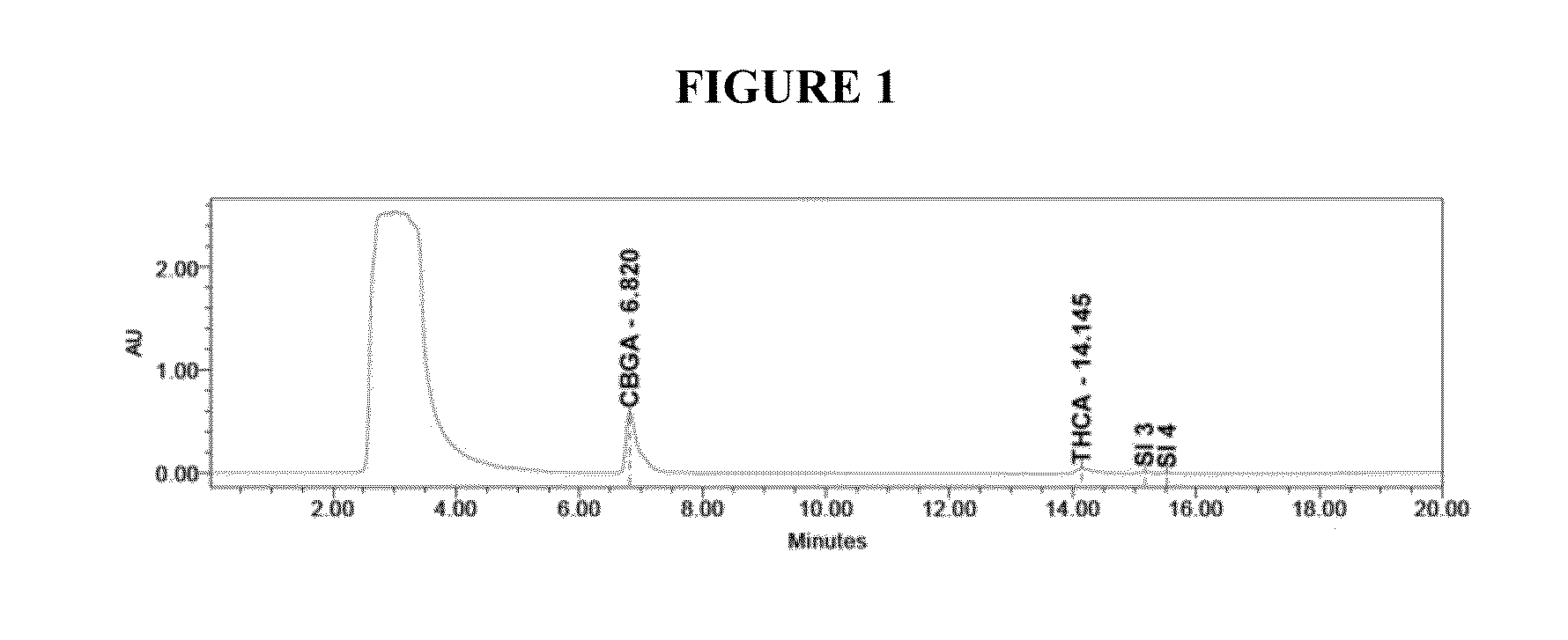
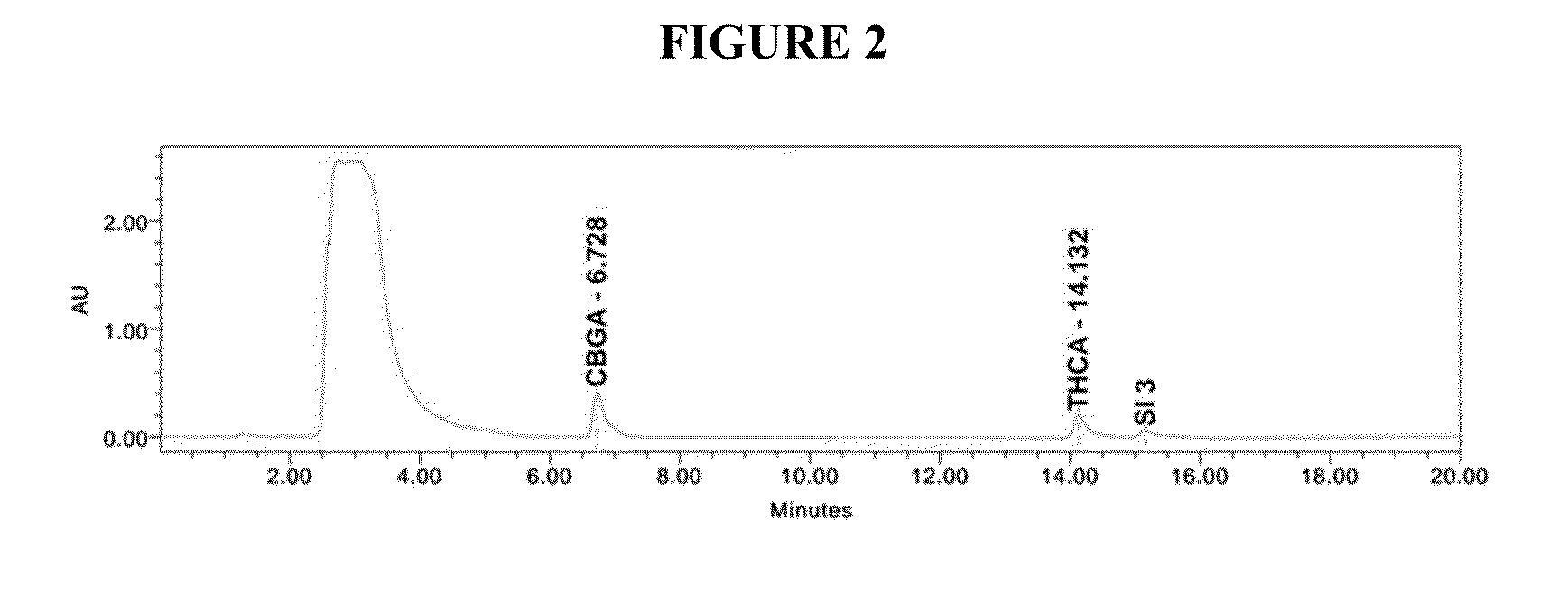
