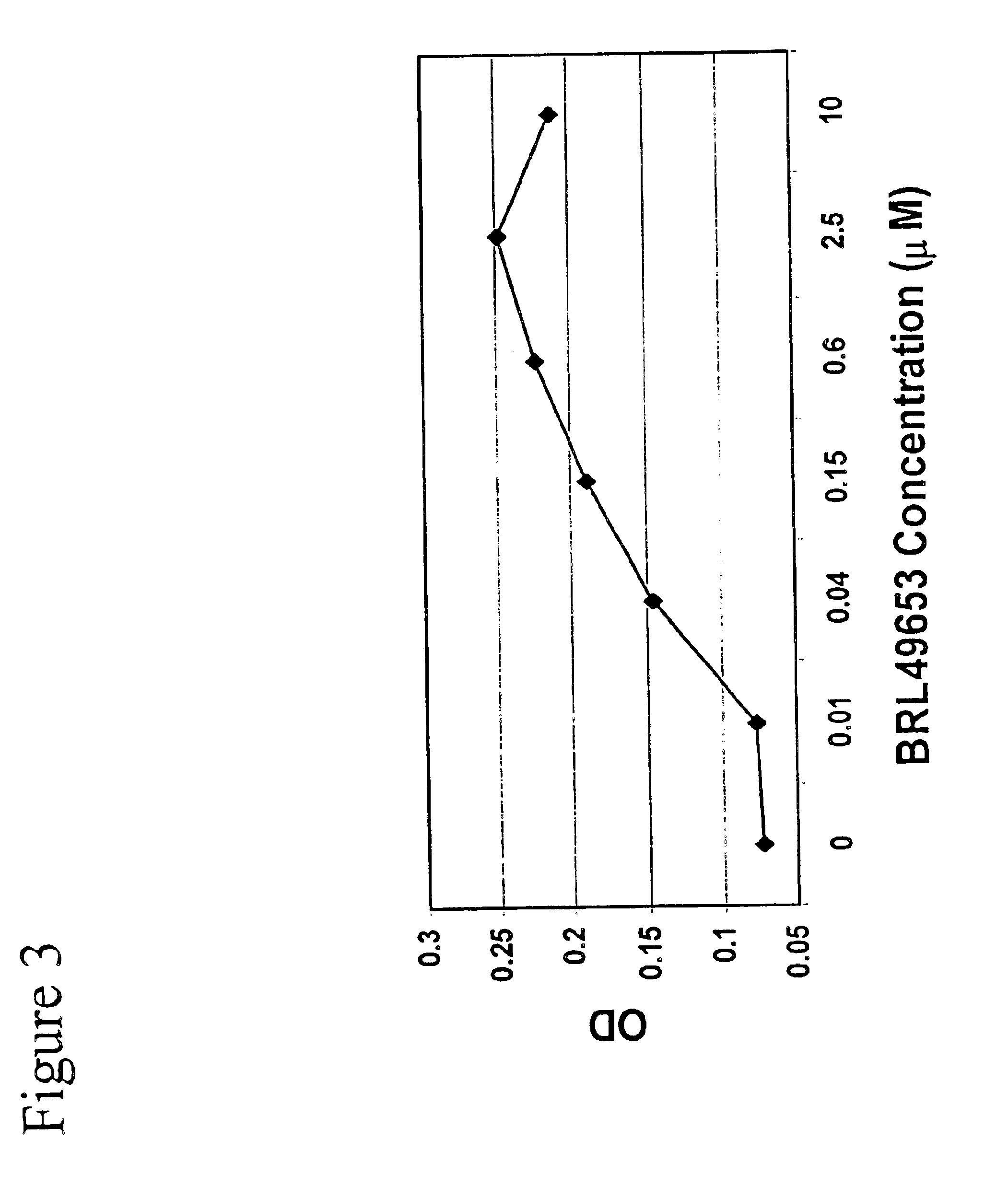
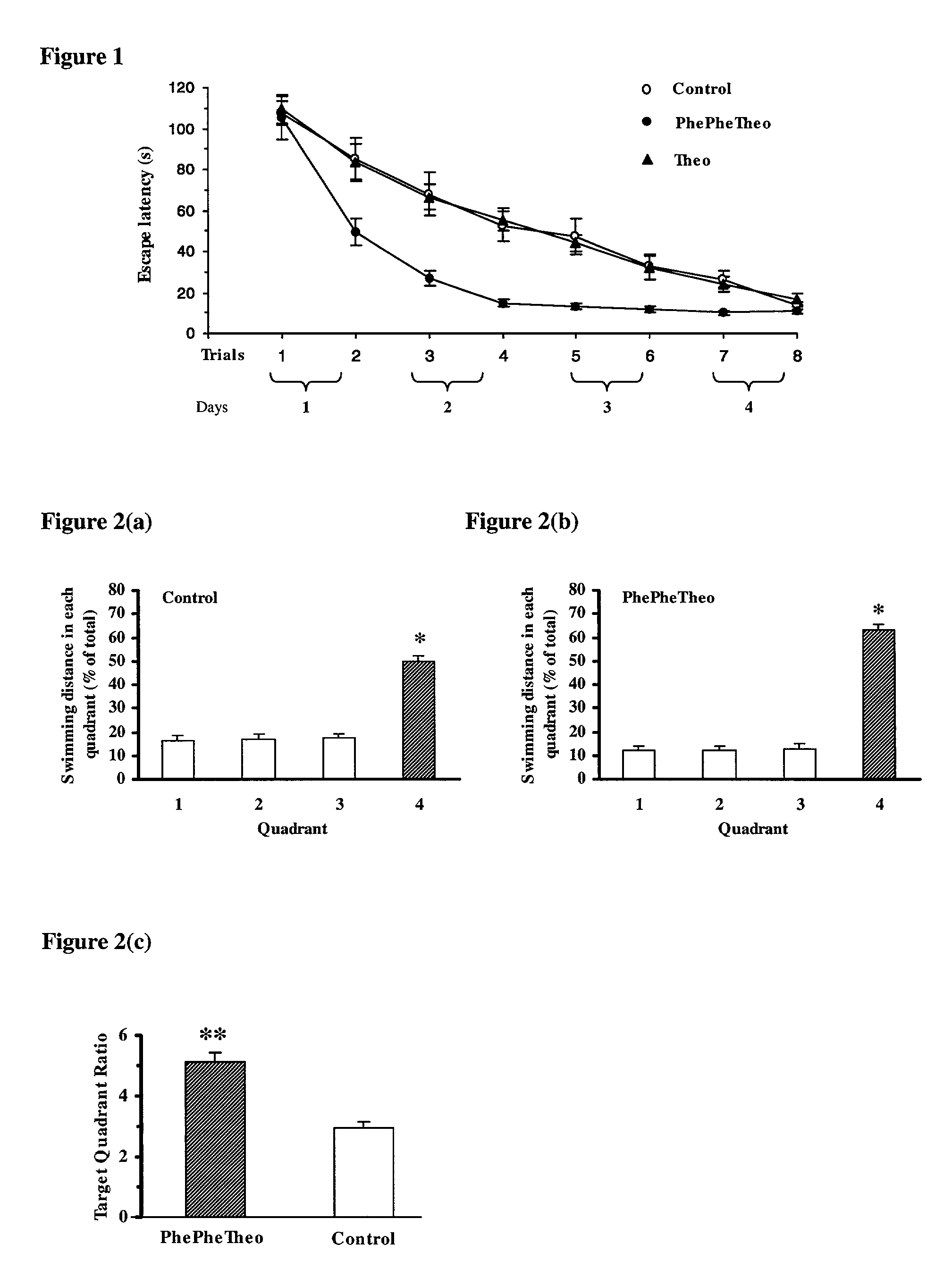
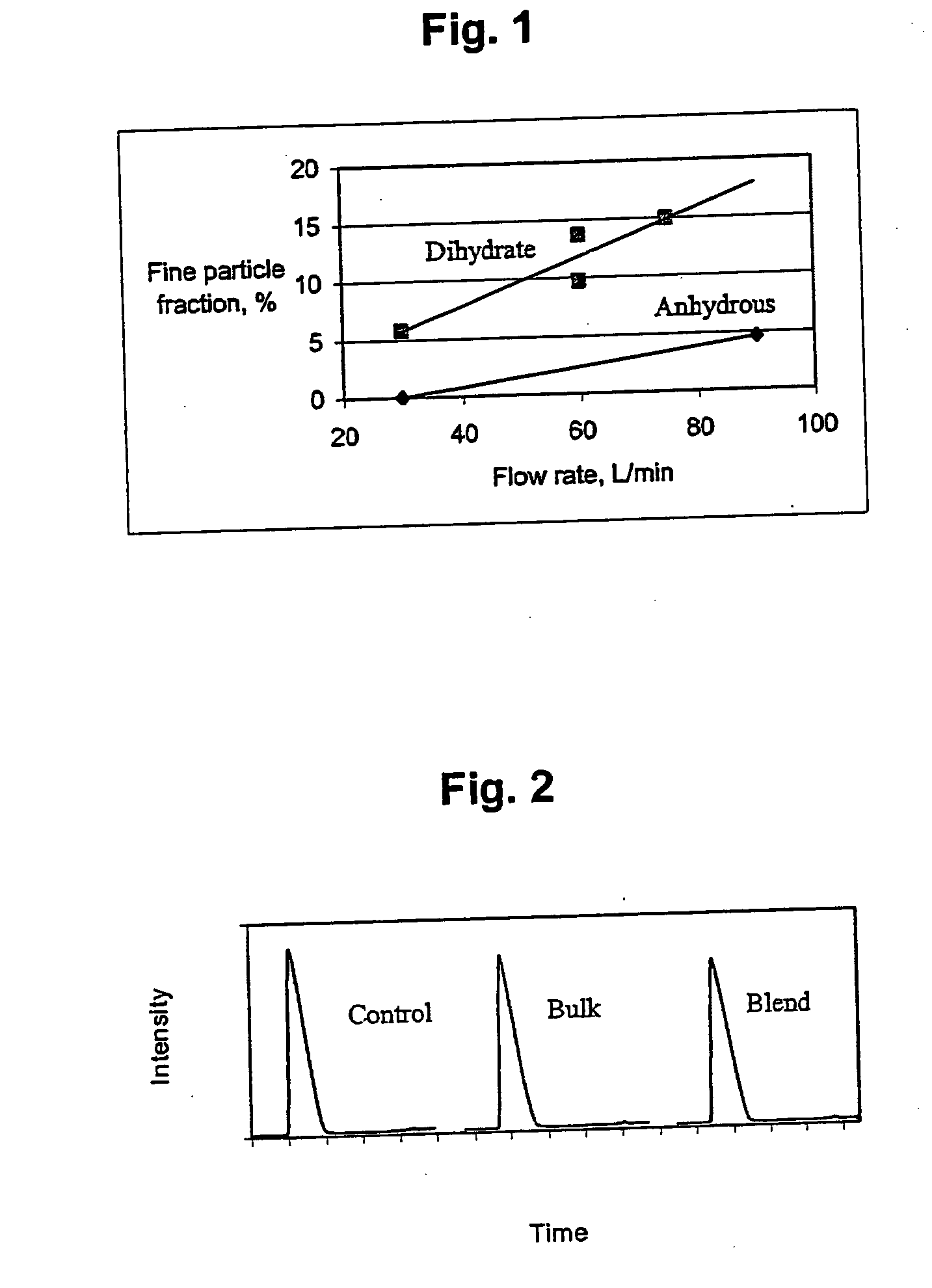
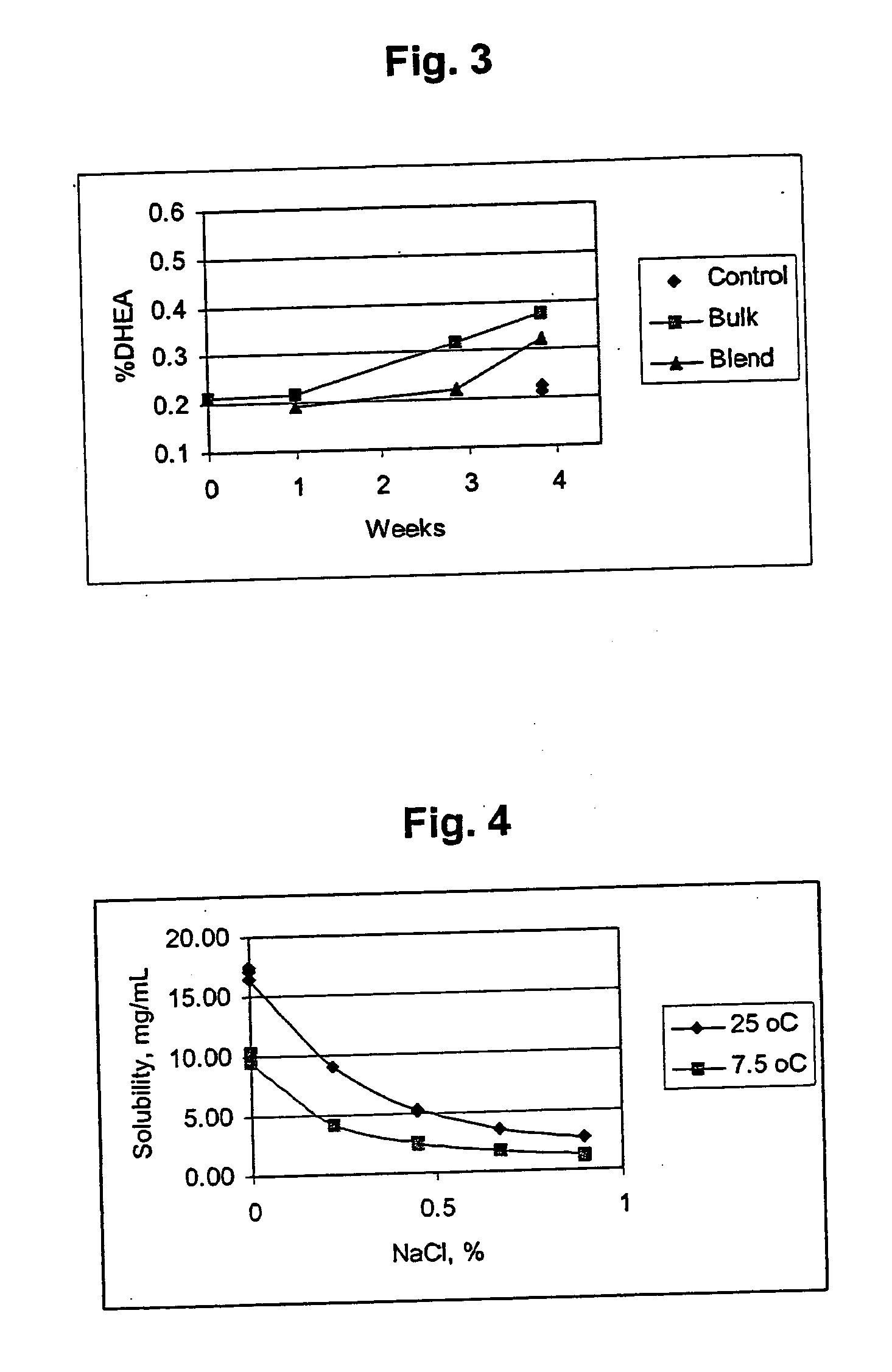
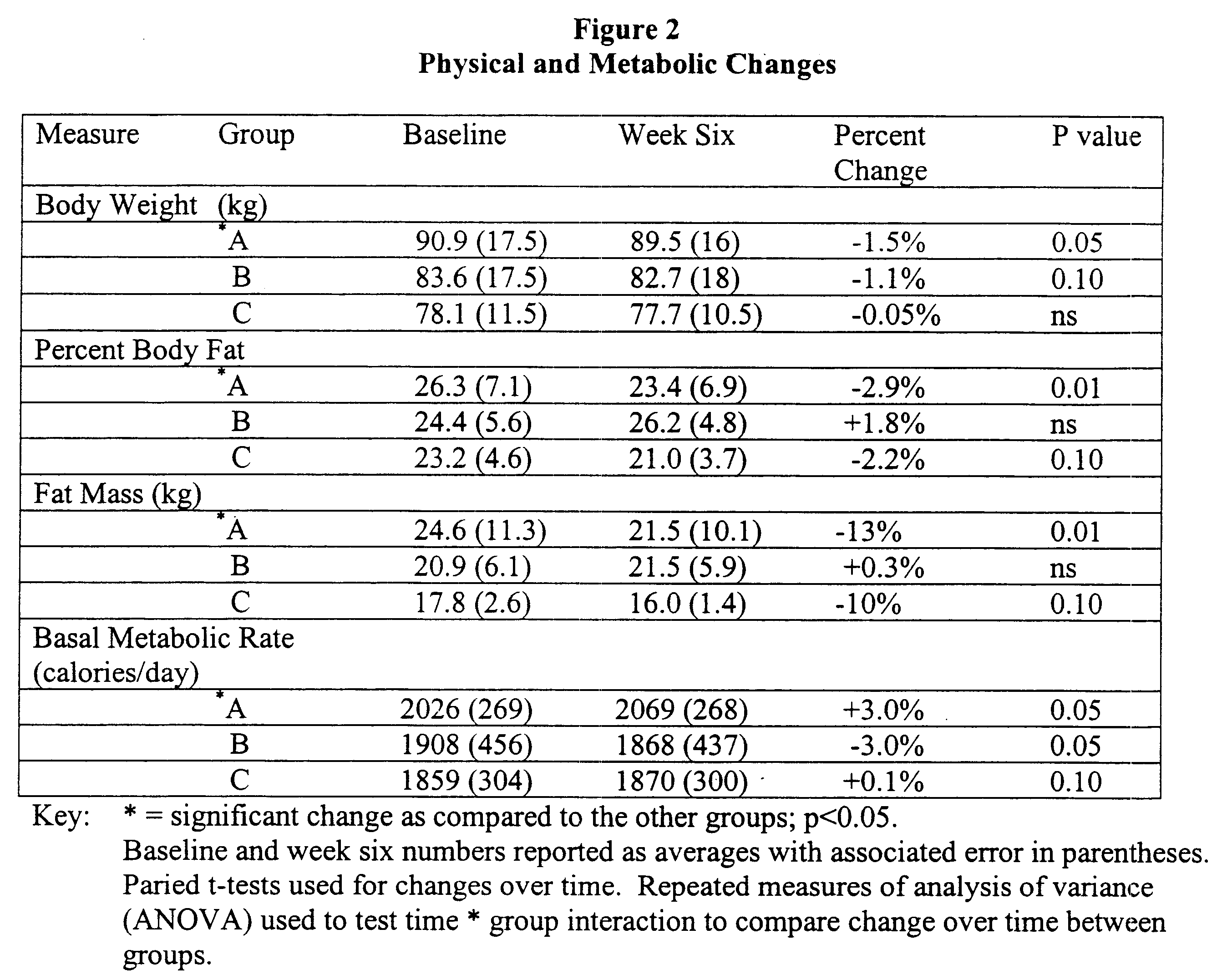
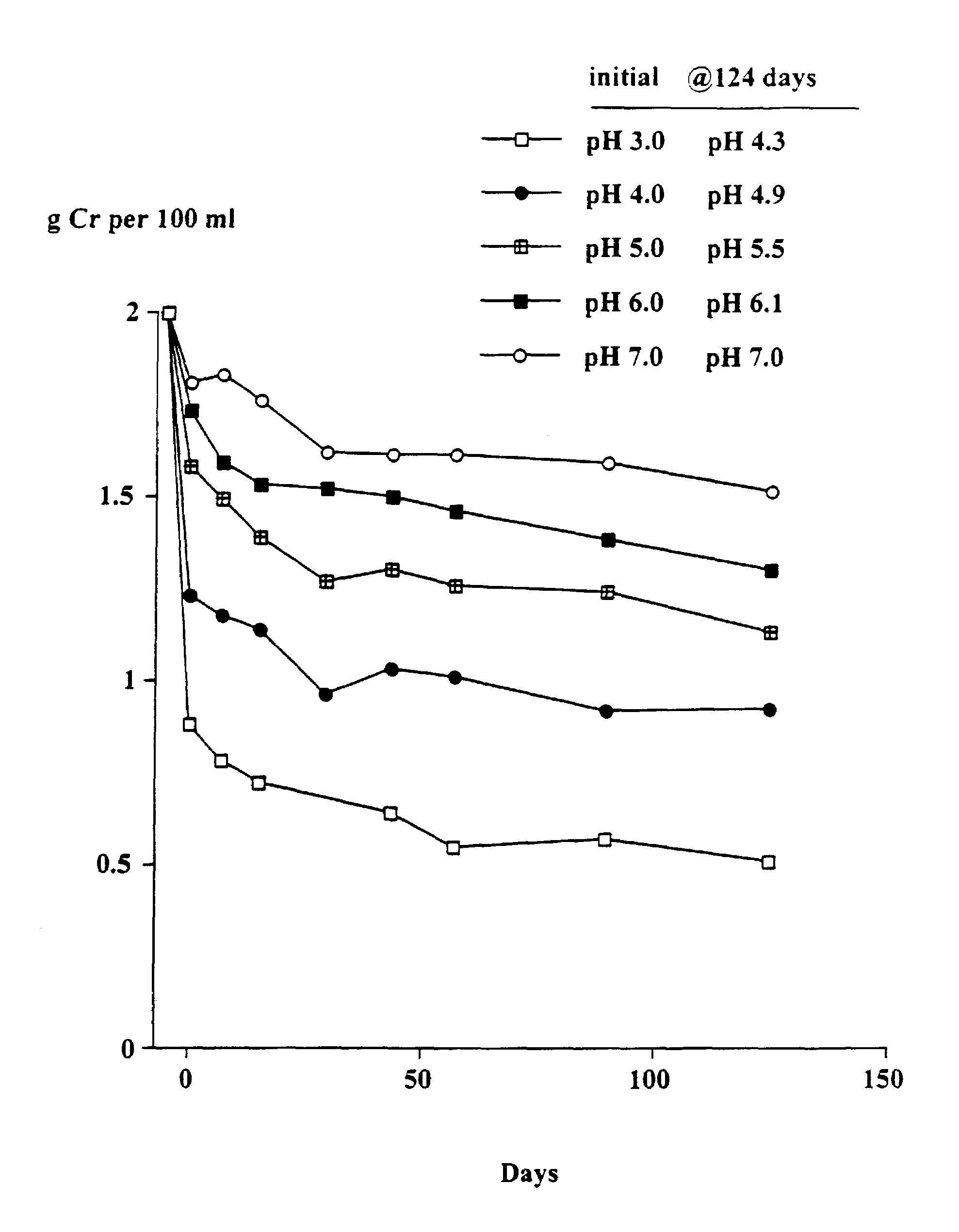
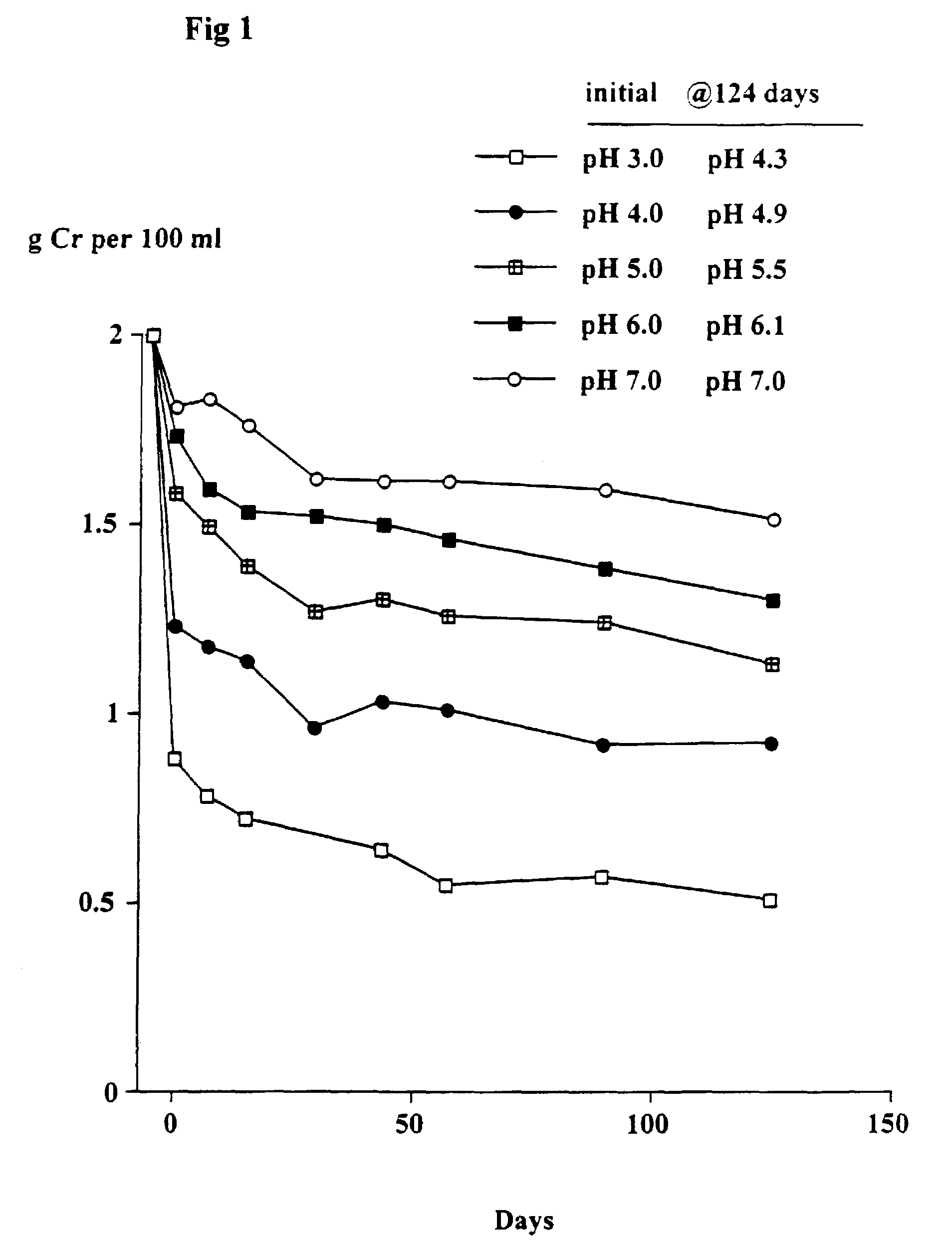
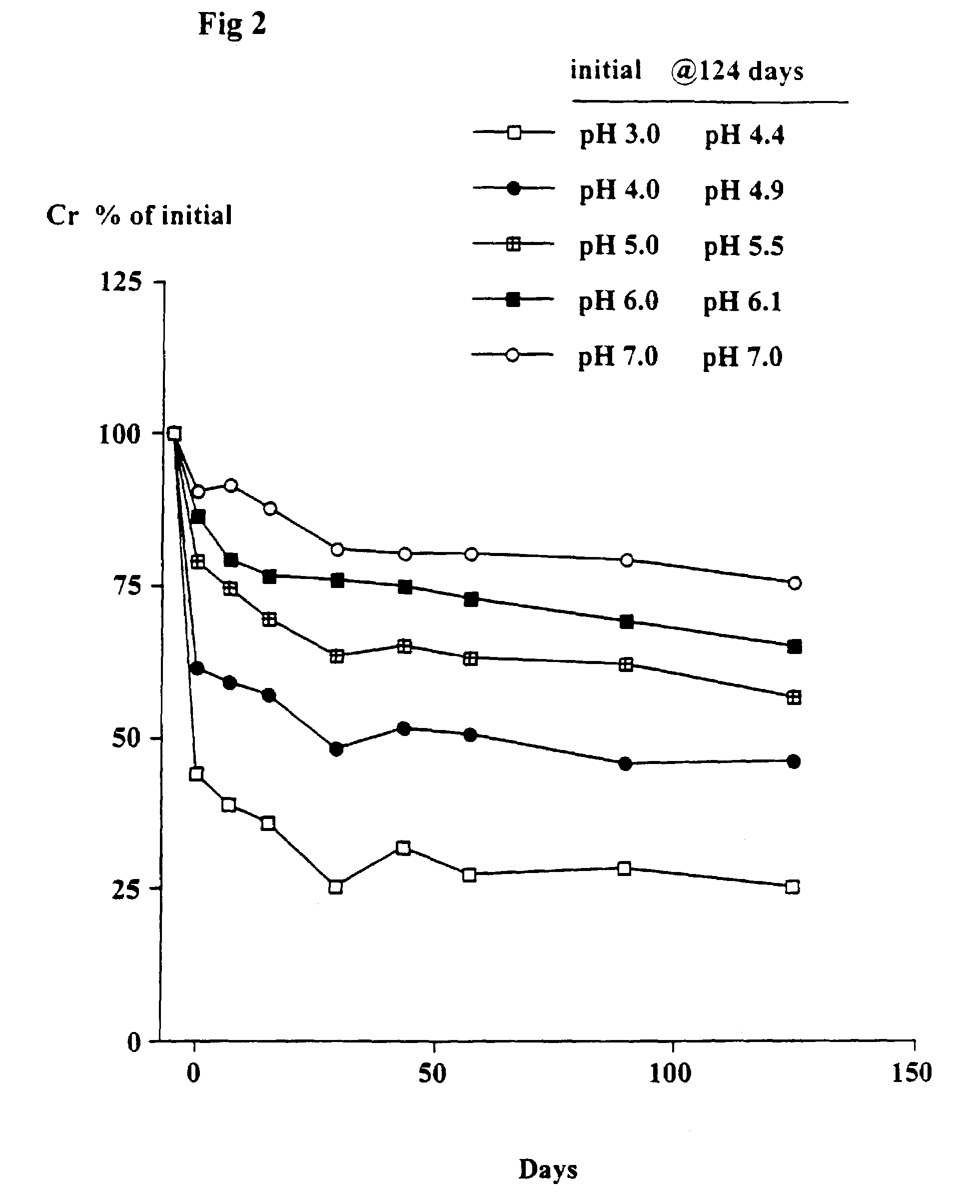
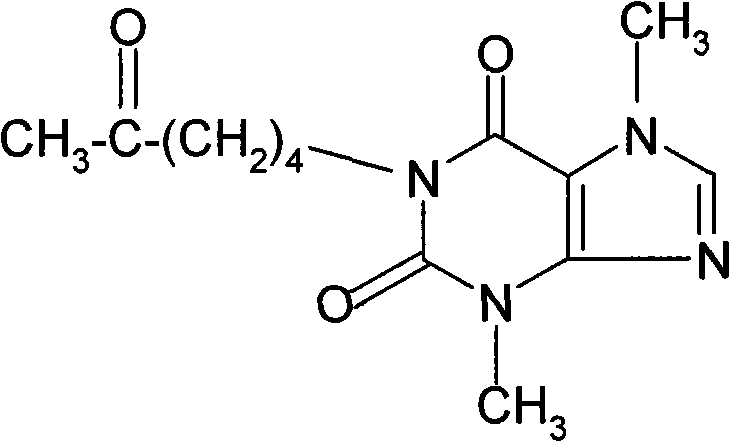
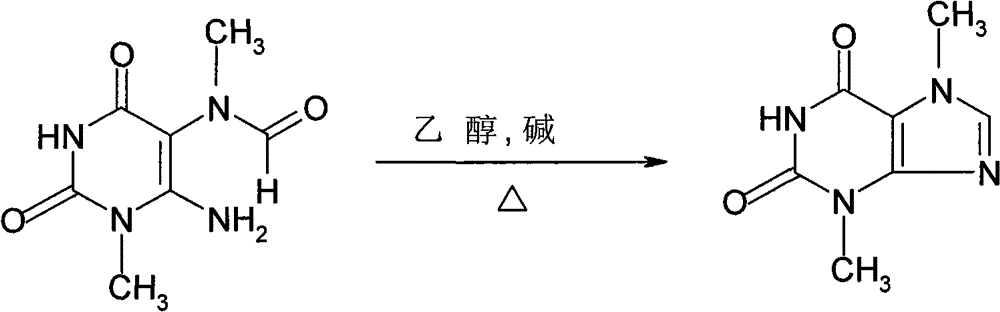
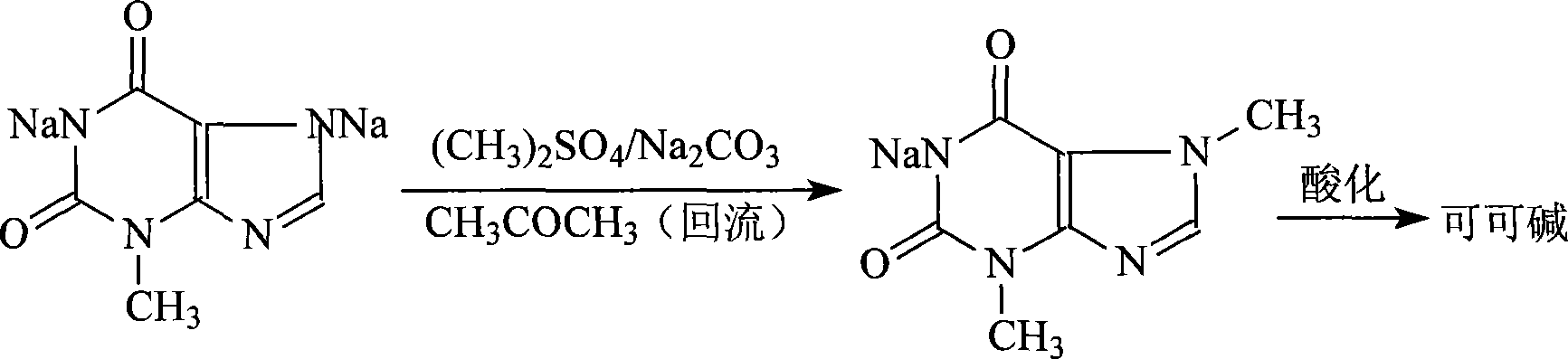Patents
Literature
114 results about "Methyl xanthine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
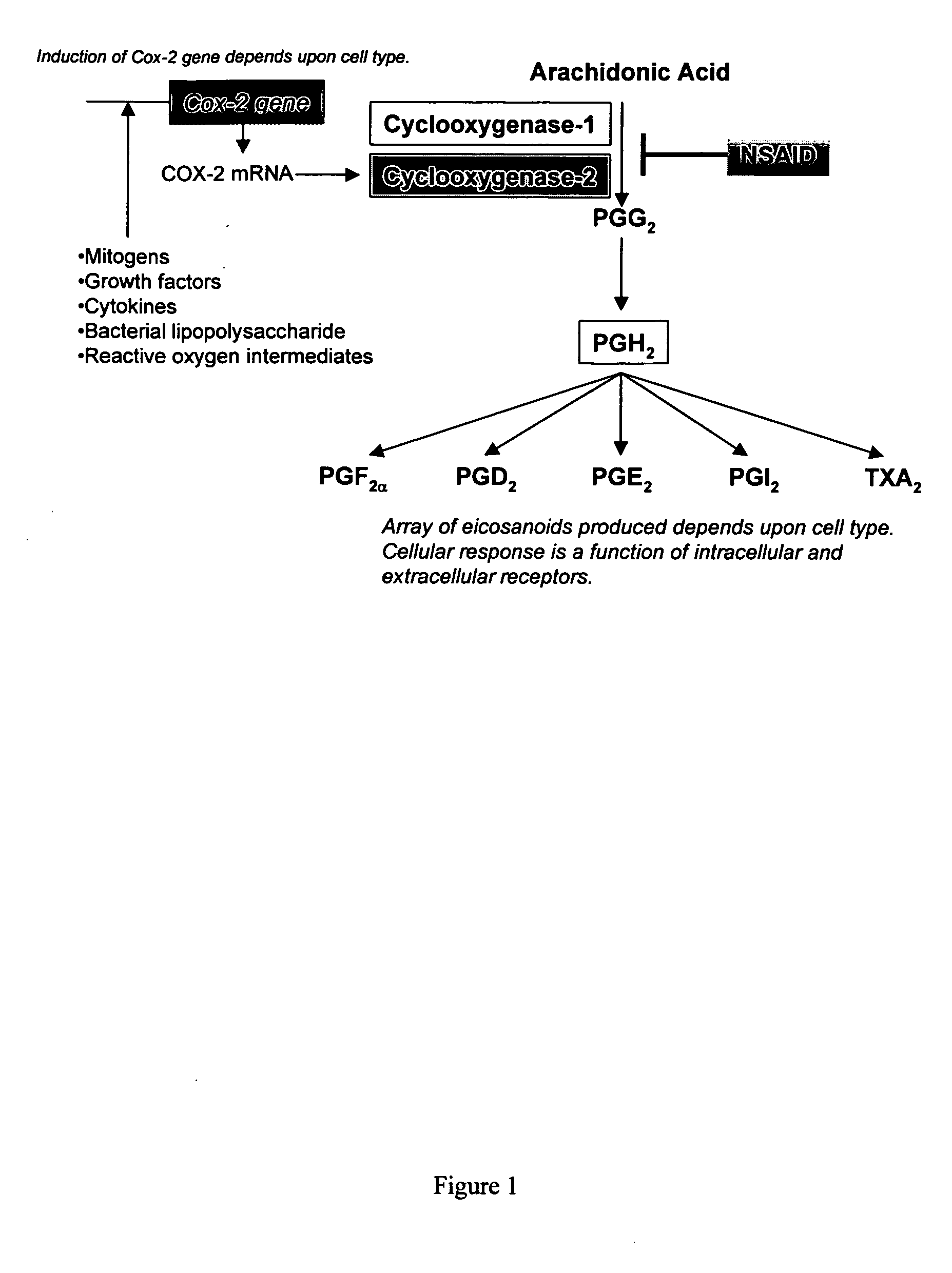
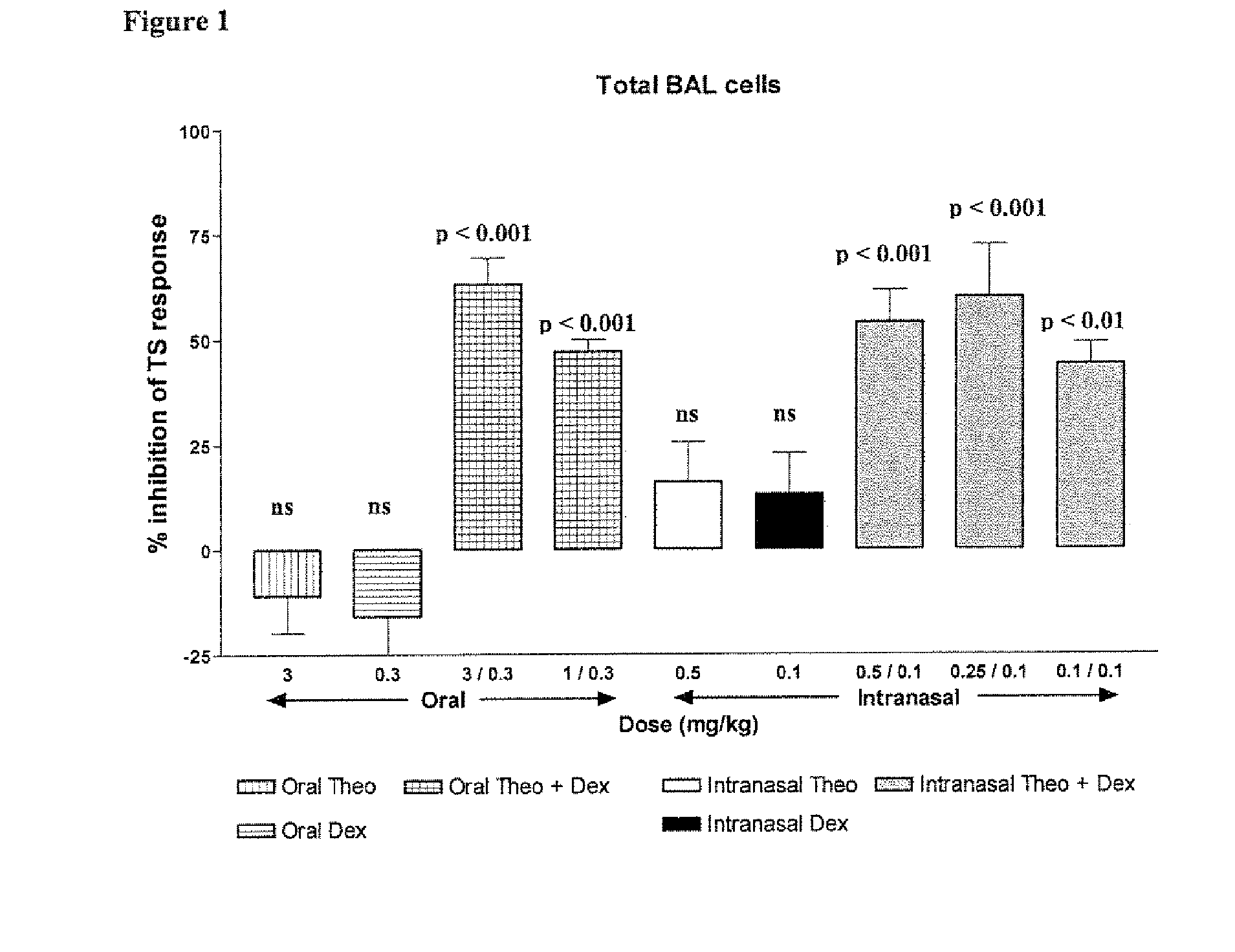
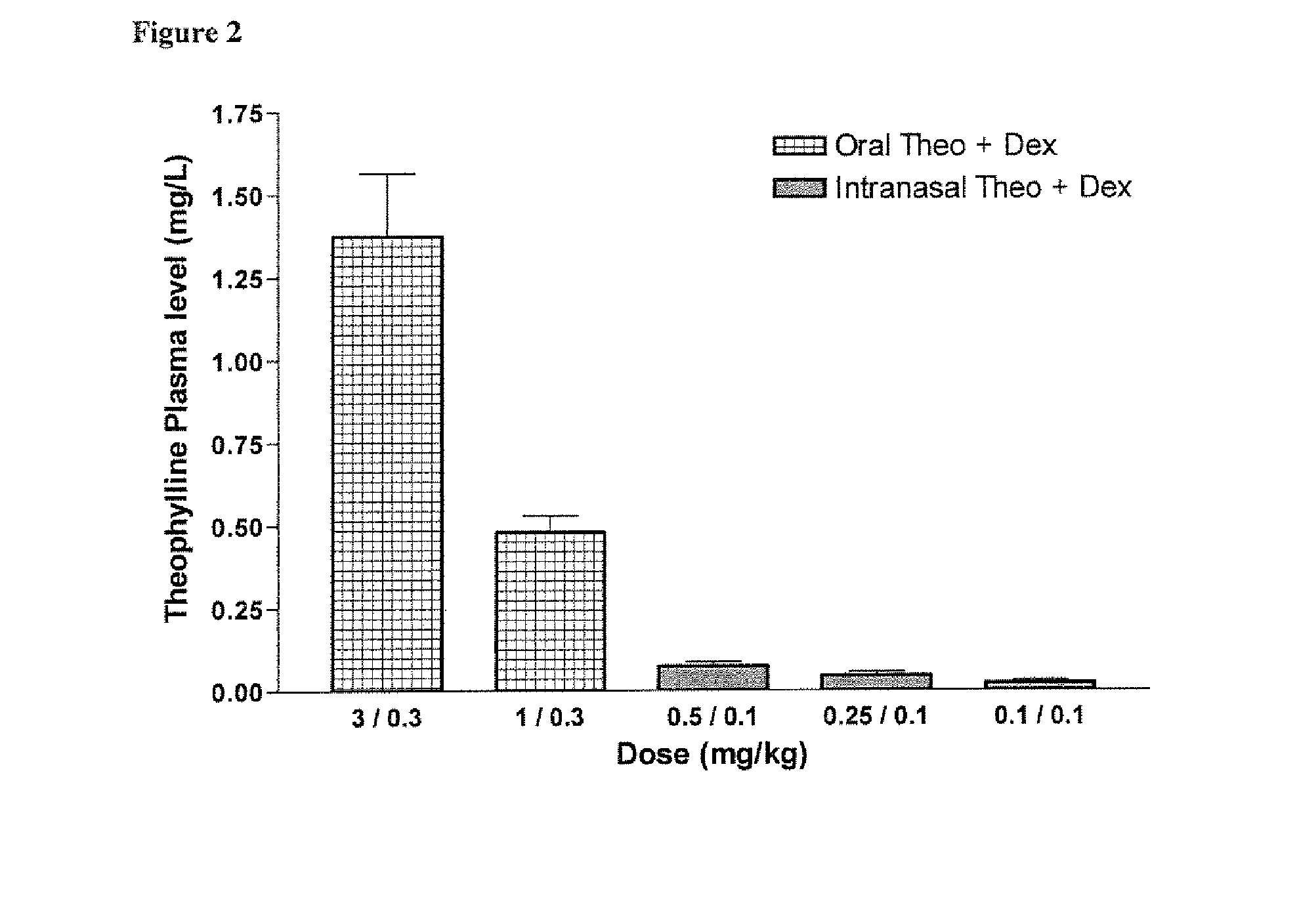
