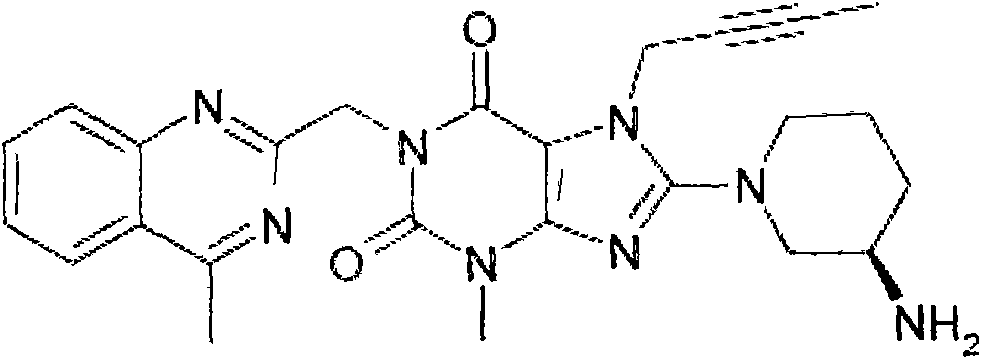
Linagliptin impurity, and preparation method and application thereof
A technology of impurities and uses, which is used as an impurity reference substance in the preparation method and quality control, in the field of linagliptin impurities, can solve problems affecting drug efficacy, side effects, etc., and achieve the effect of ensuring safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] step one:
[0027]
[0028] Add N-methylpyrrolidone (1000ml) into the reaction kettle, add L409-1101 (166g, 0.18mol) and sodium carbonate (180g, 1.7mol) sequentially under stirring, and start adding L409-1102 (99.5g , 0.75mol) of N-methylpyrrolidone (375ml), and added dropwise within 1 hour, monitored by TLC, after the reaction was complete, L409-11 was obtained.
[0029] Step two:
[0030]
[0031] Heat the reaction solution in Step 1 to 100°C, add L409-2203 (144g, 0.75mol) into the reaction kettle, and wash the kettle wall with N-methylpyrrolidone (208ml); react at 115-120°C for 3-6h, monitor by HPLC, After the reaction was complete, post-treatment was performed to obtain 222 g of a yellow-brown solid (L409-22) with a yield of 72%.
[0032] Step three:
[0033]
[0034] Add the product obtained in Step 2 (222g, 0.48mol) into the reaction kettle, add N-methylpyrrolidone (666ml), stir slowly, raise the temperature to 115-120°C, and add N,N-diiso Propylethyl...
Embodiment 2
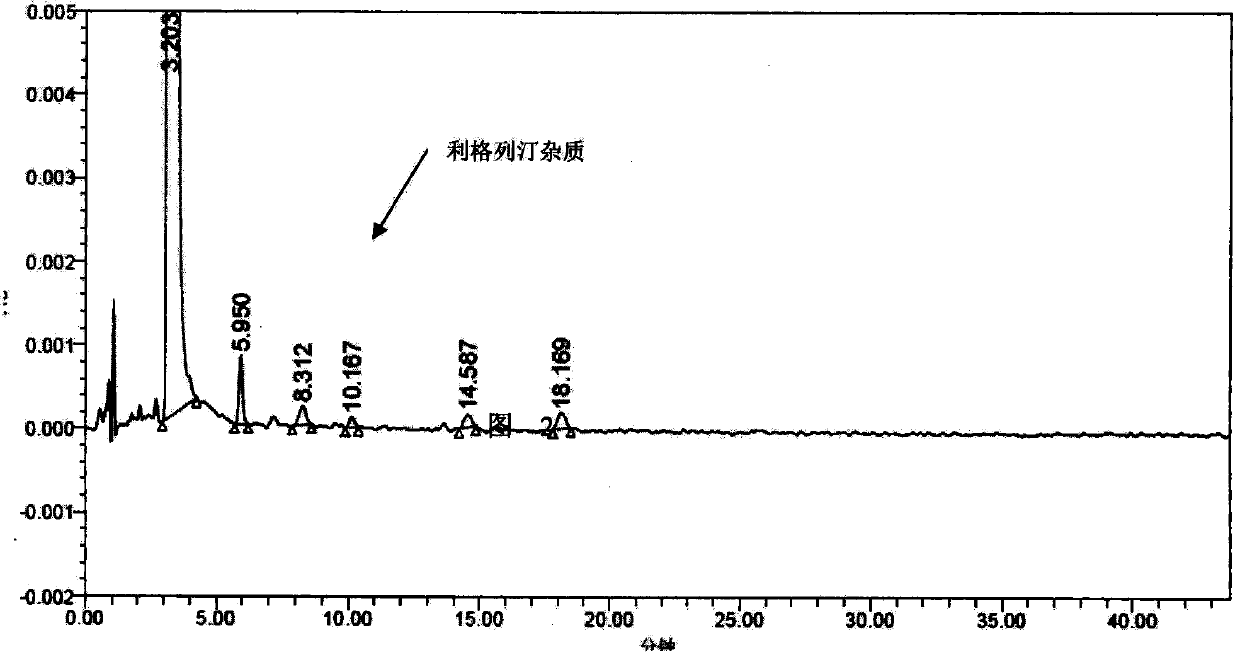
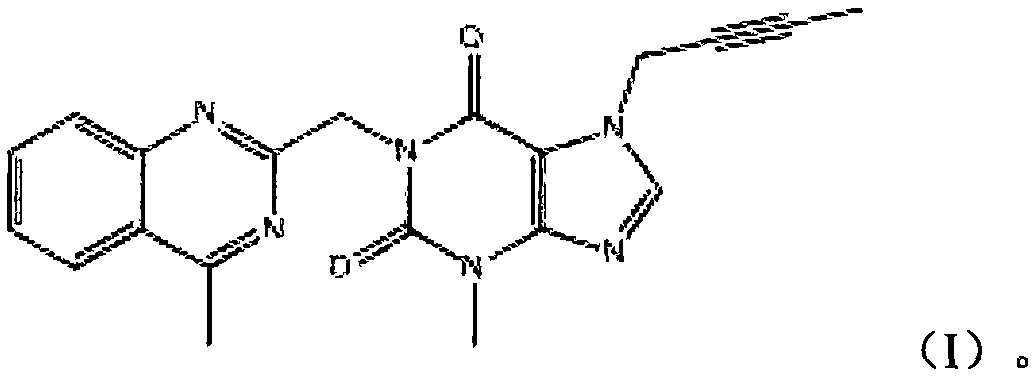
[0036] The impurity of formula (I) is used as a reference substance of related substances to determine related substances of linagliptin.