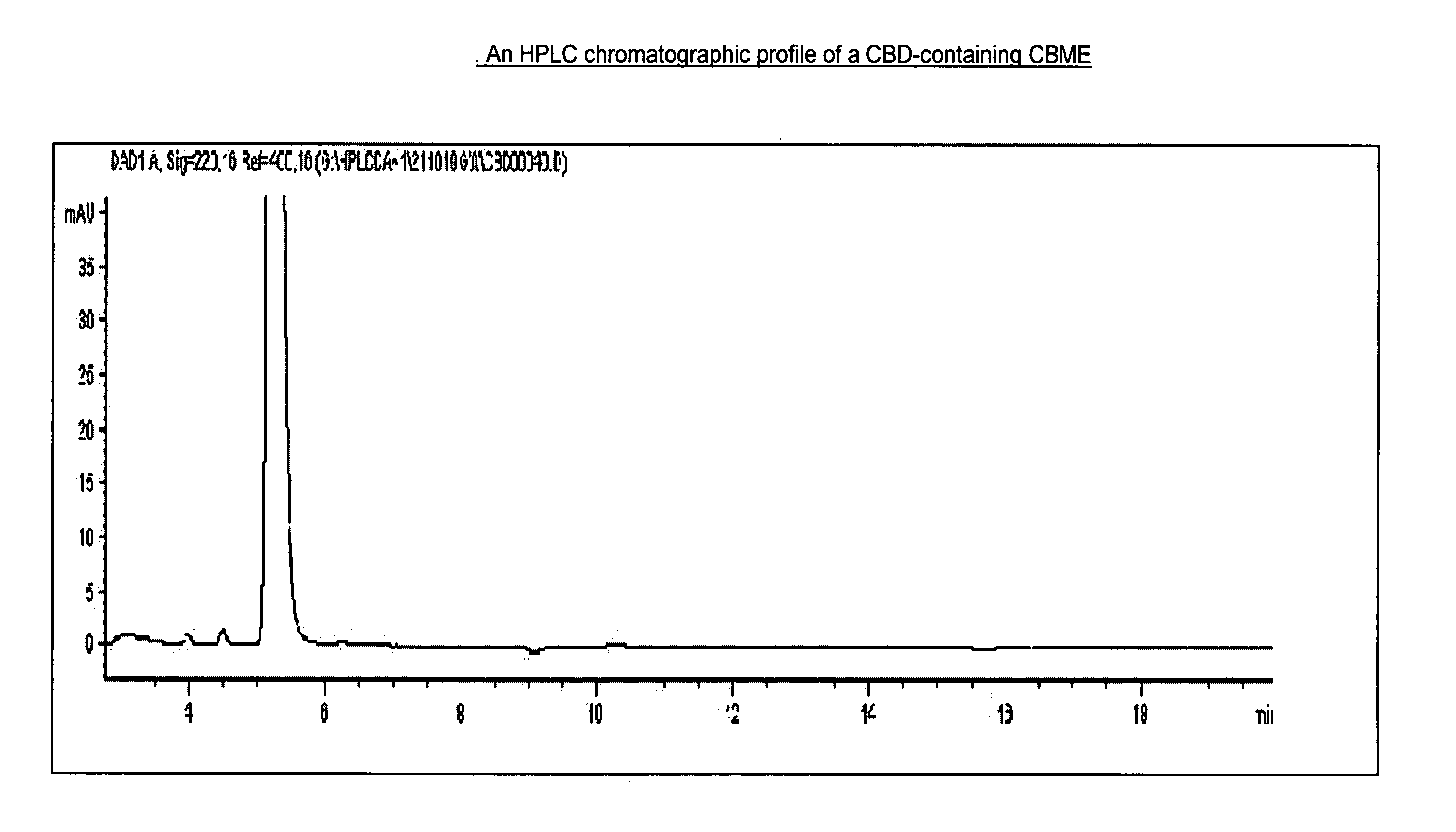
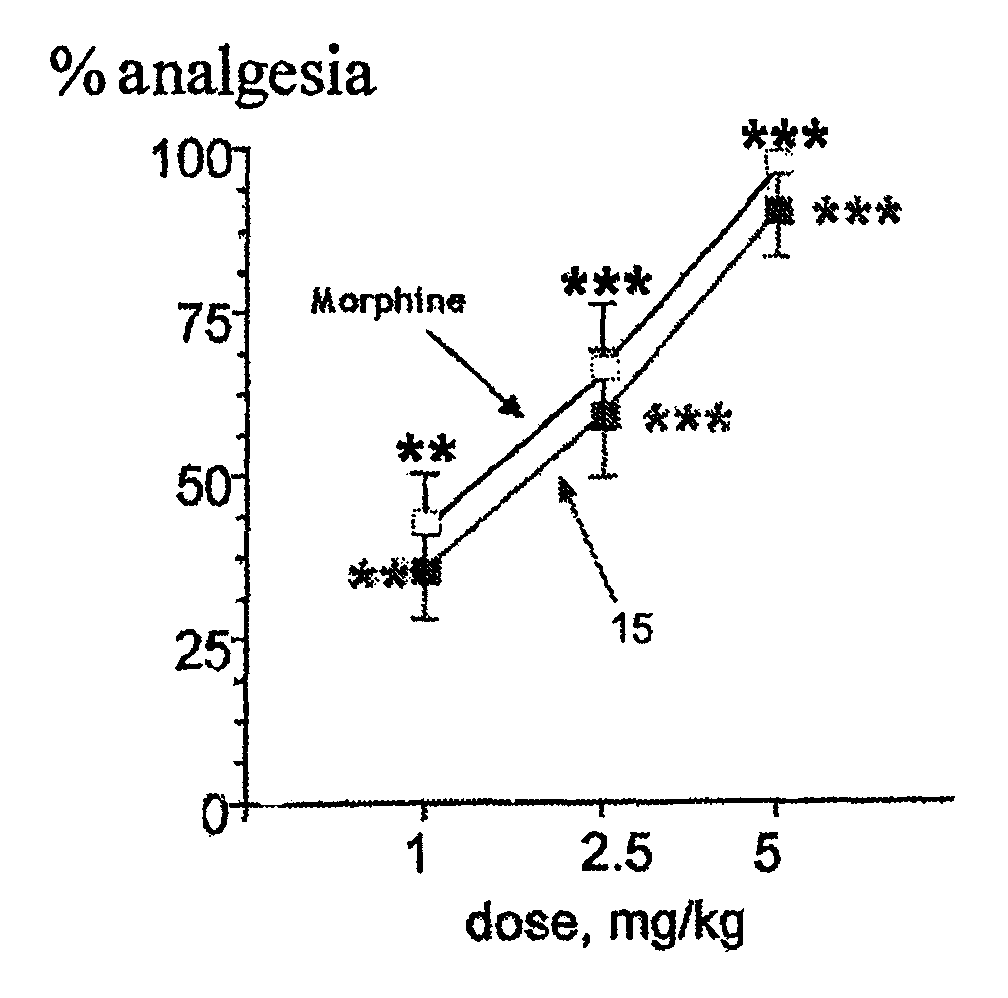