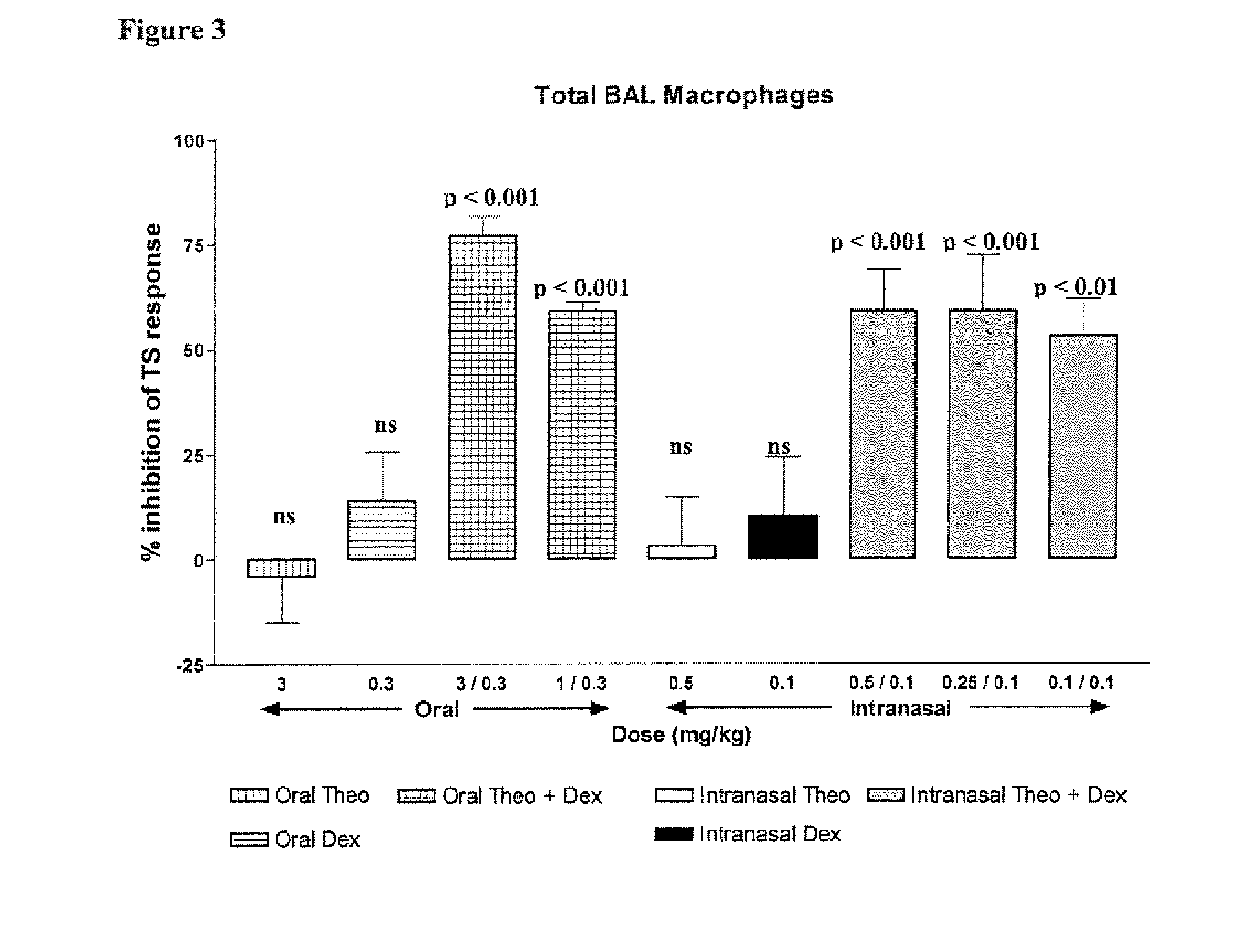
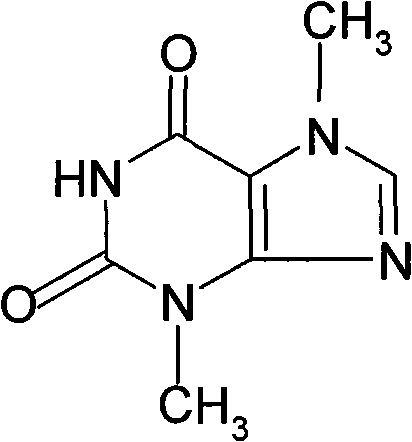
Xanthine is produced naturally by both plants and animals. The methylxanthines, theophylline, and dyphylline are used in the treatment of airways obstruction caused by conditions such as asthma, chronic bronchitis, or emphysema.
Combination Products
InactiveUS20080020018A1Sufficient reliefExtended stayAntibacterial agentsOrganic active ingredientsMethyl xanthineBULK ACTIVE INGREDIENT
A pharmaceutical formulation comprises a plurality of seamless minicapsules having a diameter of from 0.5 mm to 5 mm, at least some of the minicapsules containing a