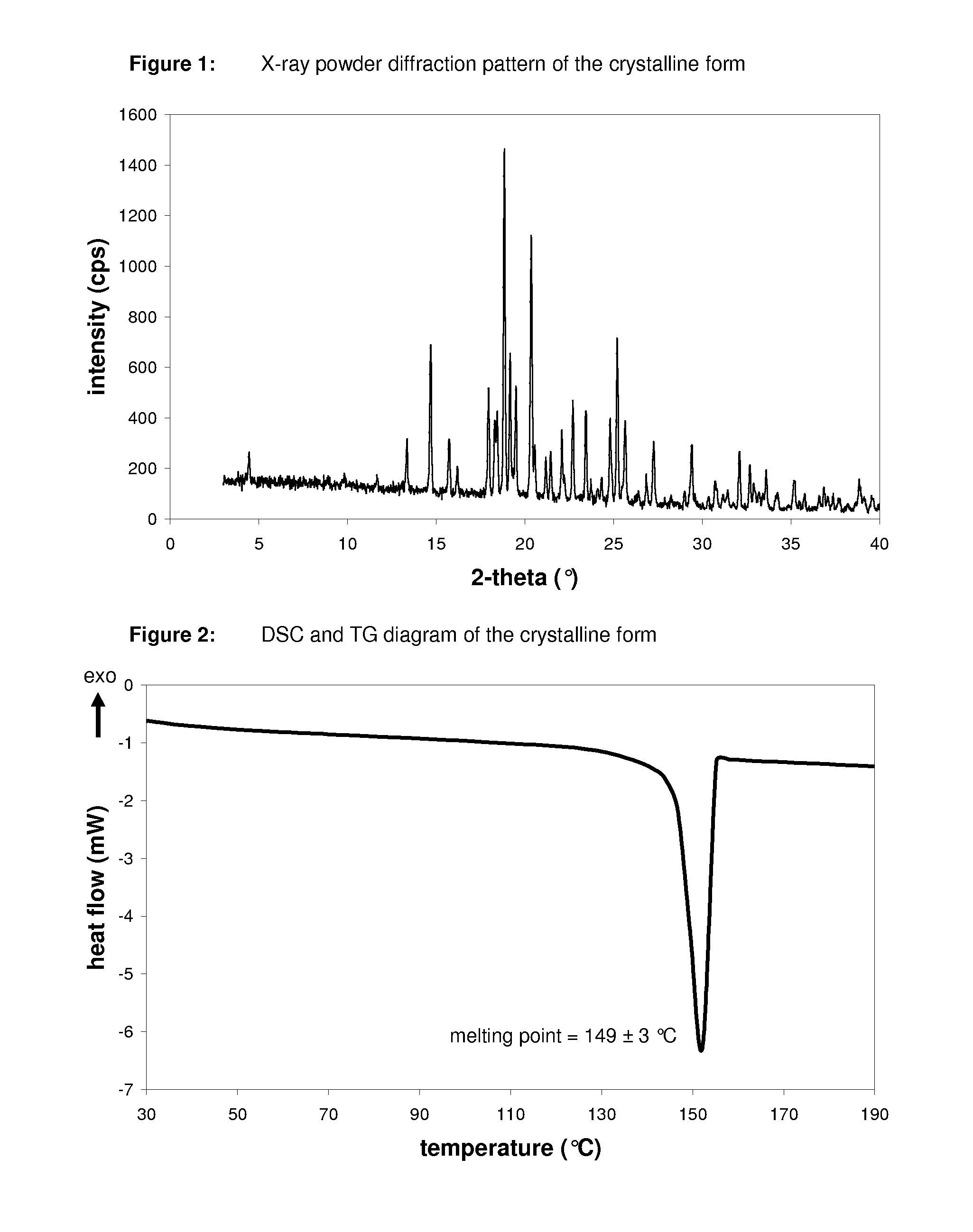
The invention discloses a linagliptin new crystal form and a preparation method thereof, belonging to the technical field of medicinal chemistry. X-ray powder diffraction, infrared analysis, melting point measurement, differential scanning calorimetry, thermogravimetric analysis, and nuclear magnetic resonance are performed on the new crystal form. The linagliptin new crystal form is obviously different from the linagliptin crystal form in the prior art. The new crystal form has good chemical stability and crystal form purity, is easy to prepare in a large scale, is simple to operate, and has a broad application prospect.