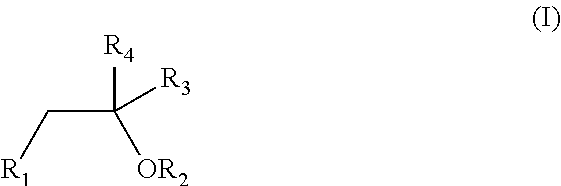Patents
Literature
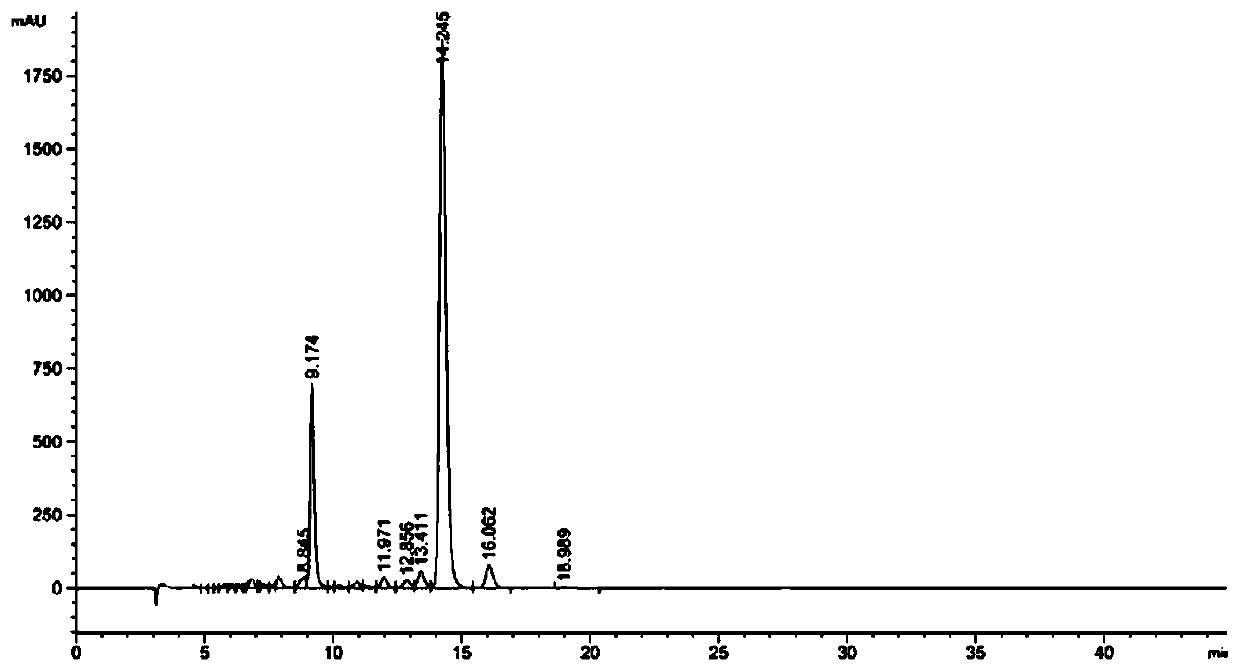