methyxanthine as one active ingredient, and at least some of the minicapsules containing a corticosteriod as another active ingredient.
Owner:SIGMOID PHARM LIMITED
Synergistic anti-inflammatory pharmaceutical compositions and related methods using curcuminoids or methylxanthines
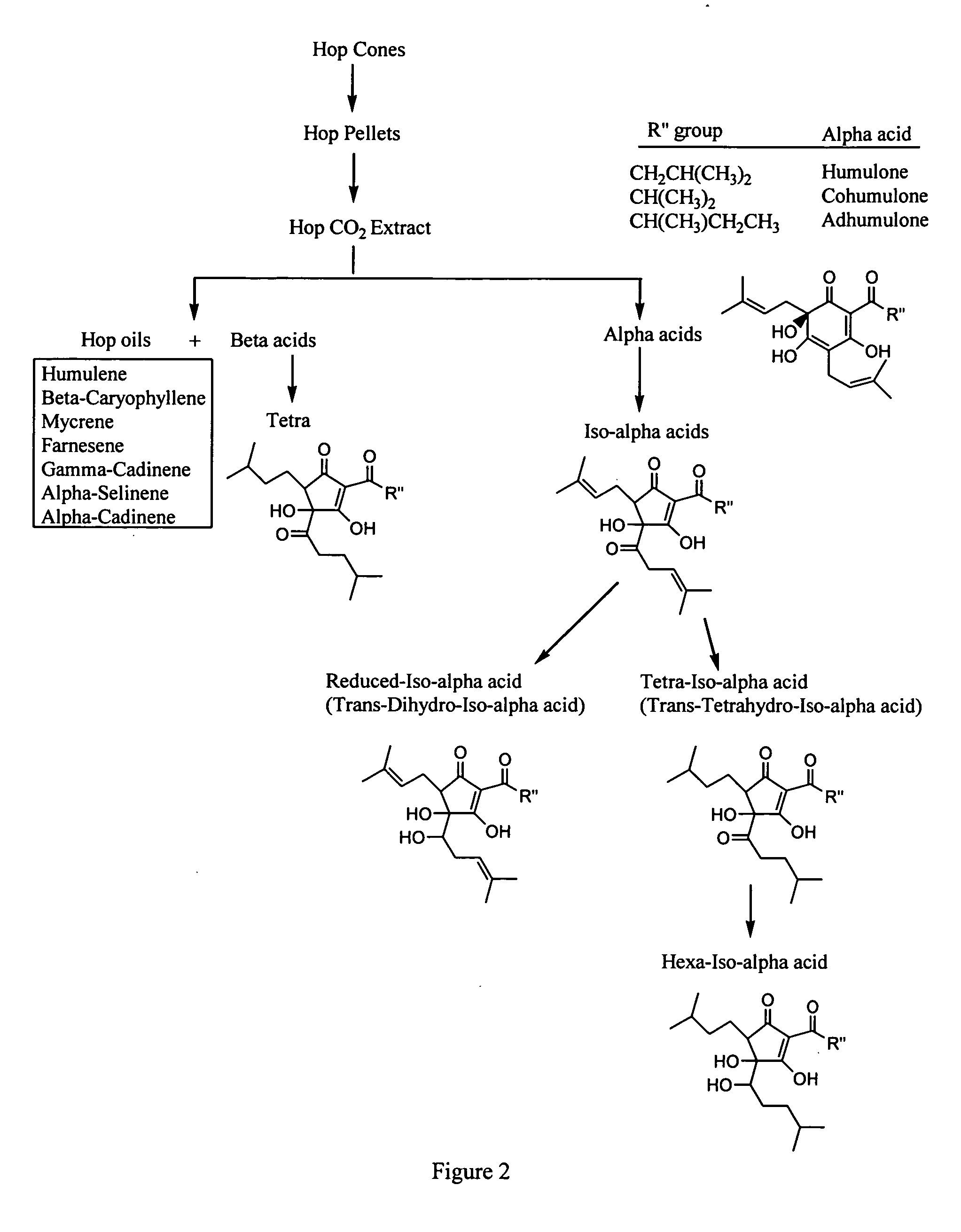
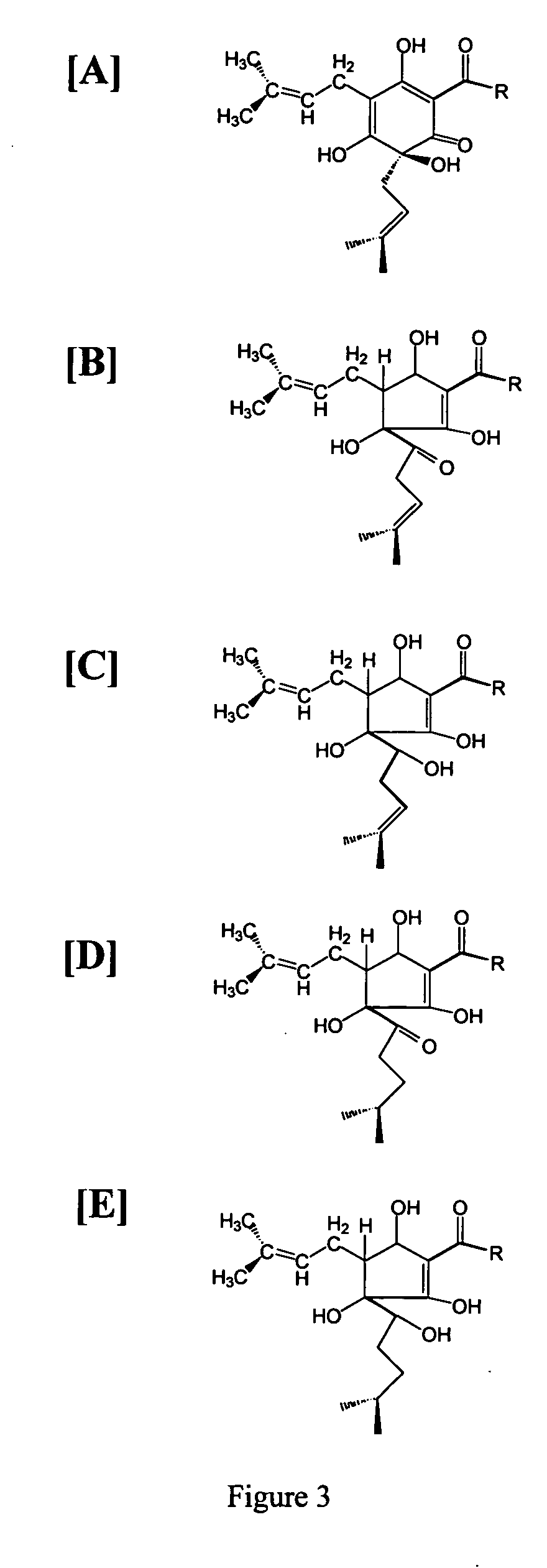
The invention provides compositions containing a fraction isolated or derived from hops and a methylxanthine. The invention additionally provides compositions containing a fraction derived from hops and a curcuminoid. The invention also provides methods of using such compositions to reduce inflammation.