Owner:ZHUHAI UNITED LAB
Conversion method for dimers
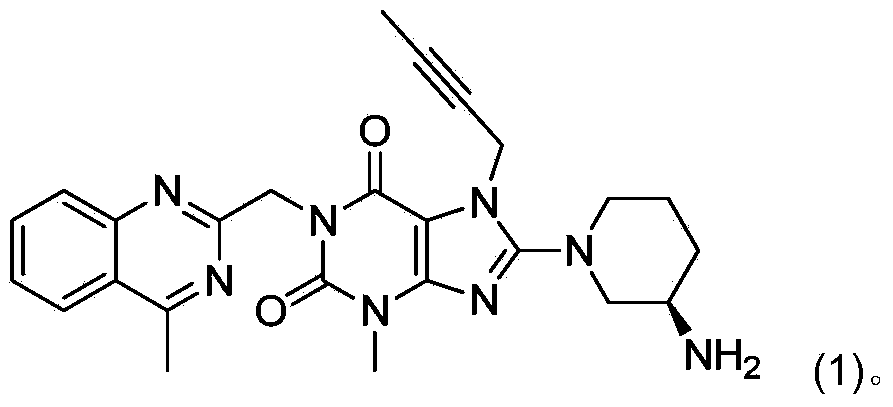
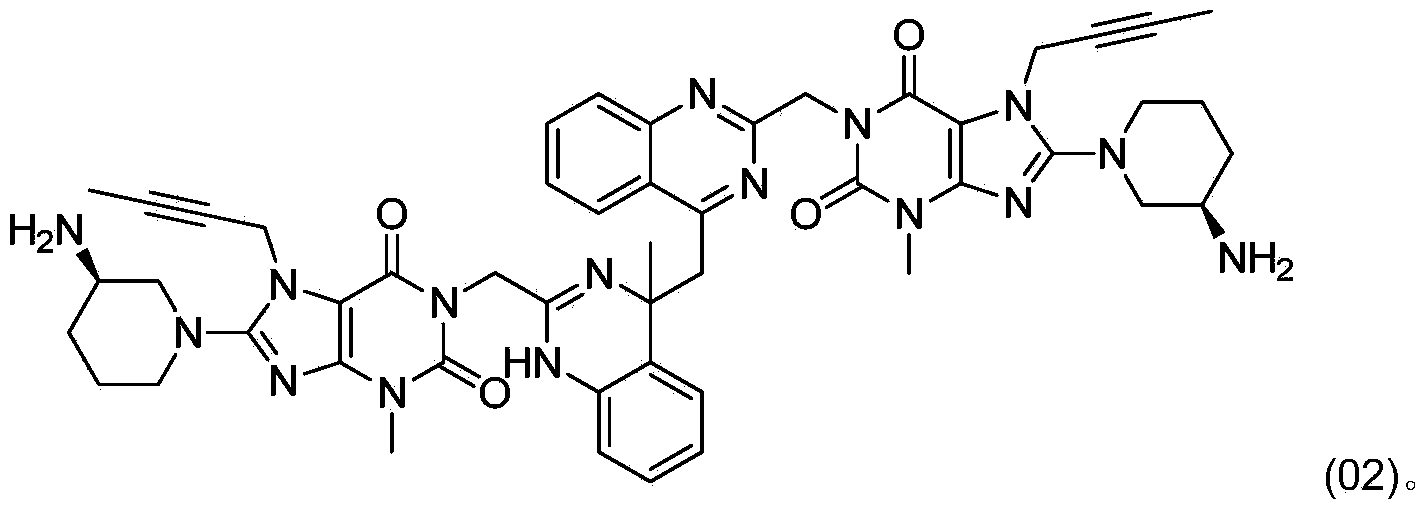
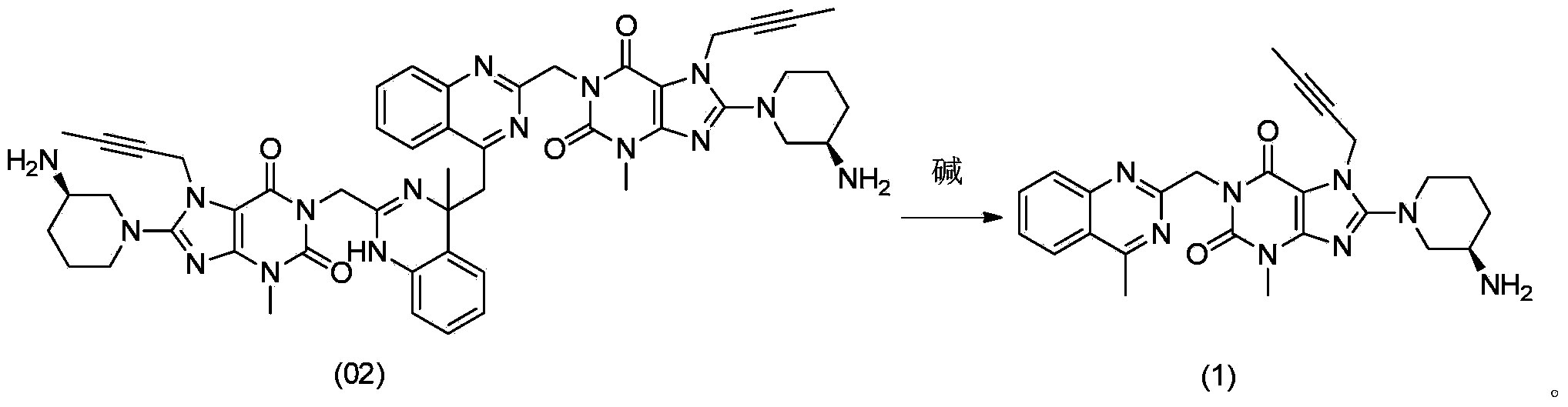
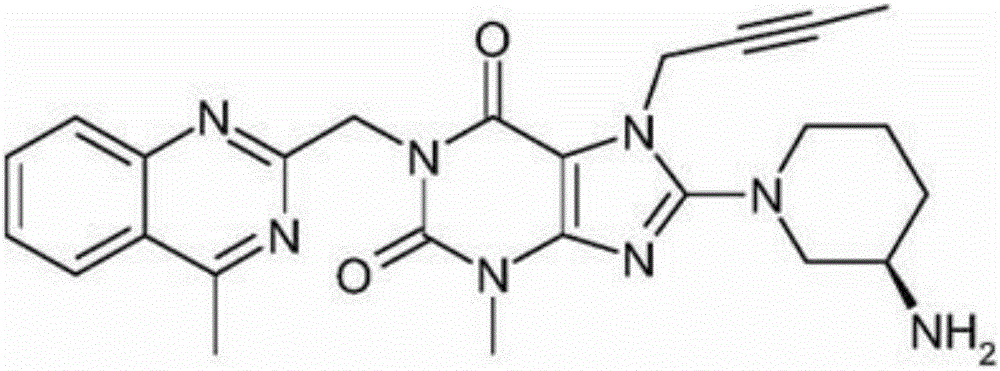
The invention relates to a method for converting dimers of linagliptin into linagliptin. Under the alkaline condition that the pH value is not less than 8, dimers can be converted into a product linagliptin under the action of alkali, so that impurities are removed, and the yield is increased. The method is simple in process, reduces the production cost, and facilitates the industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of linagliptin
ActiveCN105906628AHigh purityPurity does not affectOrganic chemistryTert-Butyloxycarbonyl protecting groupReaction temperature
The invention discloses a preparation method of linagliptin. The preparation method comprises putting t-butyloxycarbonyl-linagliptin (g) into a methanol aqueous solution and carrying out stirring so that the solution undergoes a reaction along with heating reflux in an inert atmosphere at a temperature of 25-50 DEG C for 3-12h, wherein a weight ratio of the t-butyloxycarbonyl-linagliptin (g) to the methanol aqueous solution is 100: (400-550). The preparation method carries out Boc protection base removal in a methanol aqueous solution in an inert gas protective atmosphere, is free of expensive trifluoroacetic acid-DCM in a Boc protection base removal reaction, only utilizes a cheap methanol-water system and has simple after-treatment processes. A trifluoroacetic acid-DCM system reaction produces more impurities and needs a very complex post-treatment purifying process. The preparation method prevents strong acid-produced intermediate (g) impurities and linagliptin amide bond fracture impurities.