[0037] Chromatographic conditions: use octadecylsilane bonded silica gel as filler; use phosphate buffer as mobile phase A, use methanol-acetonitrile (55:45) as mobile phase B, and A-B (65:35) as mobile phase; The column temperature is 55°C; the detection wavelength is 225nm.
[0038] Need testing solution: take appropriate amount of this product, accurately weighed, add mobile phase A-acetonitrile (70:30) ultrasonically dissolve and quantitatively dilute to make a solution containing 0.5mg in every 1ml, as need testing solution.
[0039] Impurity reference substance solution: take an appropriate amount of linagliptin impurity shown in formula (I), accurately weighed, add mobile phase A-acetonitrile (70: 30) to dissolve and quantitatively dilute to make a solution containing 0.5 μg in every 1ml, as Impurity reference substance solution. ...
Embodiment 3
[0044] The impurity of formula (I) was used as a reference substance for related substances to determine related substances in linagliptin tablets.
[0045] Measure according to high performance liquid chromatography (Chinese Pharmacopoeia 2010 edition two appendix VD), chromatographic conditions refer to embodiment 2.
[0046] Need testing solution: take an appropriate amount of linagliptin tablet fine powder, accurately weighed, add mobile phase A-acetonitrile (70:30) to ultrasonically dissolve and quantitatively dilute to make a solution containing 0.5 mg per 1 ml, as the test product solution.
[0047]Impurity reference substance solution: take an appropriate amount of linagliptin impurity shown in formula (I), accurately weighed, add mobile phase A-acetonitrile (70: 30) to dissolve and quantitatively dilute to make a solution containing 0.5 μg in every 1ml, as Impurity reference substance solution.
[0048] Determination method: Precisely measure 10 μl each of the test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com