Owner:METAPROTEOMICS
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
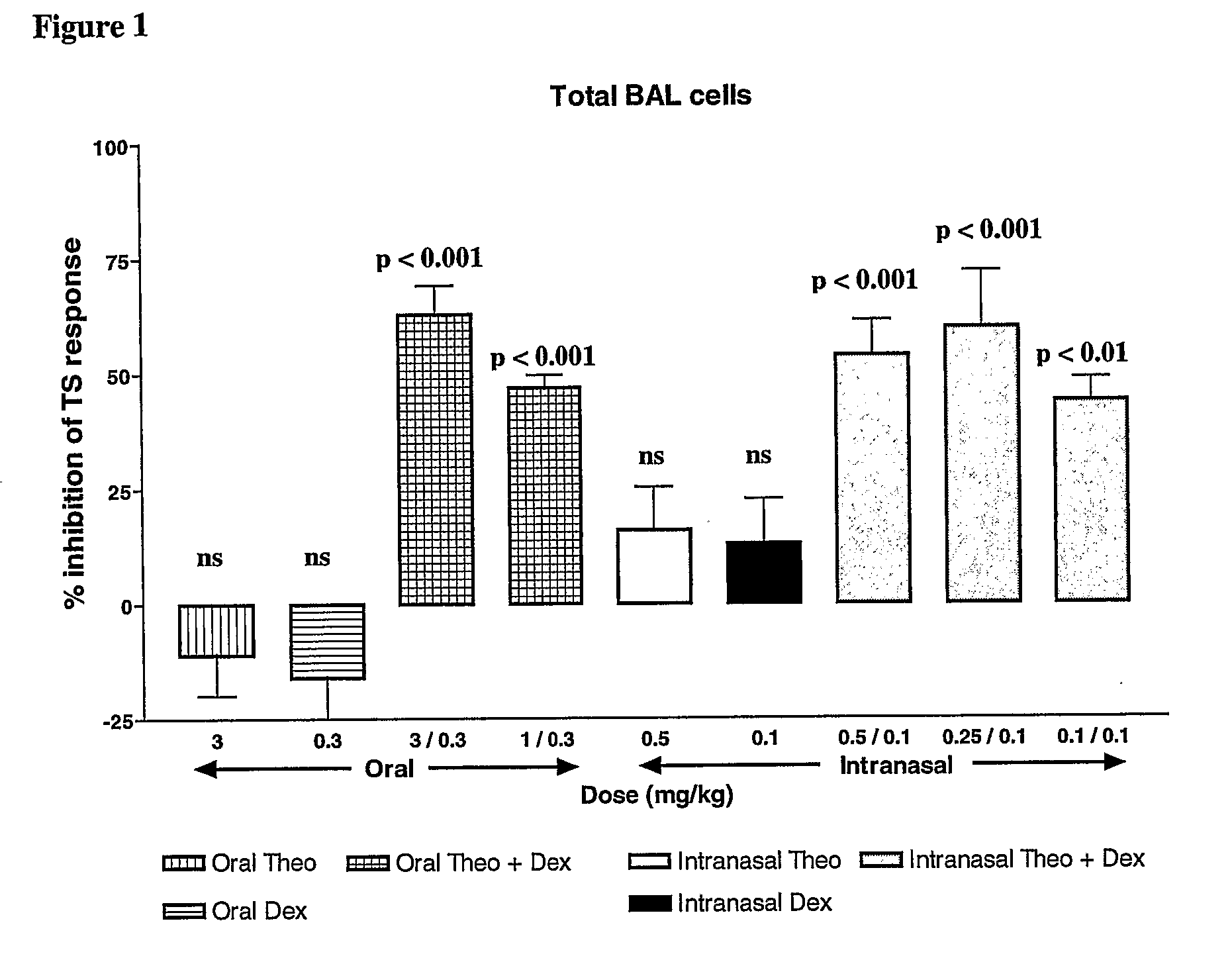
Combination of Methylxanthine Compounds and Steroids to Treat Chronic Respiratory Diseases
InactiveUS20080318913A1Reduce the number of cellsCell count is reducedBiocideAnimal repellantsDiseaseMethyl xanthine
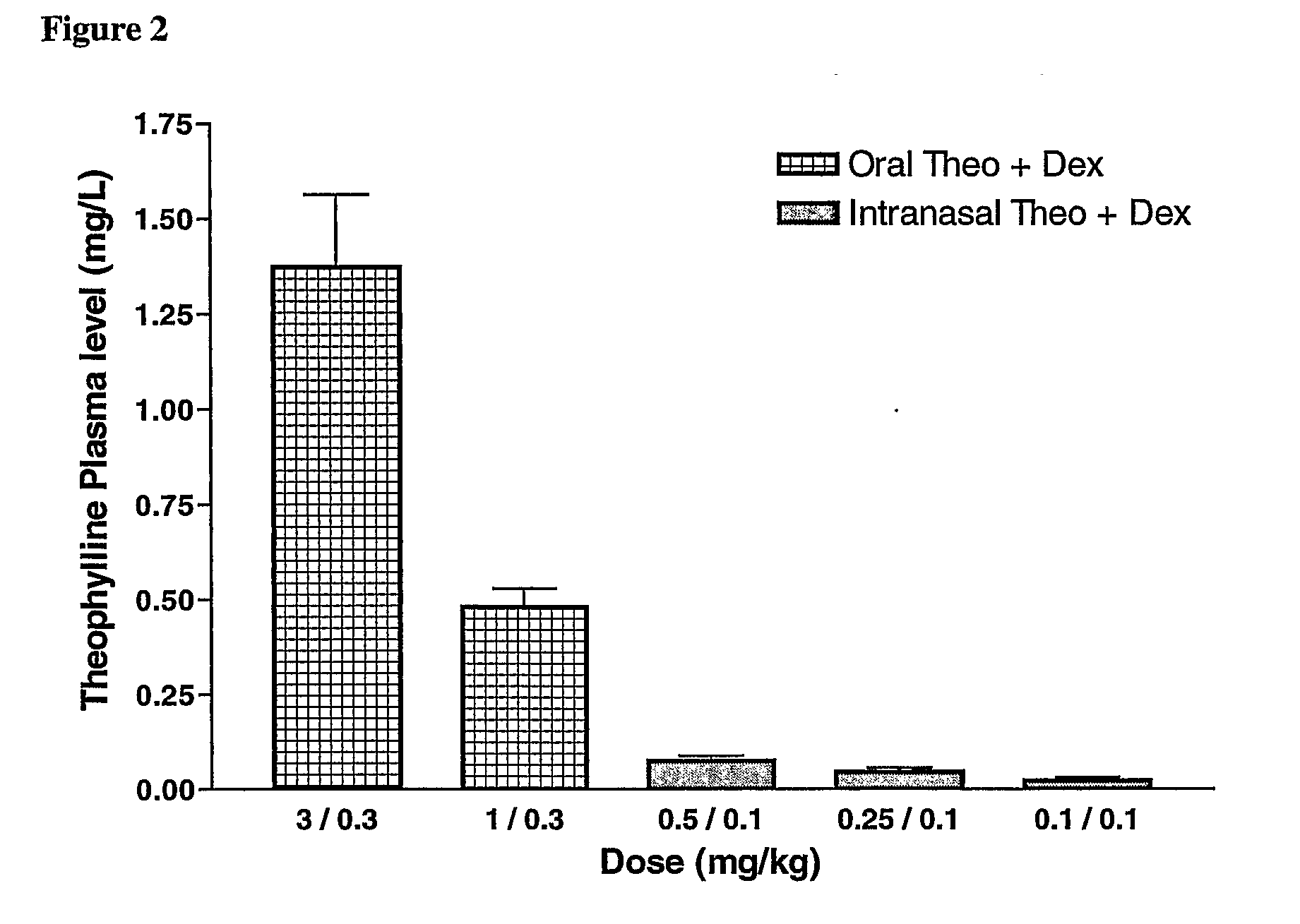
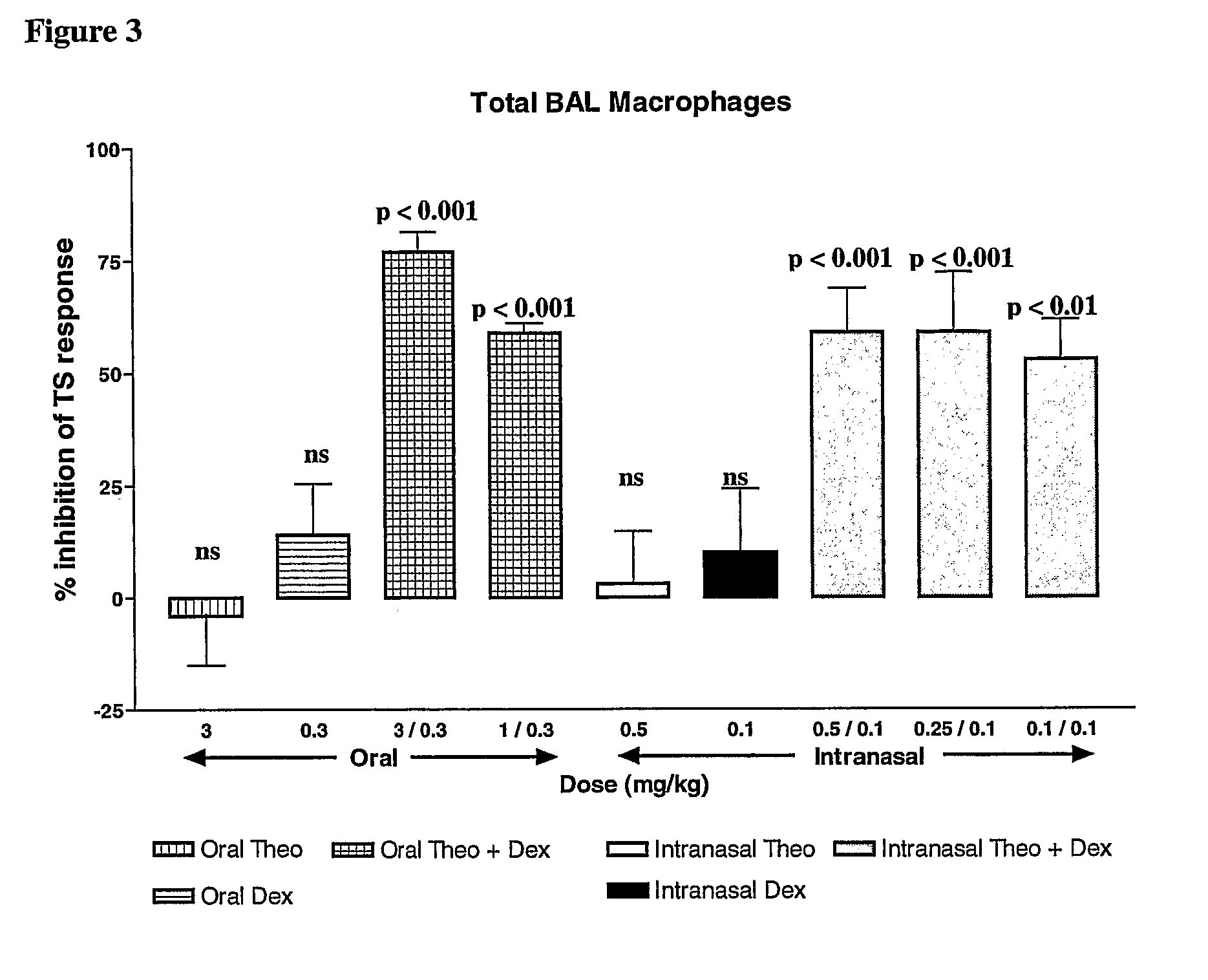
There is provided the use of a methylxanthine derivative such as theophylline and a steroid in a synergistic combination for the treatment of chronic obstructive pulmonary disease, wherein the combination is administered by the inhaled route for pulmonary delivery.