Owner:赤峰赛林泰药业有限公司
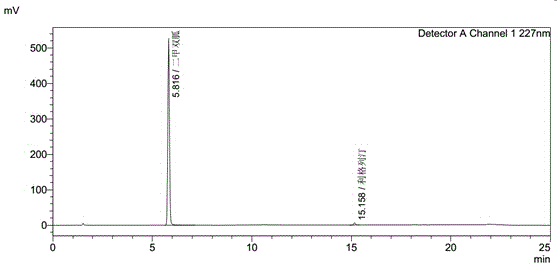
High performance liquid chromatography method for simultaneous determination of linagliptin and metformin contents
InactiveCN105277644ASatisfy R&DMeet production needsComponent separationTest analysisAnalysis method
The present invention discloses a high performance liquid chromatography method for simultaneous determination of linagliptin and metformin contents, a reverse-phase high performance liquid chromatography method is used, a to-be-tested solution and a contrast solution are prepared, 10mul of the to-be-tested solution and 10mul of the contrast solution are respectively taken by measuring, and injected into a chromatographic instrument, chromatogram is recorded, and determination of linagliptin and metformin contents can be completed by peak area calculation and conversion into dry content by external standard method; the advantages of the method are that: the method fills the blank of no analysis method for determination of linagliptin and metformin contents in the prior art, can satisfy the needs of research and development and production, and provides the necessary technical support for linagliptin drug to come into the market early.
Owner:JIANGSU SINOBIOPHARMA
Industrial production method of linagliptin
InactiveCN106008508AHigh purityGood effect of removing impuritiesOrganic chemistryFiltrationTert-Butyloxycarbonyl protecting group
An industrial production method of linagliptin. According to the molar ratio, 8-bromo-7-(2-butyne)-3-methylxanthine and 2-chloromethyl-4-methylquinazoline are added to the dipolar aprotic organic solvent, and then the base is added and potassium iodide, crystallized, filtered and dried to obtain intermediate I; according to the molar ratio, intermediate I and (R)-3-tert-butoxycarbonylaminopiperidine were added to the dipolar aprotic organic solvent, and alkali was added to analyze crystallized, filtered and washed to remove inorganic salts, and the solvent was removed under reduced pressure to obtain the crude product, which was then crystallized from methanol and isopropanol to obtain intermediate II; according to the volume ratio, intermediate II and the deprotection reagent were added to the reaction solvent, and crystallized to obtain The crude product was recrystallized with ethanol, filtered and dried to obtain the final product of linagliptin. The method of the invention can greatly shorten the production cycle, save production cost, obtain intermediates with higher purity, and better effect of removing impurities. The method is safe, reliable, simple and easy to operate, and has good repeatability.
Owner:合肥远志医药科技开发有限公司
Stable linagliptin medicine composition
InactiveCN106138059AAvoid degradationKeep dryMetabolism disorderPharmaceutical non-active ingredientsBioavailabilityPharmaceutical Substances
The invention discloses a stable linagliptin medicine composition and a preparation method thereof. According to the formula, besides active components, through selection on a diluent and a disintegrating agent in special composition with ratio of all components being controlled, the linagliptin has excellent stability and bioavailability.
Owner:TIANJIN HANRUI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com