247 results about "Cannabivarin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
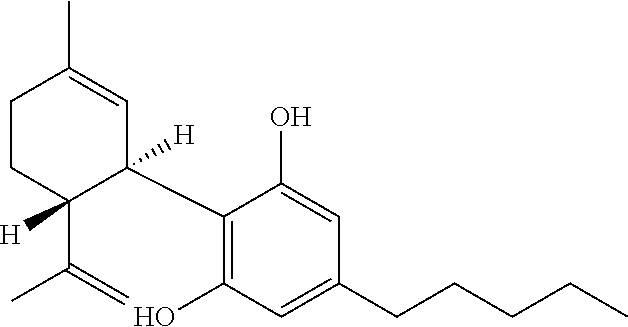
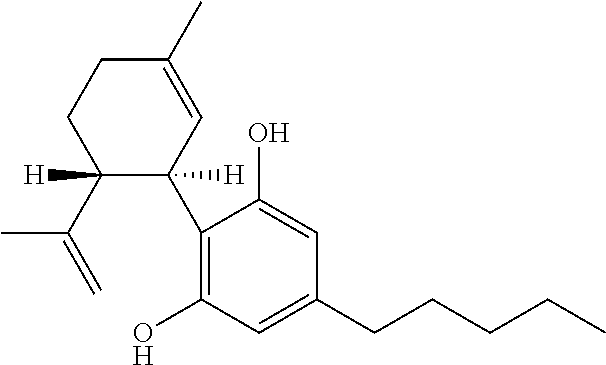