Owner:PULMAGEN THERAPEUTICS SYNERGY
Methods and compositions for the differentiation of human preadipocytes into adipocytes
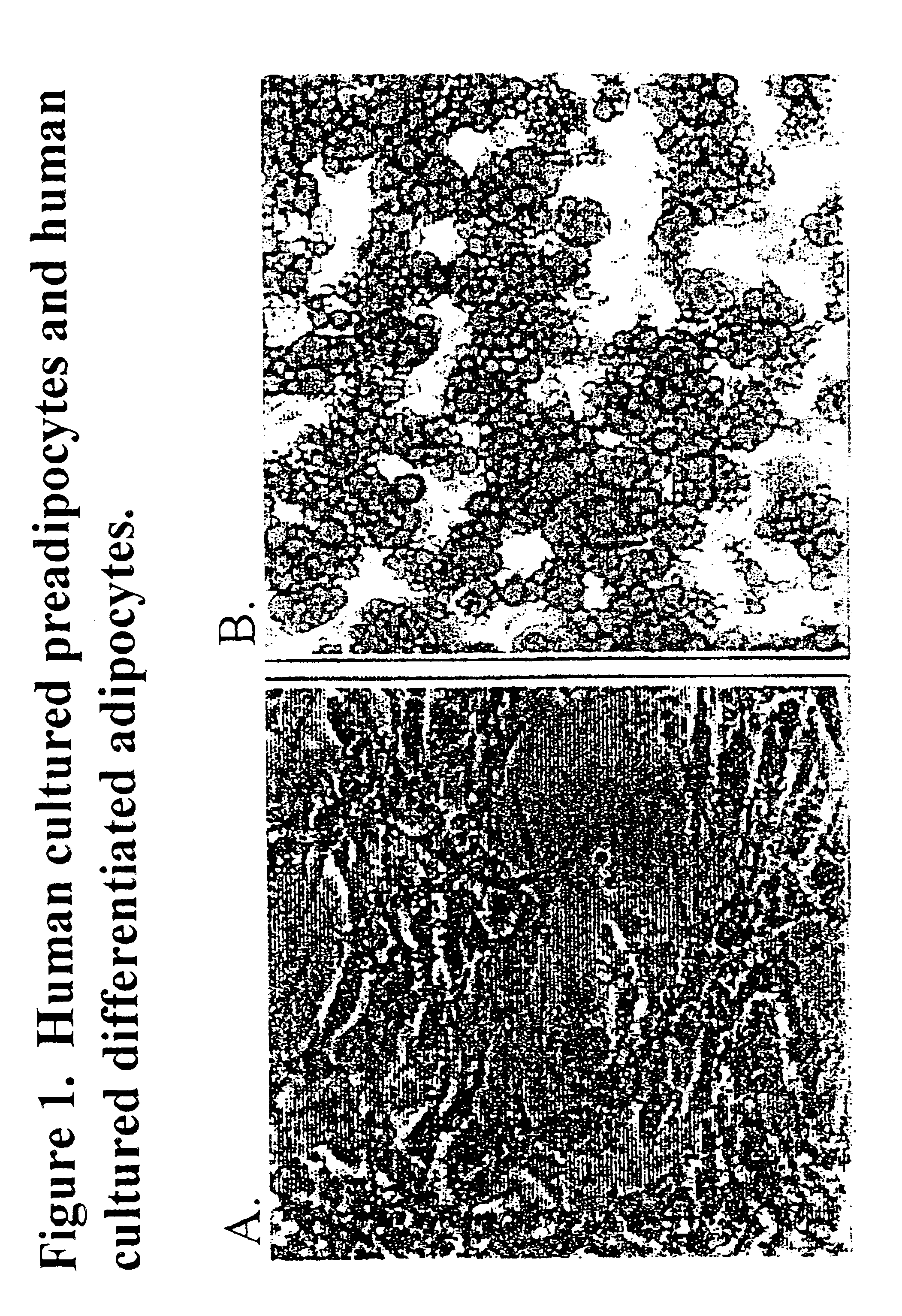
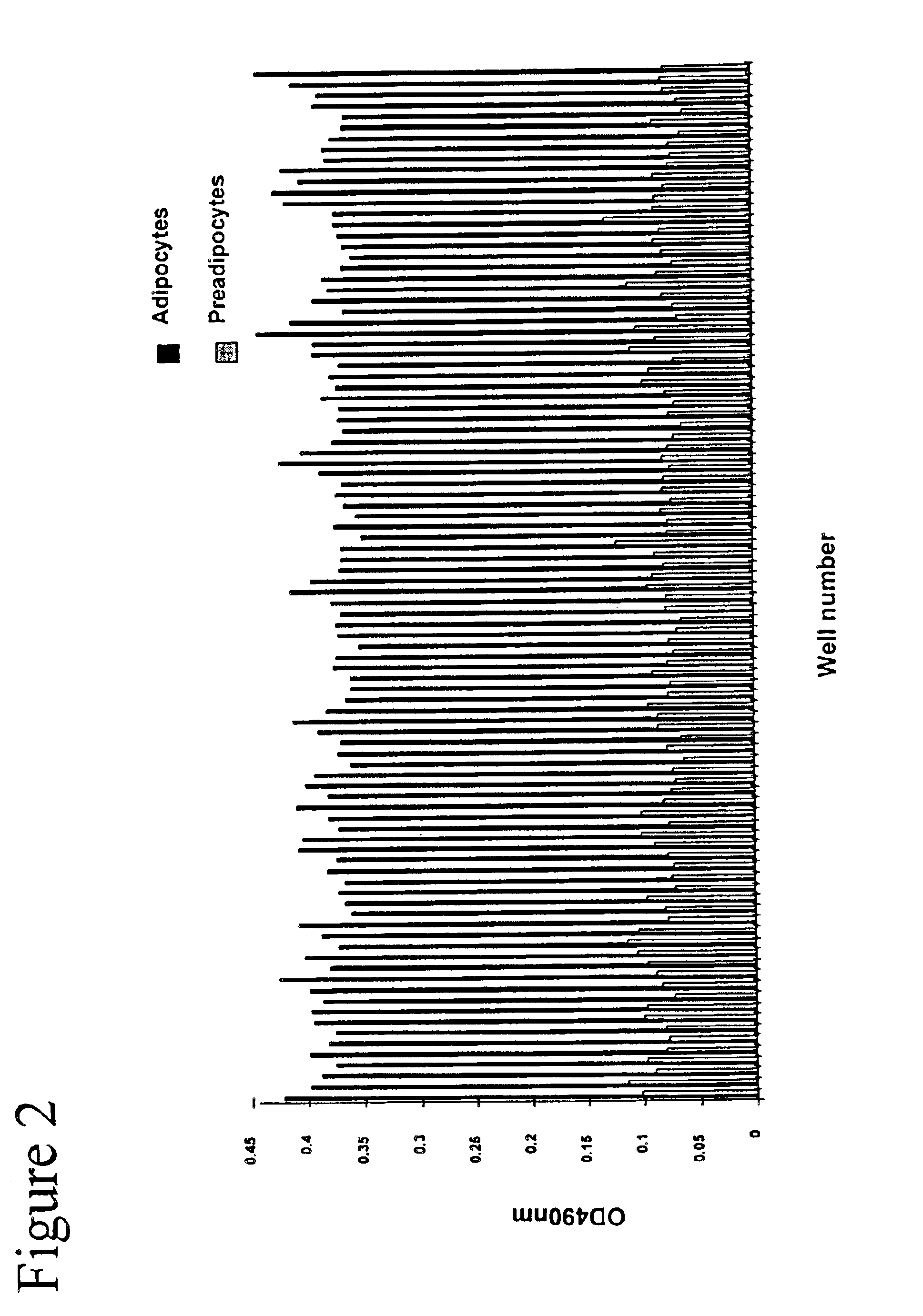
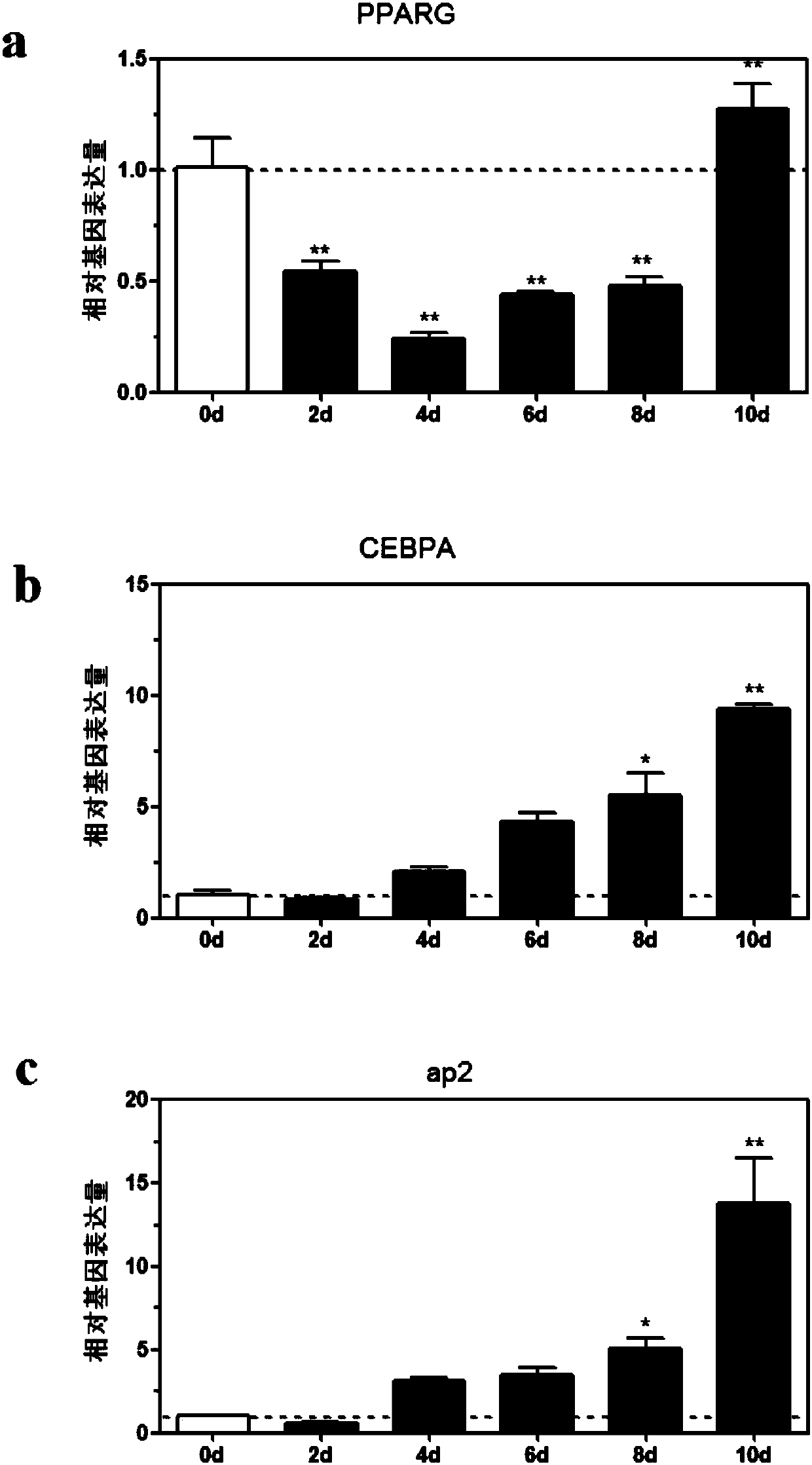
The present invention provides methods and compositions for the consistent and quantitative differentiation of human preadipocytes isolated from adipose tissue into adipocytes bearing biochemical, genetic, and physiological characteristics similar to that observed in isolated primary adipocytes. The methods of the invention comprise incubating isolated human preadipocytes, plated at least about 25,000 cells / cm2, in a medium containing, glucose, a cyclic AMP inducer such as isobutylmethylxanthine or forskolin, a glucocorticoid or glucocorticoid analogue, insulin or an insulin analogue and a PPARγ agonist or a RXR agonist. The compositions of the invention include media for the differentiation of human preadipocytes, human adipocytes differentiated by the methods of the invention and transfected adipocytes.The present invention also provides methods for determining the ability of a compound to affect the differentiation of human preadipocytes to adipocytes, for determining the ability of a compound to act as a PPARγ antagonist. a glucocorticoid, a glucocoticoid analogue, or an insulin analogue, for transfecting cultured human adipocytes, and as a means to identify novel polypeptides secreted from human adipocytes into the conditioned medium. The methods and compositions have use in the drug discovery of compounds having relevance to the disease states of diabetes, obesity, and cardiovascular disease and in the studies of these diseases.
Owner:SEED INTPROP LAW GRP
Synergistic enhancement of cognitive ability
The present invention relates to the combination of a methylxanthine and a carbonic anhydrase activator to provide synergistic effects. The invention further relates to the improved / enhanced cognitive ability of individuals, particularly those suffering from various disorders, such as Alzheimer's Disease, stroke, hypoxia, general dementia, ADHD, mental retardation, and "sun down" syndrome.
Owner:WEST VIRGINIA UNIVERSITY
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a methylxanthine derivative for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026848A1Alleviate different aspectConvenient treatmentOrganic active ingredientsBiocideDiseaseDehydroepiandrosterone sulfate
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a methylxanthine derivative for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Compositions containing creatine and creatinine and a methyl xanthine
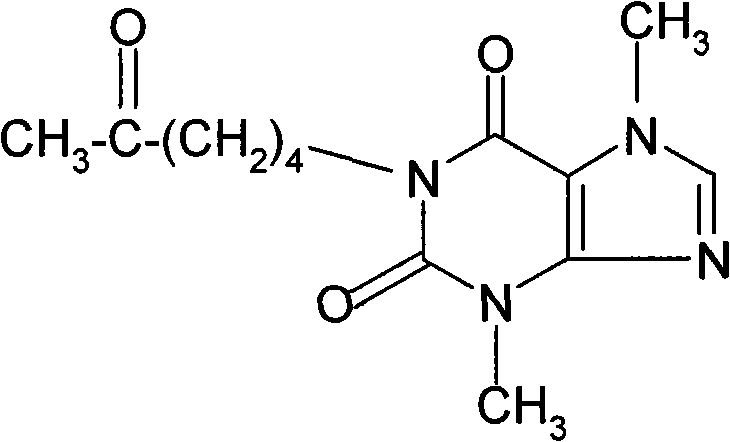
InactiveUS20030219500A1Improve solubilityLarge doseBiocideVitamin food ingredientsCreatinine riseMethyl xanthine
A composition for human consumption, comprising creatine and creatinine, the latter being in sufficient quantity to render creatine in an aqueous medium substantially stable, and a methyl xanthine; and a method of making the composition is provided.
Owner:ORIGINAL CREATINE PATENT CO LTD THE
Inhaled combination therapy
InactiveUS20100324002A1Function increaseImprove inflammationBiocideRespiratory disorderMethyl xanthineObstructive Pulmonary Diseases
Owner:PULMAGEN THERAPEUTICS SYNERGY
Dietary supplement and method of using same
InactiveUS6576272B1Good for weight lossMaintaining and preserving lean body massBiocideStringersCitrus aurantium extractDietary supplement
Dietary supplements comprising Citrus aurantium extract and a methylxanthine, with or without St. John's wort extract and L-Phenylalanine for controlling weight wherein fat is lost and lean body mass preserved.
Owner:ISI BRANDS +11
Compositions containing creatine and creatinine and a methyl xanthine
A composition for human consumption, comprising creatine and creatinine, the latter being in sufficient quantity to render creatine in an aqueous medium substantially stable, and a methyl xanthine; and a method of making the composition is provided.
Owner:ORIGINAL CREATINE PATENT CO LTD THE
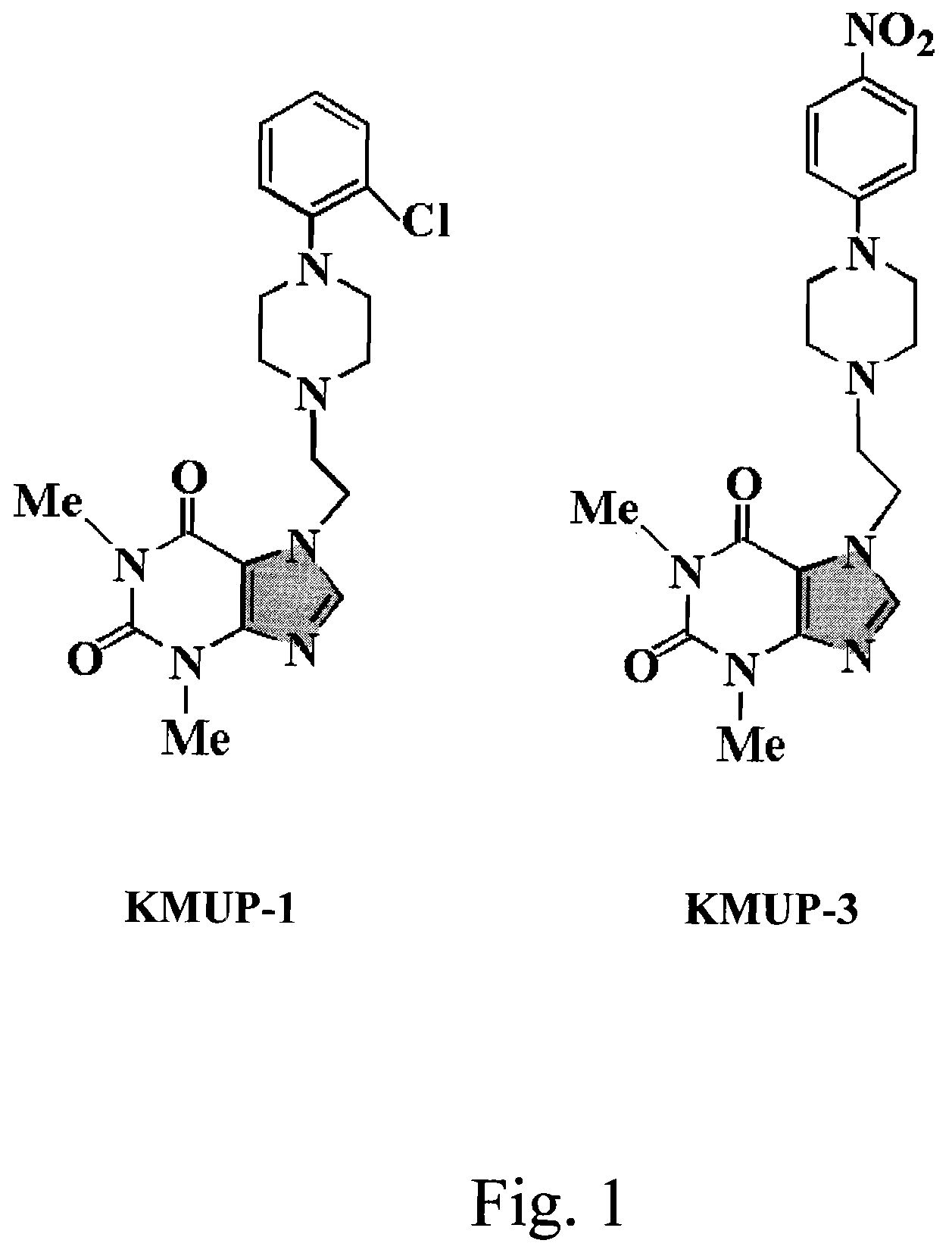
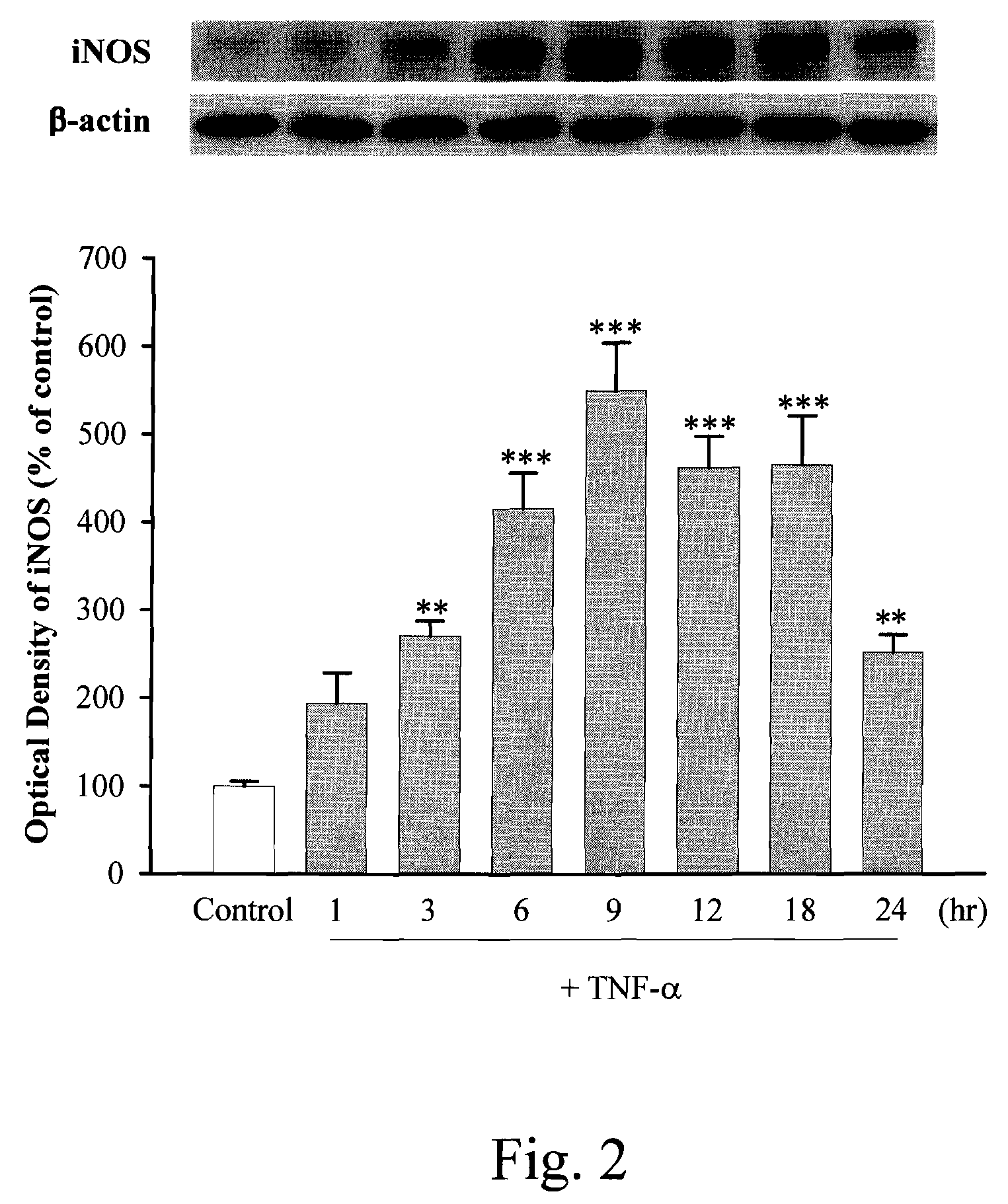
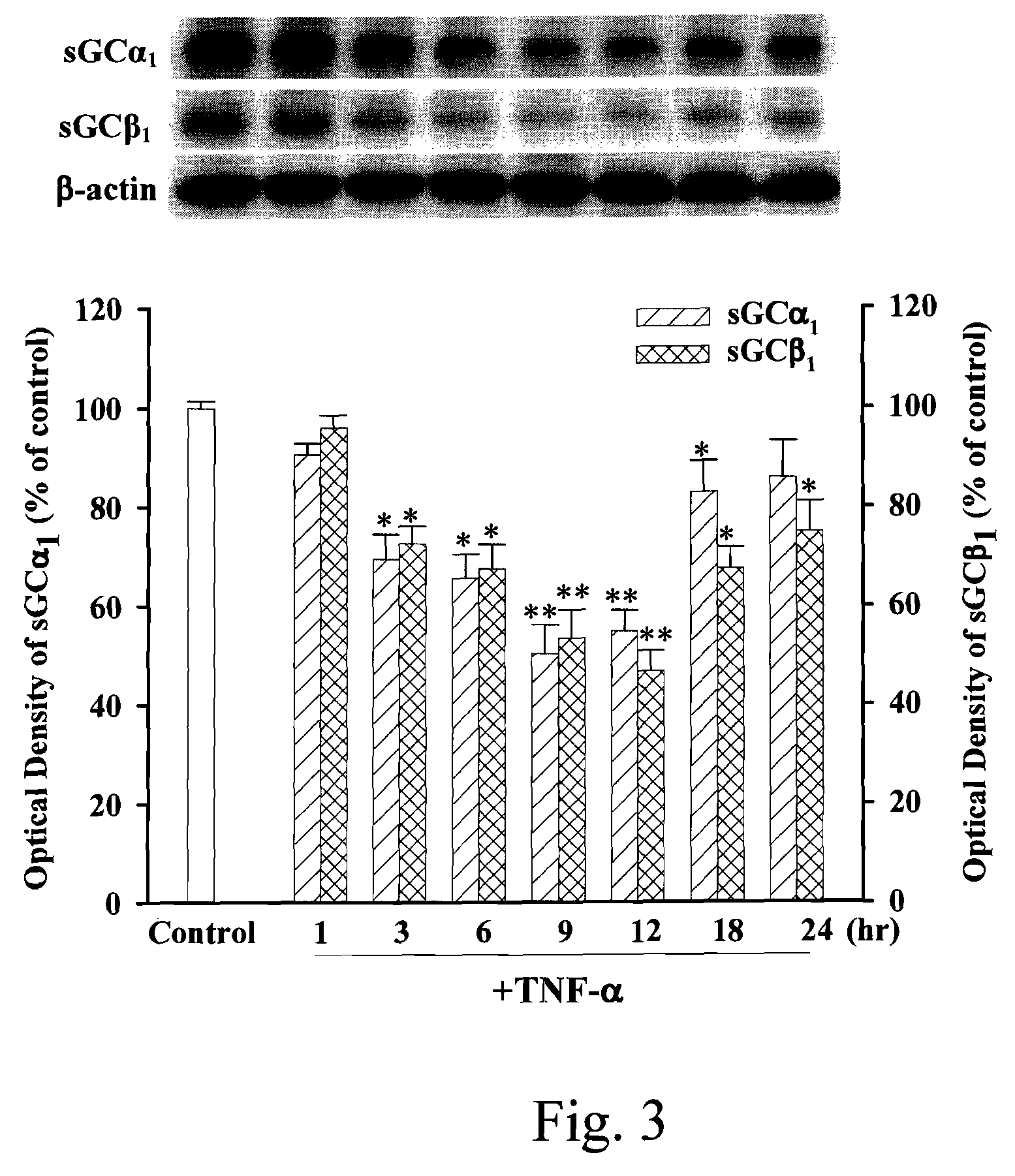
Anti-inflammation activity of newly synthesized xanthine derivatives kmup-1 and kmup-3
InactiveUS20080081816A1Inhibit expressionPreventing airway constrictionOrganic chemistryHeterocyclic compound active ingredientsChlorobenzeneNitrobenzene
