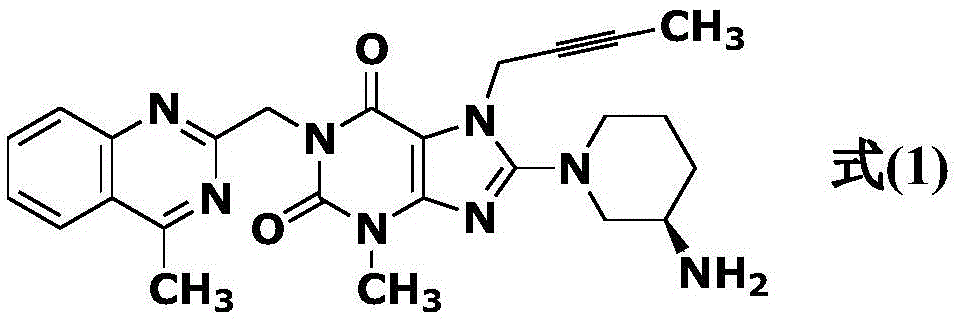
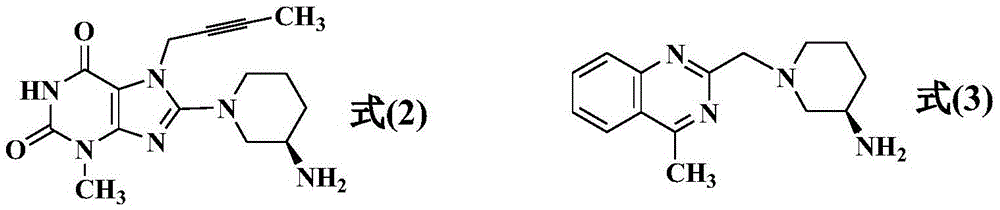
Simple preparation method of high-purity linagliptin
A high-purity, simple technology, applied in the field of pharmaceuticals, can solve the problems of inability to control key intermediates, unsuitable for raw material drug declaration and production, etc., to achieve the effects of ensuring chiral purity, simplifying operations, and avoiding production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
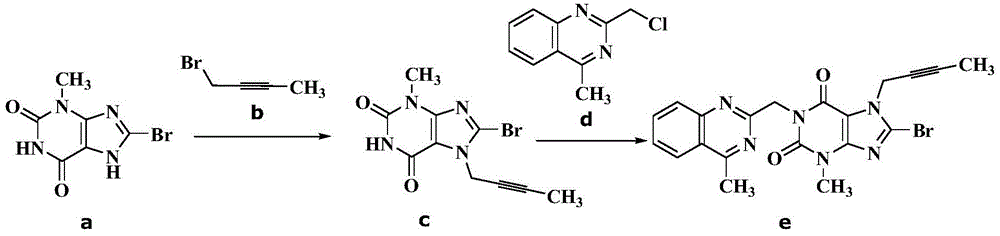
[0029] 60.0g (0.245mol) compound a (8-bromo-3-methylxanthine), 32.6g (0.245mol) compound b (1-bromo-2-butyne), 63.3g (0.490mol ) diisopropylethylamine and 570gN, N-dimethylformamide, warming up to 95-105 ℃, insulated and stirred for 4 hours, TLC monitoring without 8-bromo-3-methylxanthine residue (Rf (8-bromo -3-methylxanthine)=0.4, Rf (reaction solution)=0.6, developer: toluene / dehydrated alcohol=6 / 1, volume ratio), add 47.2g (0.245mol) compound d (2 -(chloromethyl)-4-methylquinazoline), continue to insulate and stir at 95-105°C for 3 hours, TLC monitors that there is no compound c(8-bromo-7-but-2-yn-1-yl-3 -Methyl-3,7-dihydro-1H-purine-2,6-dione) remaining (Rf (compound c) = 0.6, Rf (reaction solution) = 0.7, developer: toluene / absolute ethanol = 6 / 1, volume ratio), cooling the reaction solution to 5-25°C, slowly adding 600g of tap water dropwise to the system, stirring at 15-25°C for 0.5 hours, filtering, rinsing with toluene, and vacuum-drying the obtained solid at 70°C ...
Embodiment 2
[0034] 60.0g (0.245mol) compound a (8-bromo-3-methylxanthine), 32.6g (0.245mol) compound b (1-bromo-2-butyne), 63.3g (0.490mol ) diisopropylethylamine and 570gN, N-dimethylformamide, warming up to 90-100 ℃, insulated and stirred for 5 hours, TLC monitoring without 8-bromo-3-methylxanthine residue (Rf (8-bromo -3-methylxanthine)=0.4, Rf (reaction solution)=0.6, developer: toluene / dehydrated alcohol=6 / 1, volume ratio), add 47.2g (0.245mol) compound d (2-(chloro Methyl)-4-methylquinazoline), continue to stir at 90-100°C for 3 hours, TLC monitors that there is no compound c (8-bromo-7-but-2-yn-1-yl-3-methyl -3,7-dihydro-1H-purine-2,6-dione) remaining (Rf (compound c) = 0.6, Rf (reaction solution) = 0.7, developer: toluene / absolute ethanol = 6 / 1, volume ratio), the reaction solution was cooled to 5-25°C, and 600g of tap water was slowly added dropwise to the system, stirred at 15-25°C for 0.5 hours, filtered, rinsed with toluene, and the obtained solid was vacuum-dried at 70°C to ...
Embodiment 3
[0037]60.0g (0.245mol) compound a (8-bromo-3-methylxanthine), 32.6g (0.245mol) compound b (1-bromo-2-butyne), 49.5g (0.490mol ) triethylamine and 570gN-methylpyrrolidone, warming up to 90-100 ℃, insulated and stirred for 5 hours, TLC monitoring without 8-bromo-3-methylxanthine remaining (Rf (8-bromo-3-methylxanthine )=0.4, Rf (reaction solution)=0.6, developer: toluene / dehydrated alcohol=6 / 1, volume ratio), add 47.2g (0.245mol) compound d (2-(chloromethyl)-4-methyl Quinazoline), continue to stir at 90-100 ° C for 3 hours, TLC monitoring no compound c (8-bromo-7-but-2-yn-1-yl-3-methyl-3,7-dihydro -1H-purine-2,6-dione) remaining (Rf (compound c) = 0.6, Rf (reaction solution) = 0.7, developer: toluene / absolute ethanol = 6 / 1, volume ratio), the reaction solution Cool down to 5-25°C, slowly add 600g of tap water dropwise to the system, stir at 15-25°C for 0.5 hours, filter, rinse with toluene, and dry the obtained solid in vacuum at 70°C to obtain compound e (8-bromo-7-(2 -butyn-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com