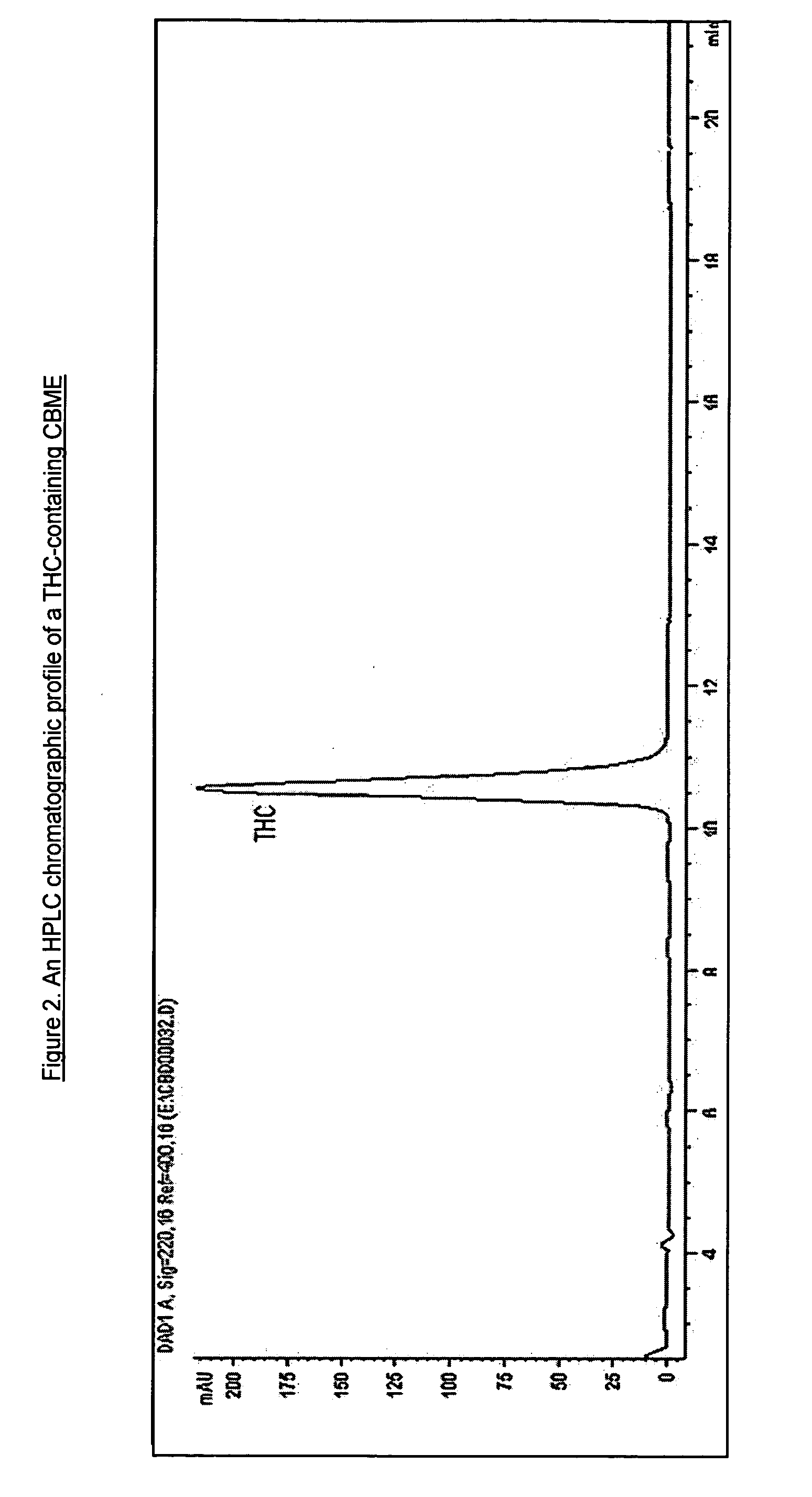
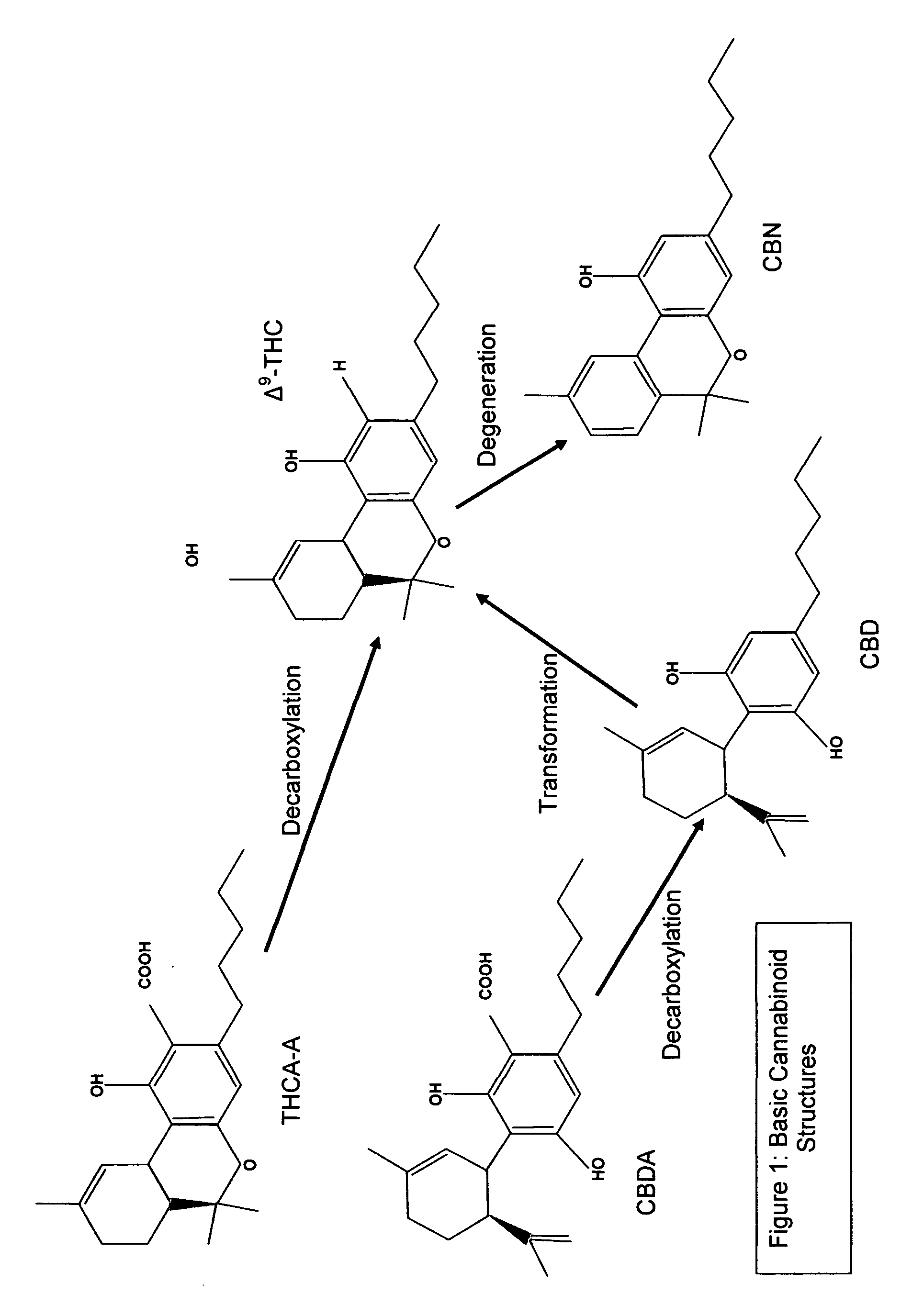
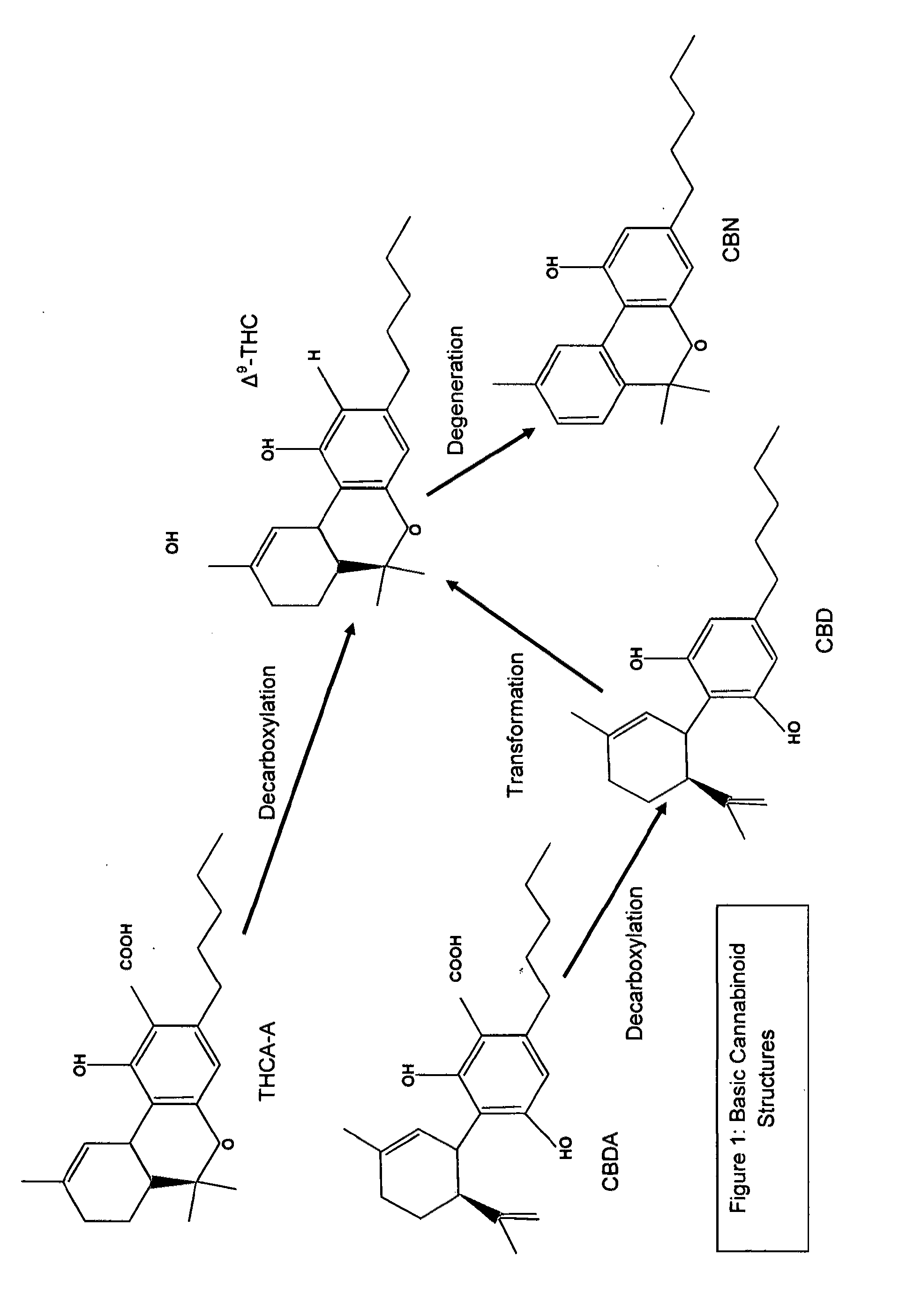