An anti-inflammation substrate for decreasing the proinflammation induced by the cytokines and inhibiting the lung function degeneration is provided. The anti-inflammation substrate includes one selected from the group consisting of a 7-[2-[4-(2-chlorobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine, a 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine, a respective pharmaceutical acceptable salt thereof, and a combination thereof.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
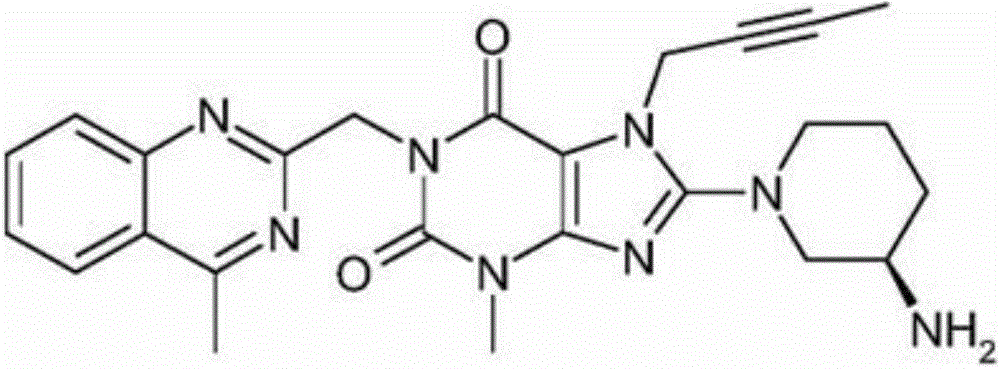
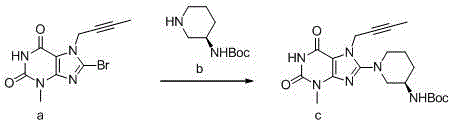
Synthetic method of linagliptin
ActiveCN105936634AHigh purityPurity does not affectOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupXanthine
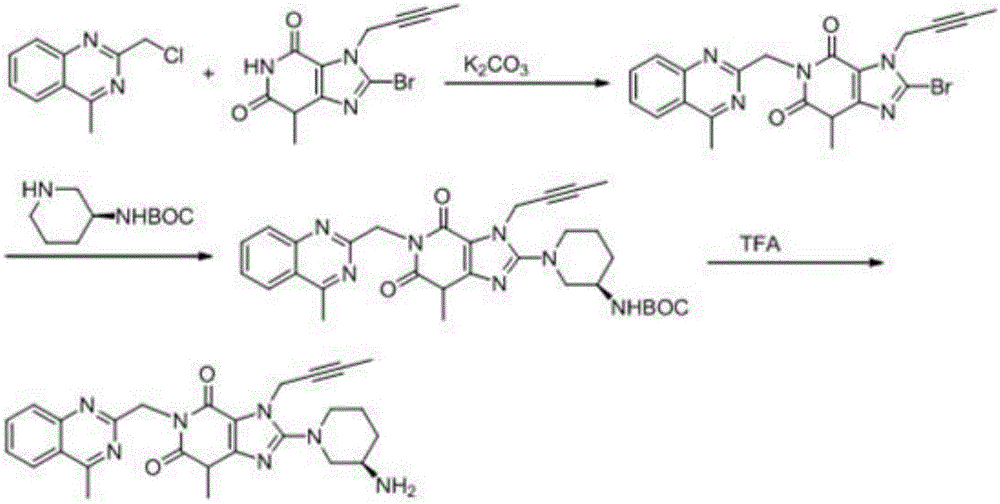
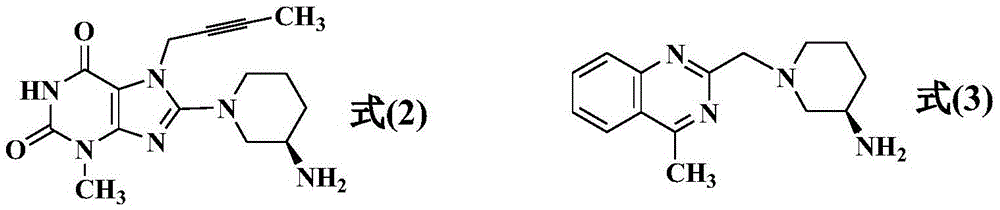
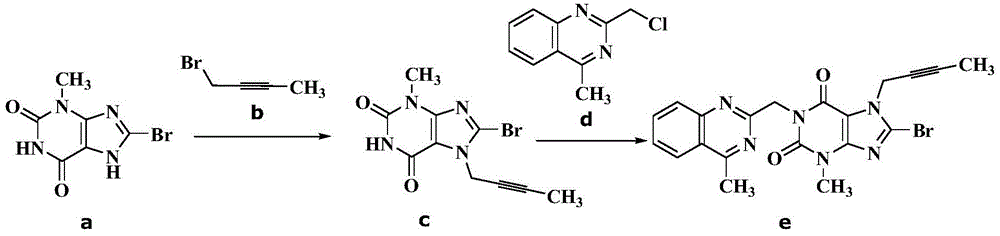
The invention discloses a synthetic method of linagliptin, wherein the method includes the following steps: (1) carrying out a reaction of 8-bromo-3-methylxanthine (a) and 1-bromo-2-butyne (b), to obtain 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine (c); (2) carrying out a reaction of 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine (c) and 2-chloromethyl-4-methylquinazoline (d), to obtain 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e); (3) adding 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e), (R)-3-Boc-aminopiperidine (f), potassium carbonate and acetonitrile into a reactor and mixing evenly, and carrying out a reaction under a state of heating reflux, to obtain t-butyloxycarboryl-linagliptin (g); and (4) removing a Boc protective group of t-butyloxycarboryl-linagliptin (g) in a methanol aqueous solution, to obtain linagliptin. The synthetic method has the advantages of environmental protection, no pollution, high production rate, and no impurities.
Owner:赤峰赛林泰药业有限公司
Personal care compositions and methods for the beautification of mammalian skin and hair
Owner:THE PROCTER & GAMBLE COMPANY
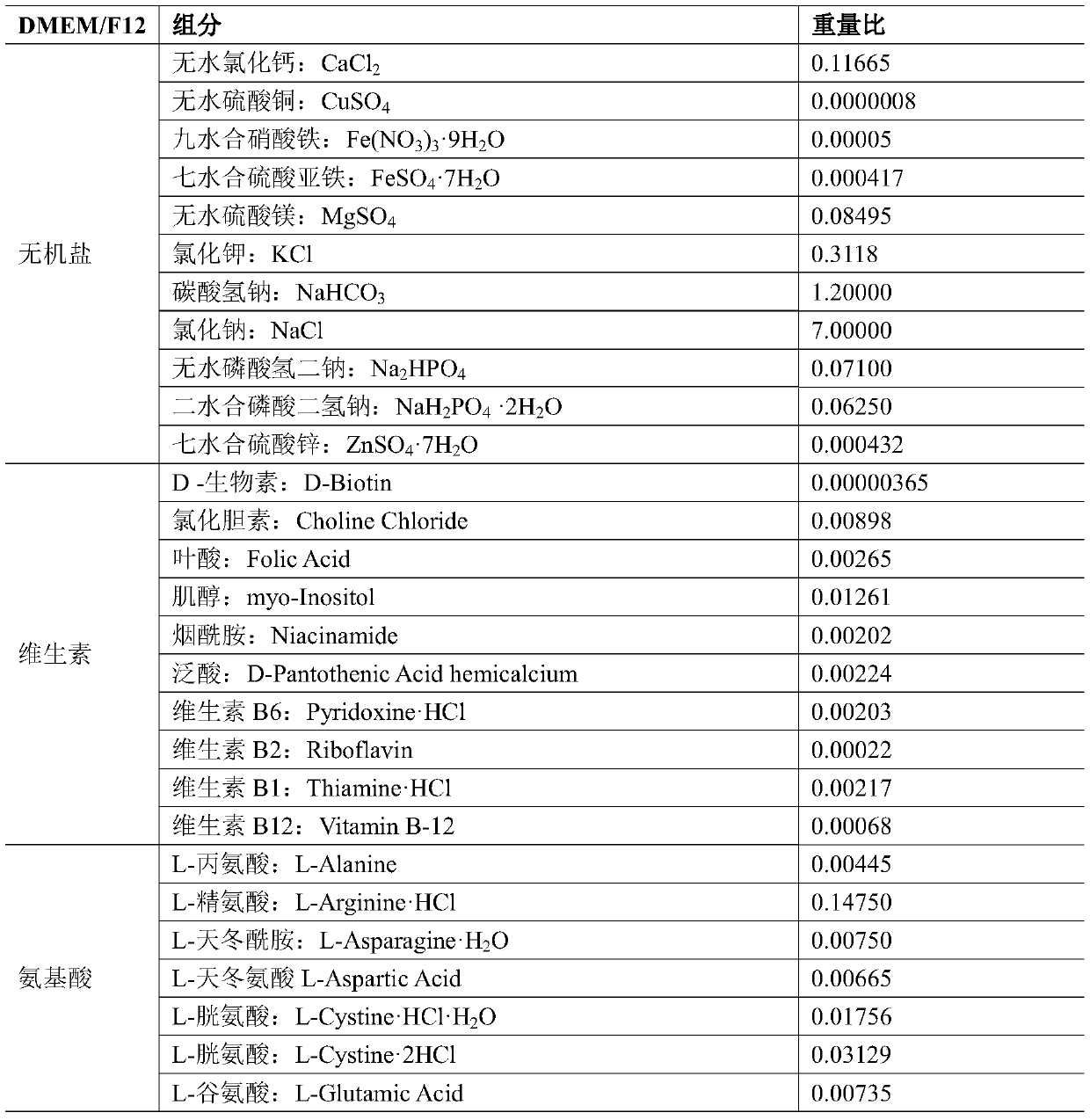
Mesenchymal stem cell adipogenic differentiation culture medium and preparation method thereof
InactiveCN104830757AImprove expression levelStrong specificitySkeletal/connective tissue cellsIndometacinMethyl xanthine
The present invention provides a mesenchymal stem cell adipogenic differentiation culture medium, and belongs to the technical field of stem cells. The mesenchymal stem cell adipogenic differentiation culture medium comprises a DMEM / F12 culture medium, and further comprises FBS with a volume percentage of 5-50%, glutamine with a volume percentage of 0.5-5%, antibiotic with a volume percentage of 0.5-5%, 50-500 [mu]M indometacin, 50-500 nM insulin, 5-50 nM dexamethasone, 0.5-5 [mu]M 3-isobutyl-1-methylxanthine, and 0.05-0.5 [mu]M fasudil hydrochloride. The mesenchymal stem cell adipogenic differentiation culture medium of the present invention has advantages of high inducing efficiency and the like.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Industrial preparation process of linagliptin
The invention relates to the technical field of medicines and particularly relates to an industrial preparation process of linagliptin. The industrial preparation process comprises the steps of adding a reactant a (2-chloromethyl-4-methyl-quinazoline), an equal molar ratio of reactant b (8-bromo-7-(2-butynyl)-3-methylxanthine), an acid-binding agent and a proper amount of solvent into a reaction kettle to react at 0-140 DEG C for 3-8 hours, after TLC detection reaction is finished, directly adding a reactant c ((R)-3-phthalimide piperidine-tartaric acid) and an acid-binding agent, namely N,N-diisopropylethylamine without processing a reaction mother liquid to react at 0-125 DEG C for 3-10 hours, after the TLC detection reaction is finished, adding ethanolamine without processing the reaction mother liquid to react for 2-10 hours, after the TLC detection reaction is finished, dropwise adding purified water, carrying out suction filtration to obtain a linagliptin rough product, and refining by virtue of a refining method disclosed in a patent CN101437823A, so as to obtain a linagliptin refined product. According to the industrial preparation process, linagliptin is synthesized by virtue of a one-pot continuous feeding method, the consumption of the solvent is low, and the operation is easy; and the industrial preparation process is suitable for industrial production.
Owner:SHOUGUANG FUKANG PHARMA
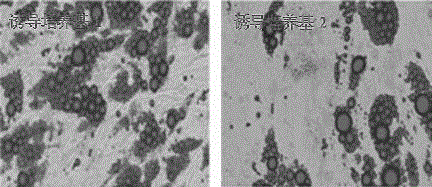
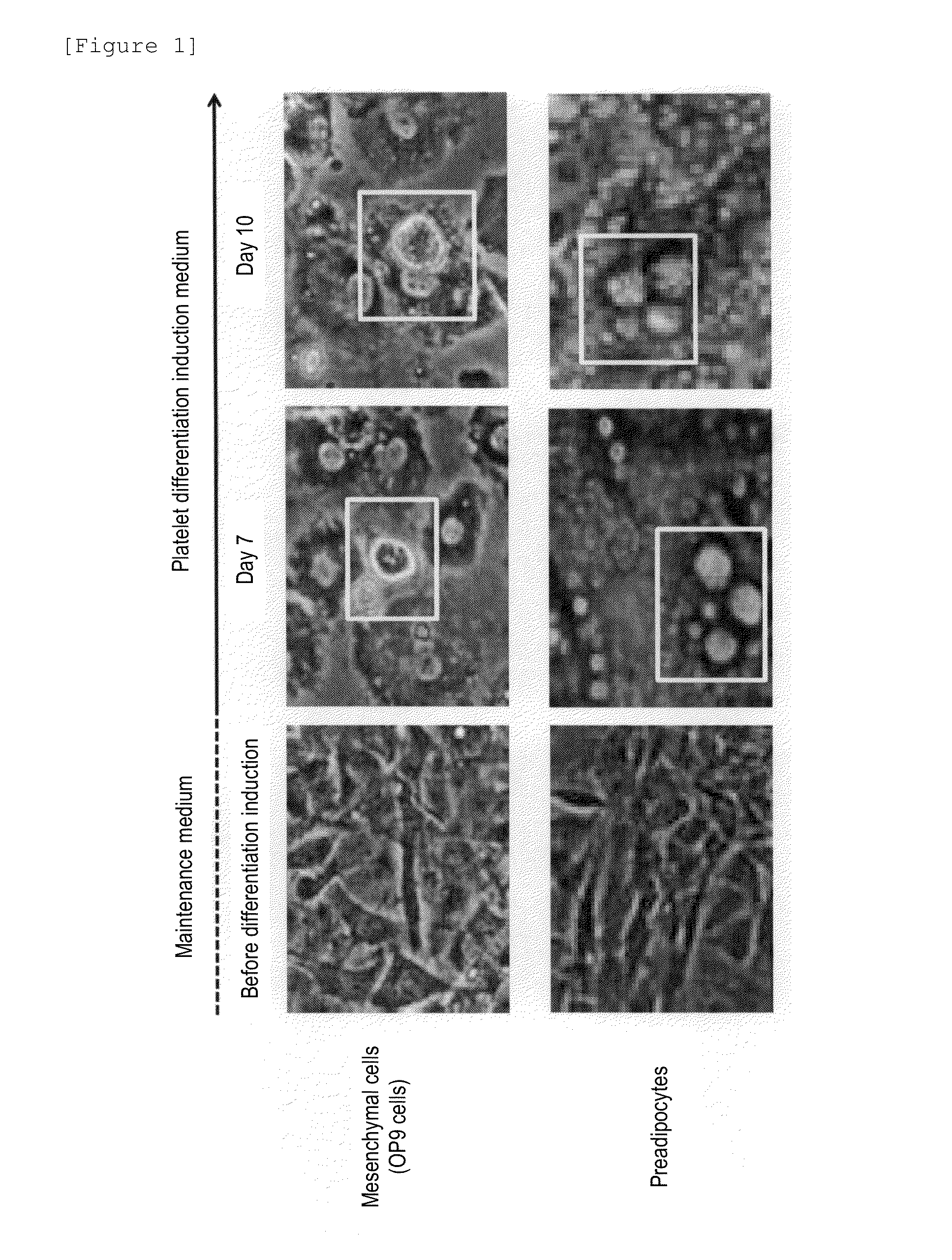
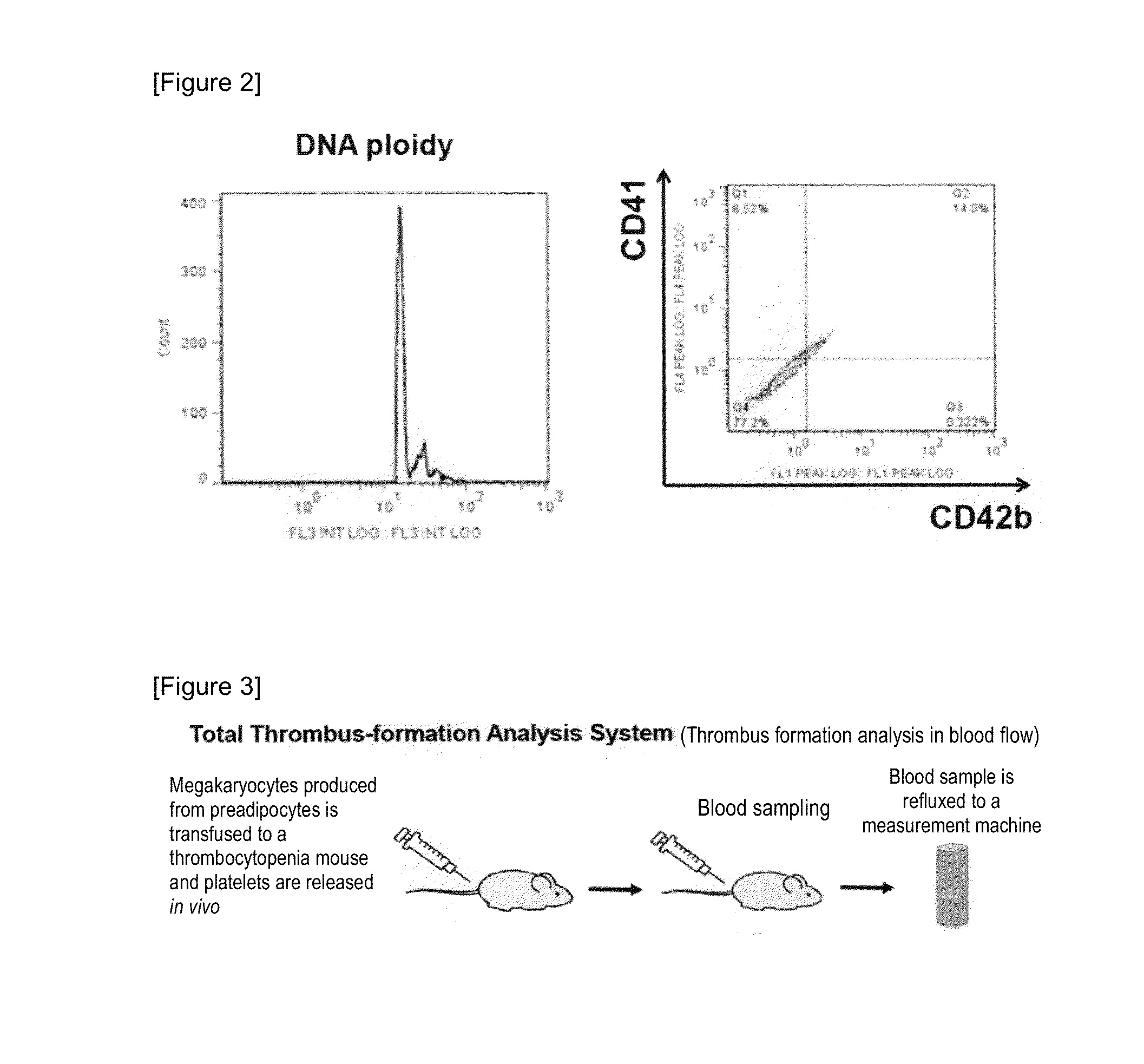
Method for producing megakaryocytes, platelets and/or thrombopoietin using mesenchymal cells
ActiveUS20160177265A1Improve efficiencyLow costCulture processSkeletal/connective tissue cellsMethyl xanthineMegakaryocyte
Provided is a megakaryocyte and / or platelet production method, enabling to produce a megakaryocyte and / or platelet from mesenchymal cells such as preadipocytes in a relatively short period of time, simply, in a large amount and at lower cost or more efficiently in vitro and a method for producing TPO simply and in a larger amount. A first invention is a method for producing a megakaryocyte and / or platelet, comprising culturing a mesenchymal cell in a mesenchymal cell culturing basic medium containing an iron ion and an iron transporter and collecting megakaryocytes and / or platelets from a culture. A second invention is a method for producing thrombopoietin, comprising culturing a mesenchymal cell or mesenchymal cell-derived megakaryocyte in a mesenchymal cell culturing basic medium containing an iron ion and an iron transporter and collecting thrombopoietin from a culture. A third invention is a method for producing thrombopoietin, comprising culturing a preadipocyte in a preadipocyte culturing basic medium containing dexamethasone, 3-isobutyl-1-methylxanthine and insulin and collecting thrombopoietin from a culture.
Owner:ADIPOSEEDS INC
Preparation method for Trajenta
ActiveCN104672238AHigh purityReaction raw materials are readily availableOrganic chemistryCompound aBromine
Owner:CHINA RESOURCES SAIKE PHARMA
Separation, culture and induction differentiation method for precursor adipocyte in chicken muscle
InactiveCN107858327AImprove the induction effectImprove induction efficiencyCell dissociation methodsCulture processMethyl xanthineOleic Acid Triglyceride
The invention relates to a separation, culture and induction differentiation method for precursor adipocyte in chicken muscle and belongs to the technical field of cytology. According to the method, hormone mixture 3-isobutyl-1-methylxanthine, dexamethasone and insulin are used, an induction differentiation system of external-source oleic acid and rosiglitazone is added, generation of oil drop ofprecursor adipocyte in the chicken muscle is induced, and an effective research means can be provided for the quality of poultry meat and particularly fat deposition in poultry muscle. By adopting themethod, intramuscular precursor adipocyte which is even in component and exuberant in proliferation and differentiation can be obtained, the effective induction differentiation system of adipocyte inthe chicken muscle is obtained, and the method has the advantages that the method is simple and convenient, easy to operate, low in cost, high in vigor and excellent in proliferation and differentiation capacity and can achieve acquirement of a large number of cells.
Owner:HENAN AGRICULTURAL UNIVERSITY
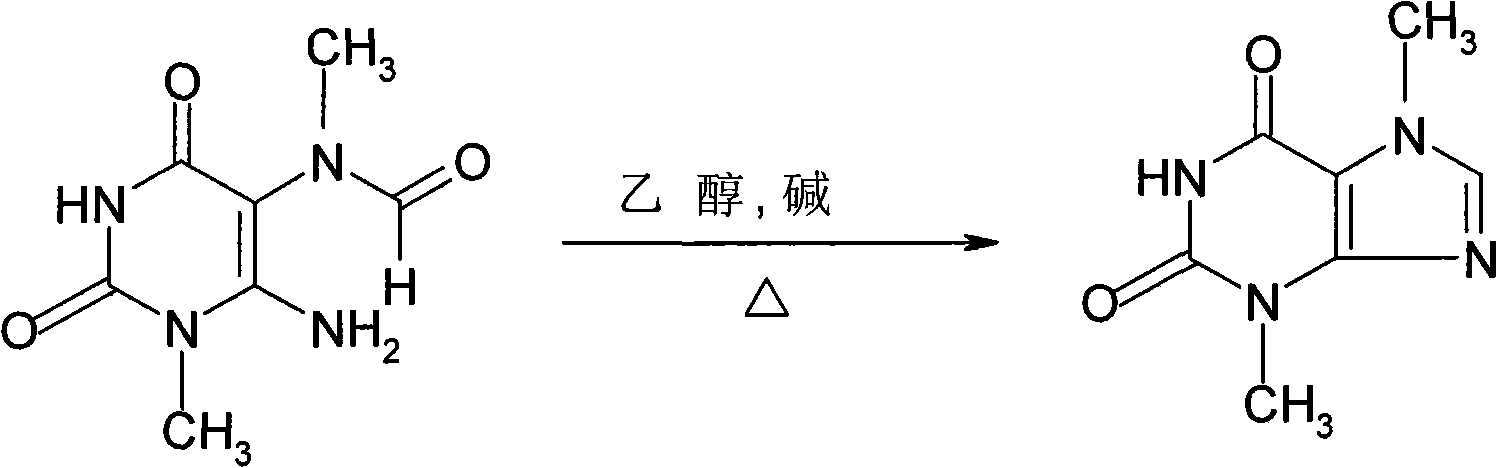
Theobromine production process
The invention provides a new method for preparing a theobromine by a 3-methyl xanthine disodium salt methylation with more production value. The theobromine is prepared by using acetone as a solvent to react with a dimethyl sulfate under the existence of a sodium carbonate and then acidified. The invention uses the acetone as the solvent and decreases the hydrolysis loss of the dimethyl sulfate; the sodium carbonate provides an alkaline condition for the methylation reaction and the Ph value is intermediate. The dimethyl sulfate is dropped to carry out the methylation reaction with the advantages of good selectivity and a yield higher than other methylation technique by more than 10 percent. The acetone is recycled and reused, the reaction is relatively complete and has little caffeine by-product, and the acquired analysis content of theobromine crude product HPLC is larger than 90 percent. The invention is extremely applicable to industrial production.The invention relates to a refining method of santheose, which is characterized in that dissolves liquid alkali into crude santheose solution, decolourizes, fitlers, and adds reduction agent into filter liquor, acidifies at 60-80 DEG C until pH=5-6, filters and dries to obtain santheose final product. The inventive method has refining yield of 90%, standard product color, high product quality, low cost and industrialization suitability.
Owner:PERRIGO TRADING (SHANGHAI) CO LTD
Mesenchymal stem cell adipogenesis induced differentiation method
InactiveCN109988746ALess componentsSimple ingredientsCulture processSkeletal/connective tissue cellsPenicillinAdipogenesis
The invention discloses a mesenchymal stem cell adipogenesis induced differentiation method. The method includes the following steps: (1) recovering the mesenchymal stem cells to grow to 70-90%; (2) digesting the mesenchymal stem cells and culturing for 1-3 days by using a complete culture medium; and (3) when the cell fusion degree reaches 70-90%, sucking off the complete culture medium, adding amesenchymal stem cell fat-forming induction differentiation culture medium (composed of DMEM / F12 basal culture medium, fetal bovine serum with volume concentration of 8-12%, 8-12 ug / ml of insulin, 1%. of penicillin, 1 %. of streptomycin, 0.8-1.2 umol / L of dexamethasone, 100-500 umol / L of 3-isobutyl-1-methylxanthine) for culture, and changing the culture medium every 3-4 days; preforming continuous induction, which depends on cell growth and adipogenesis condition. The method is proper in operation, simplifies the steps of induced differentiation and is quite high in adipogenesis induced differentiation rate.
Owner:上海葆年生物科技有限公司
Method for purifying preadipocytes derived from fetal bovine skeletal muscle tissue
ActiveCN106282100AEasy to purifyCell dissociation methodsCulture processMethyl xanthinePlatelet-Derived Growth Factor Receptor Alpha
The invention discloses a method for purifying preadipocytes derived from fetal bovine skeletal muscle tissue. According to the method, a used material is longissimus dorsi tissue of a 3-month-old bovine fetus, and used reagents contain collagenase, fetal bovine serum, the low- glucose Dulbecco's Modified Eagle's Medium, a cell sorting buffer solution, a PDGFR-alpha (platelet-derived growth factor receptor alpha) antibody, dexamethasone, 3-isobutyl-1-methylxanthine, bovine insulin and oil red O.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
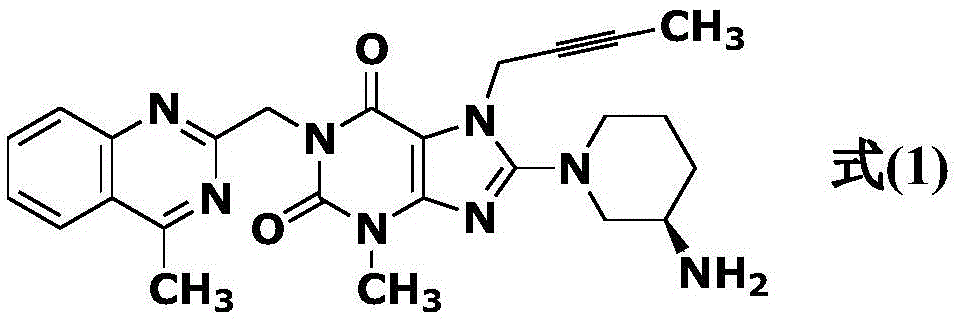
Simple preparation method of high-purity linagliptin
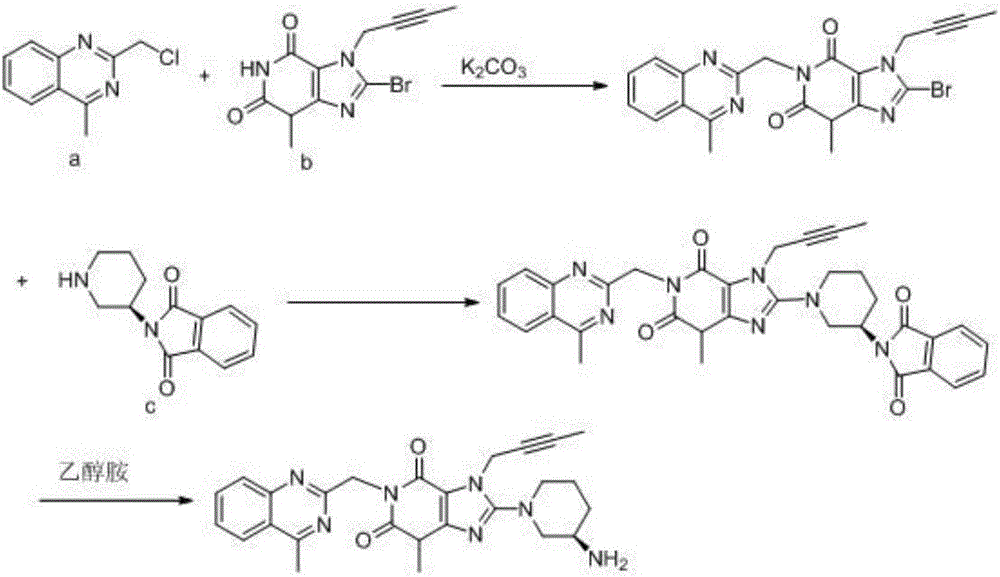
The invention relates to a simple preparation method of high-purity linagliptin. The method includes the steps of making 8-bromine-3-methyl xanthine and 1-bromo-2-butyne react, directly adding 2-chloromethyl-4-methylquinazoline without processing after reaction is completed, preparing a key intermediate 8-bromine-7-(2-butyne-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-diketone of linagliptin through a one-pot method, making the intermediate react with (R)-3-piperidinamine dihydrochloride after being filtered and separated to obtain a linagliptin solution, and obtaining a linagliptin pure product after processing the linagliptin solution. The key intermediate is prepared through the one-pot method, operation is convenient, and yield is increased; the key intermediate reacts with (R)-3-piperidinamine dihydrochloride after being separated, and therefore high-purity linagliptin is obtained, and the requirements for production and declaration of pharmaceutical enterprises are met to the maximum extent.