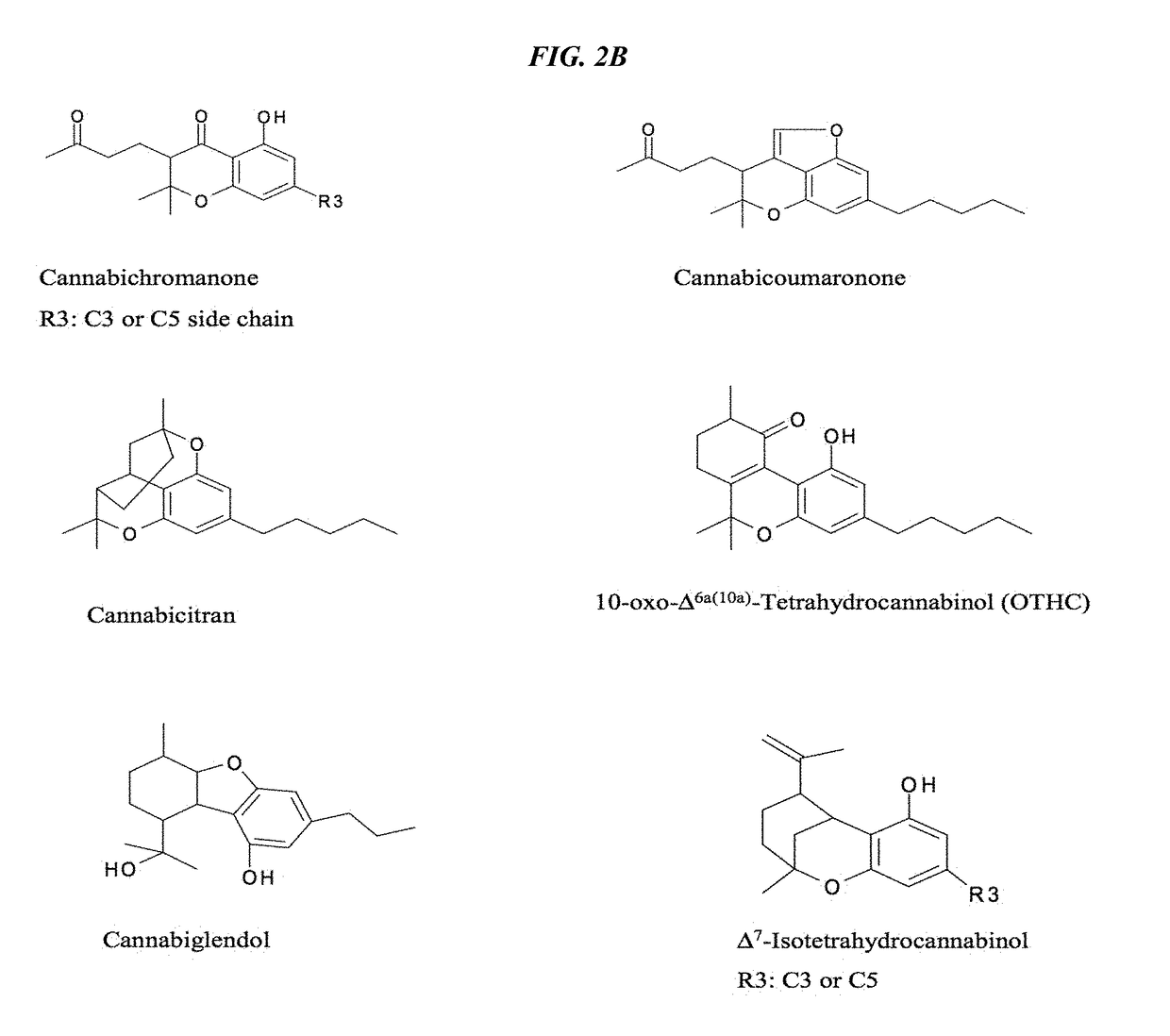
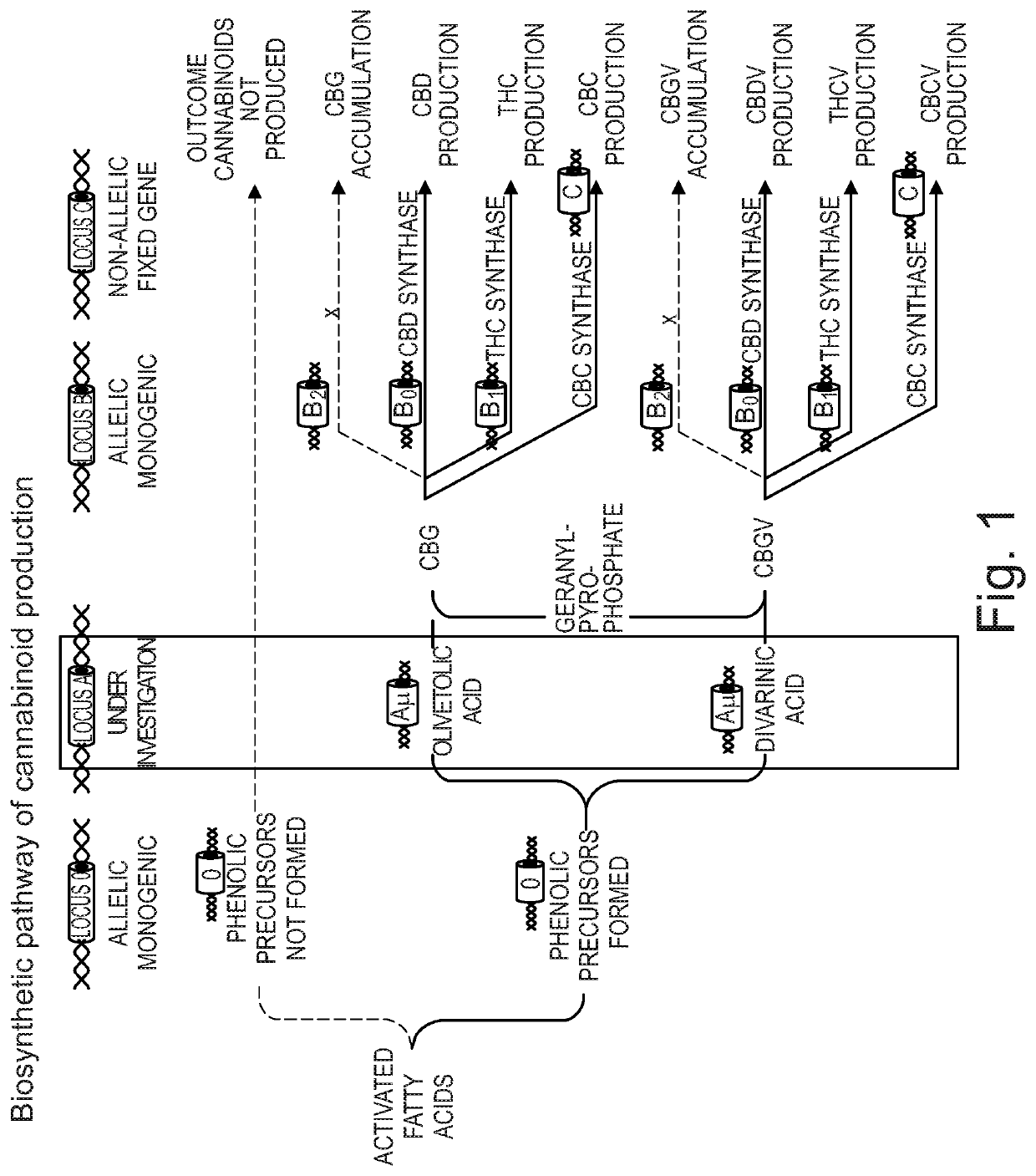