Owner:VALIANT CO LTD
Culture medium for inducing adipogenic differentiation of muscle derived stem cells of skeletal muscles, and application and adipogenic differentiation method thereof
ActiveCN106754664AIncreased rate of adipogenic differentiationCulture processSkeletal/connective tissue cellsDexamethasoneMethyl xanthine
The invention relates to the field of medicine, and discloses a culture medium for inducing adipogenic differentiation of muscle derived stem cells of skeletal muscles, and an application and an adipogenic differentiation method thereof. The culture medium disclosed by the invention comprises 3-isobutyl-1-methylxanthine, insulin, indomethacin, dexamethasone, pioglitazone and FBS in a basic culture medium. The components of the culture medium are optimized and are induced to differentiate, and the conventional component in the existing mesenchymal stem cells is replaced by pioglitazone, so that an effect of significantly improving the adipogenic differentiation rate of the muscle derived stem cells of skeletal muscles is realized.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
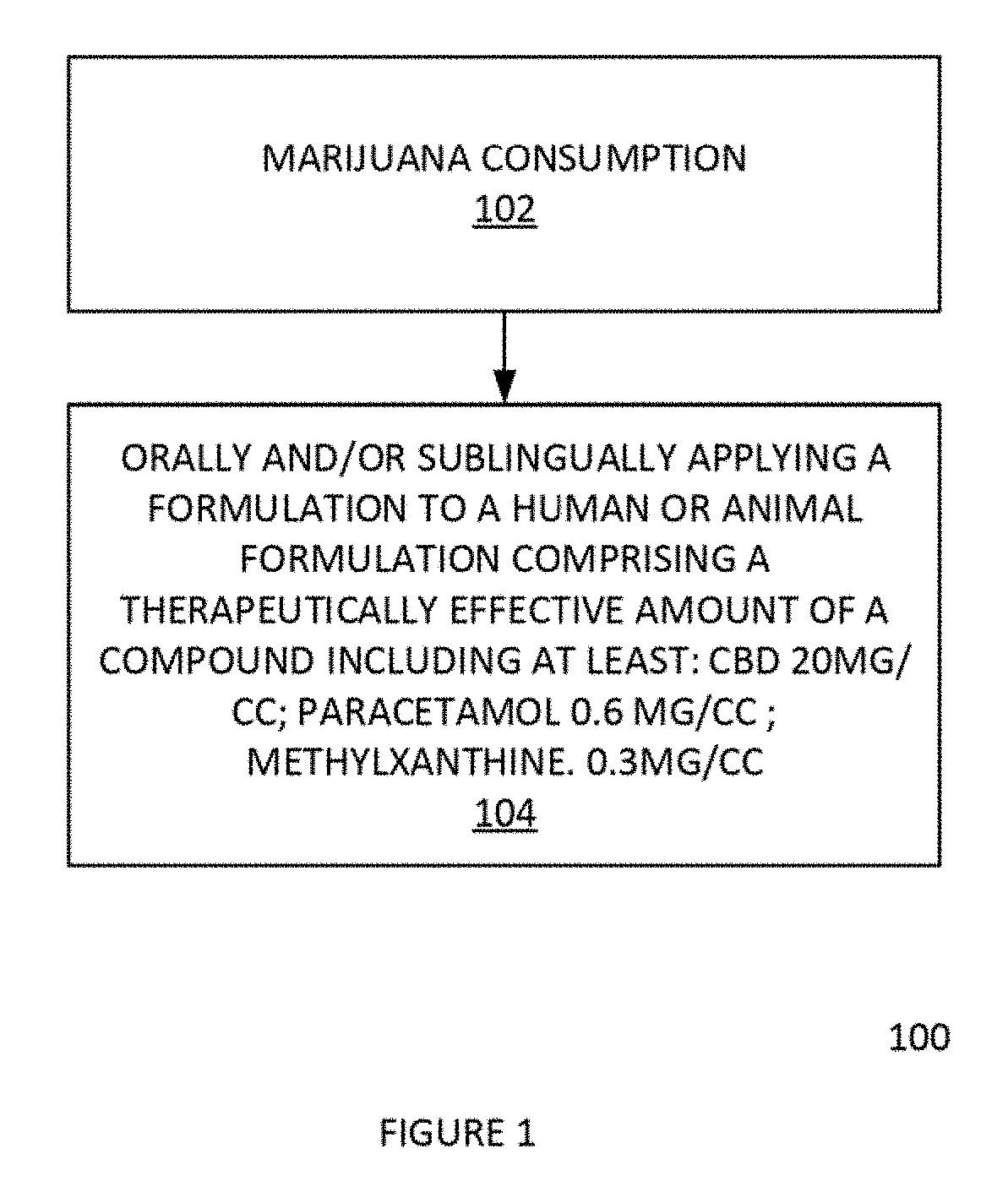
Method for reduction, suppression, or elimination of anxiety or marijuana/cannabis effects and related marijuana/cannabis product by process
InactiveUS20190134121A1Hydroxy compound active ingredientsSuppositories deliveryMonomethyl etherMedicine
In one aspect, a formulation comprising cannabinoids, paracetamol, methylxanthines, salicylates, terpenes, humulus oil, or amino acids individually or any combination or omission thereof for the reduction, alleviation, elimination, and / or suspension of effects of THC exposure and anxiety. The formulation can be the effective amount of cannabinoid is between 5 mg and 5000 mg. The formulation can be the cannabinoid comprises a Cannabidiol (CBD), a cannabidolic acid (CBDA), a Cannabinol (CBN), a Cannabigerol (CBG), a Cannabichromene (CBC), a Cannabicyclol (CBL), a Cannabivarin (CBV), a Tetrahydrocannabivarin (THCV), a Cannabidivarin (CBDV), a Cannabichromevarin (CBCV), a Cannabigerovarin (CBGV), a Cannabigerol monomethyl ether (CBGM), a Cannabielsoin (CBE), or a cannabicitran (CBT). The formulation can be the effective amount of paracetamol is between 0 mg-1000 mg.
Owner:BERMUDEZ STEVEN +1
Novel method for producing theobromine with 3_methylxanthine disodium salt methylating
The invention provides a new method for preparing theobromine by 3-methyl xanthine disodium salt methylation with more production value. The theobromine is prepared by using acetone as a solvent to react with dimethyl sulfate under the existence of sodium carbonate and then acidified. The invention uses the acetone as the solvent and decreases the hydrolysis loss of the dimethyl sulfate; the sodium carbonate provides an alkaline condition for the methylation reaction and the pH value is moderate. The dimethyl sulfate is dripped to carry out the methylation reaction with the advantages of good selectivity and a yield higher than other methylation technique by more than 10 percent. The acetone is recycled and reused; the reaction is relatively complete and has little caffeine by-product; the acquired analytic content of theobromine crude product HPLC is larger than 99 percent; therefore, the invention is extremely applicable to industrial production.
Owner:PERRIGO TRADING (SHANGHAI) CO LTD
Environment-friendly refining method of theobromine
The invention discloses an environment-friendly refining method of theobromine, and relates to the technical field of synthetic chemistry. The method comprises the following steps: (1) dissolving a crude product to prepare a sodium salt; (2) carrying out decoloration; and (3) carrying out acidification and crystallization. According to the method provided by the invention, the theobromine in a theobromine crude product is converted into a sodium salt by adding a sodium hydroxide solution and insoluble impurities are filtered out; then, the decoloration effect is ensured by use of a decolorant,and pigment impurities are removed; and then, by controlling the pH value during acidification, the residual rate of theobromine in crystallization mother liquor can be reduced and the purity of theobromine in precipitated crystals can be increased. The prepared finished product of theobromine is white or creamy in appearance, and the residual rate of 3-methylxanthine is less than 0.15% or more,and meets requirements of the quality standard of theobromine; and the refining yield reaches 95% or more, thus reducing the loss amount of theobromine in a refining process.
Owner:安徽省百花香料香精有限公司
Chocolate composition as delivery system for nutrients and medications
InactiveUS7048941B2Worsen indicationBiocideInorganic non-active ingredientsN-acylethanolaminesAdditive ingredient
A novel chocolate product for use in delivering medicaments and / or nutrients to animals, particularly humans, specially formulated so that the craving for such product by animals, particularly humans, is significantly greater than the craving for chocolate conventionally used in pharmaceutical compositions and the concentration, optimization, and the addition of endogenous and exogenous ingredients to increase such craving as well as to treat specific indications. The chocolate product contains: from about 0.5 to about 200 milligrams, more preferably from about 5 to about 20 milligrams, of one or more biogenic amines per 1 gram of the chocolate product; from about 10 to about 500 milligrams, more preferably form about 20 to about 200 milligrams, of one or more amino acids per 1 gram of the chocolate product; (C) from about 1 microgram to about 20 milligrams, more preferably from about 10 micrograms to about 10 milligrams, of one or more of: methyl tetrahydroisoquinoline, N-acylethanolamines, and / or anandamide and / or salsolinol per 1 gram of the chocolate product; (D) from about 0.2 to about 30 milligrams of at least one trace mineral per 1 gram of the chocolate product; and (E) from 0.6 to about 500 milligrams, more preferably from about 35 to about 100 milligrams, of one or more methylxanthine alkaloids per 1 gram of the chocolate product. The chocolate product used in this invention also preferably contains effective amounts of at least one chocolate aroma and at least one vanilla aroma.
Owner:NEW WORLD ENTERPRIZES
Method for efficient separation and culture of human primary melanin cells
ActiveCN108192856AImprove separation efficiencyShort training periodCell dissociation methodsEpidermal cells/skin cellsAdditive ingredientClonal growth
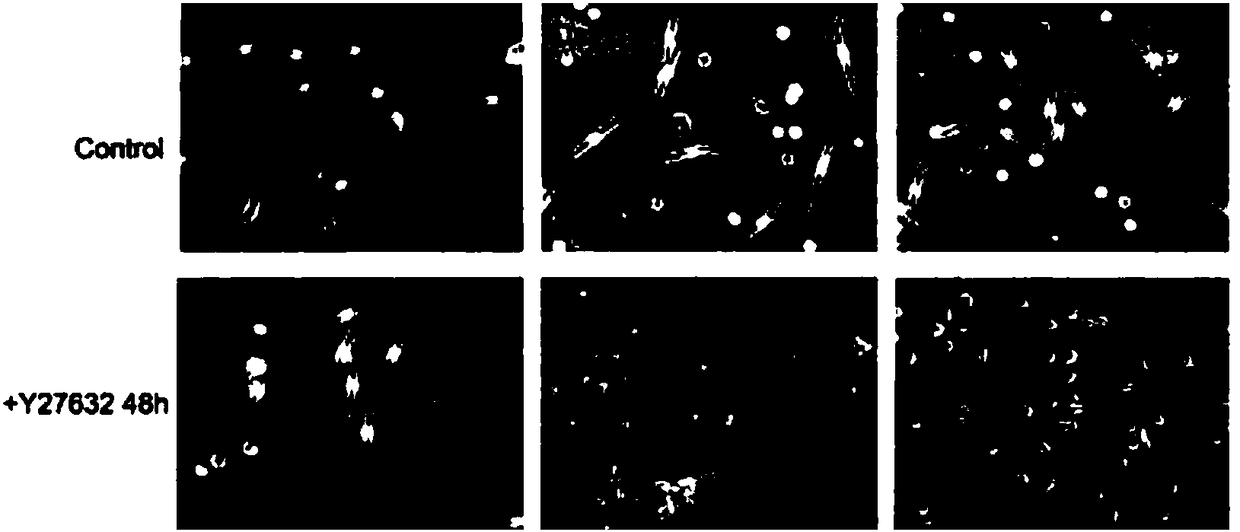
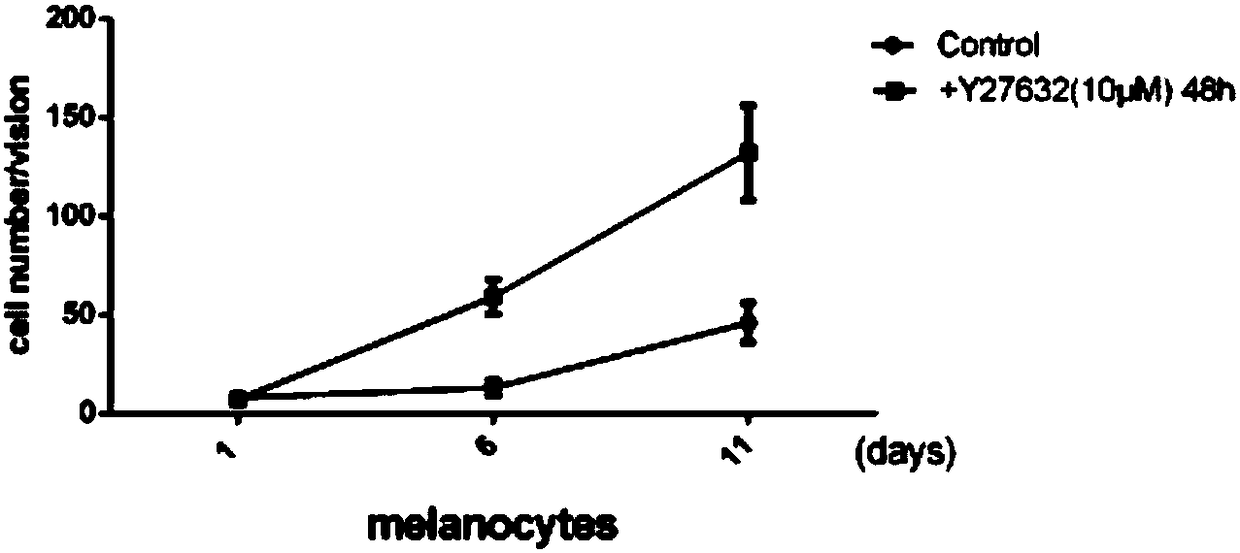
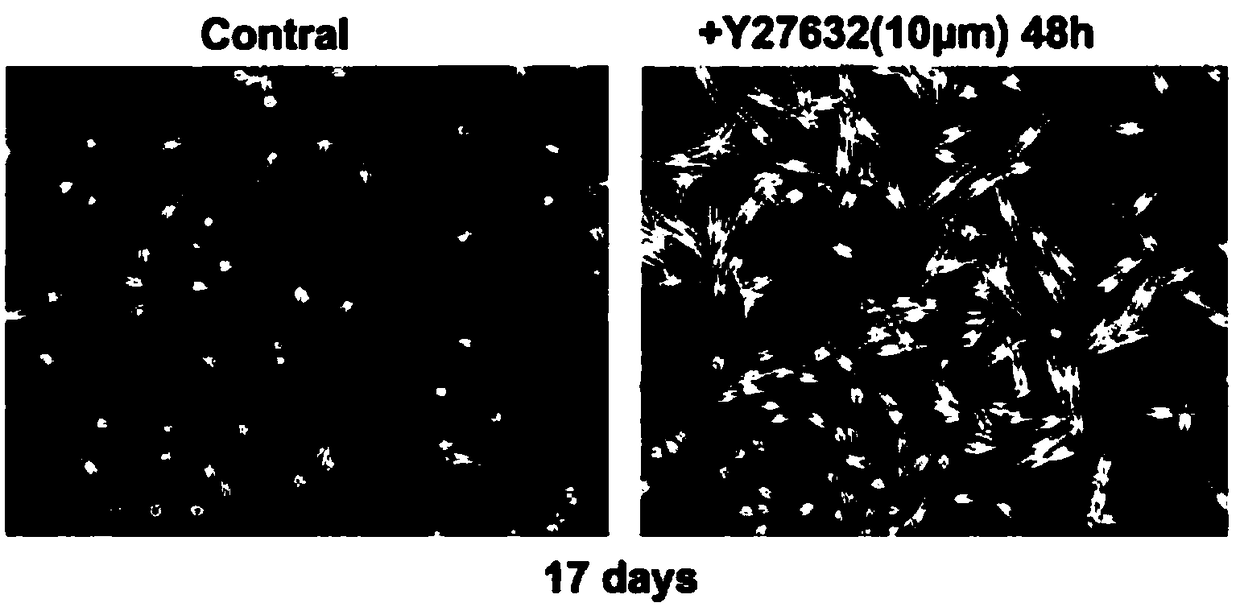
The invention belongs to the technical field of cell culture, and in particular relates to a method for efficient separation and culture of human primary melanin cells. According to the method provided by the invention, an ROCK inhibitor is added to a culture medium which consists of the following ingredients: a Ham's F-12 medium, dibutiryl cyclic adenosine monophosphate, 3-isobutyl-1-methylxanthine, sodium orthovanadate, phorbol 12-myristinate 13-acetate, fetal calf serum and double-antibody; on the basis of mutual actions of the various ingredients, the melanin cells can achieve cloning growth, so that a culture cycle can be shortened by half and separation efficiency of the melanin cells can be greatly improved; the separation and culture method provided by the invention is simple and easy to operate and is low in amount of medium ingredients; and large-scale production of the human primary melanin cells is achieved.
Owner:SHANDONG UNIV
Theobromine production process
A process for preparing theobromine by methylating 3-methyl xanthine disodium salt comprises reacting 3-methyl xanthine disodium salt with dimethyl sulfate by using acetone as solvent in the presence of sodium carbonate and acidifying. The present application also provides a process for refining theobromine which comprises dissolving coarse theobromine in liquid alkaline solution, decolorizing, filtering, adding reducing agent in filtrate, and acidifying at 60-80 °C until pH is 5-6, filtering, and drying to obtain theobromine.
Owner:PERRIGO TRADING (SHANGHAI) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com