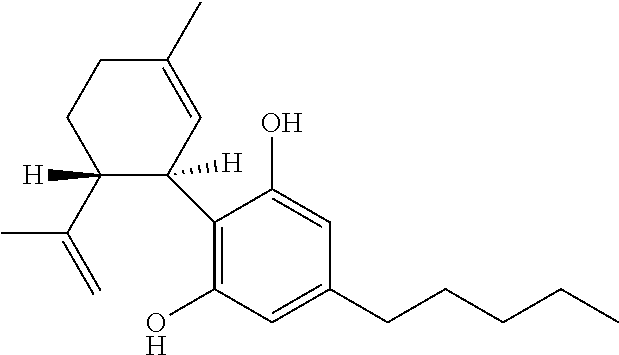
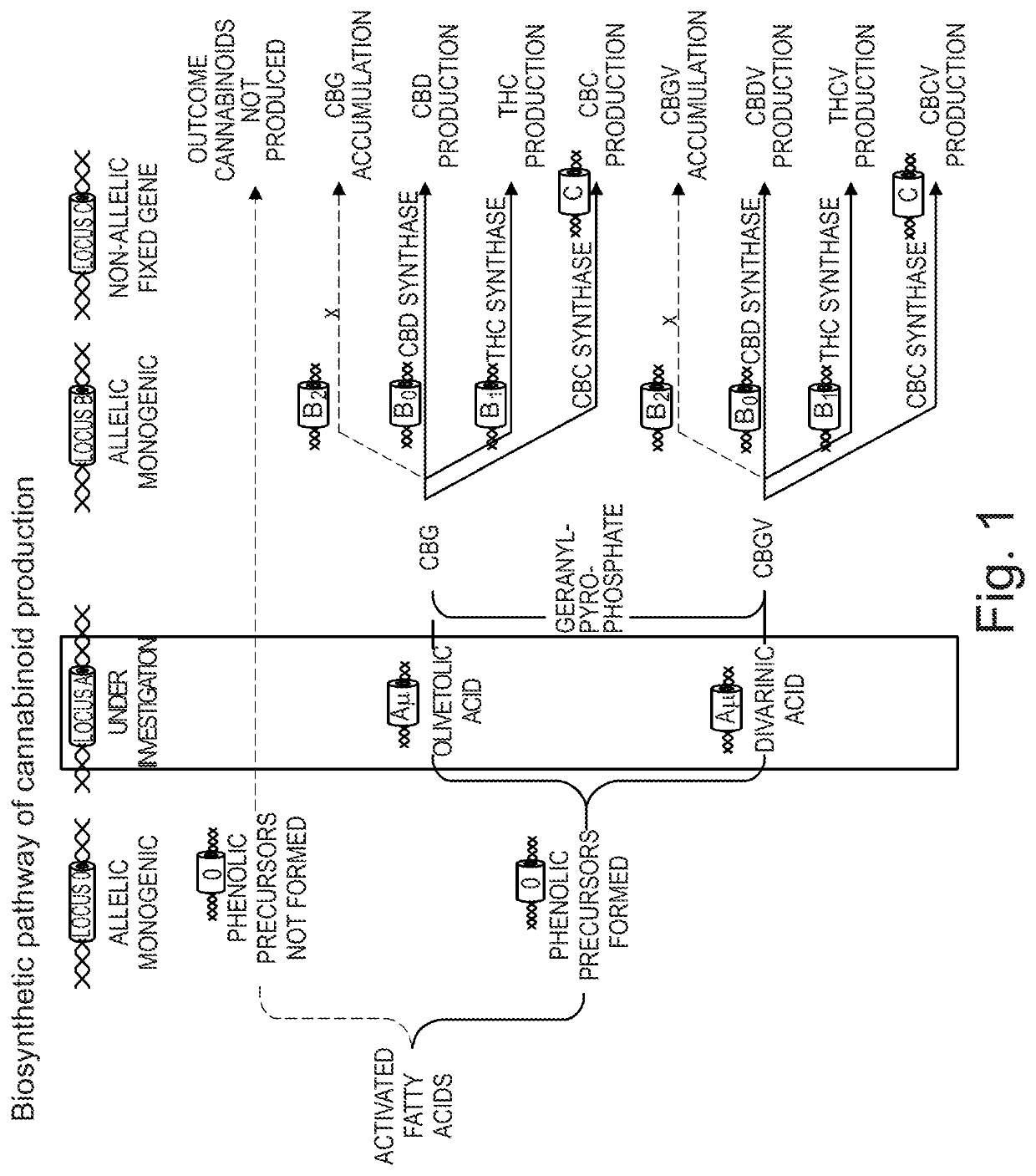
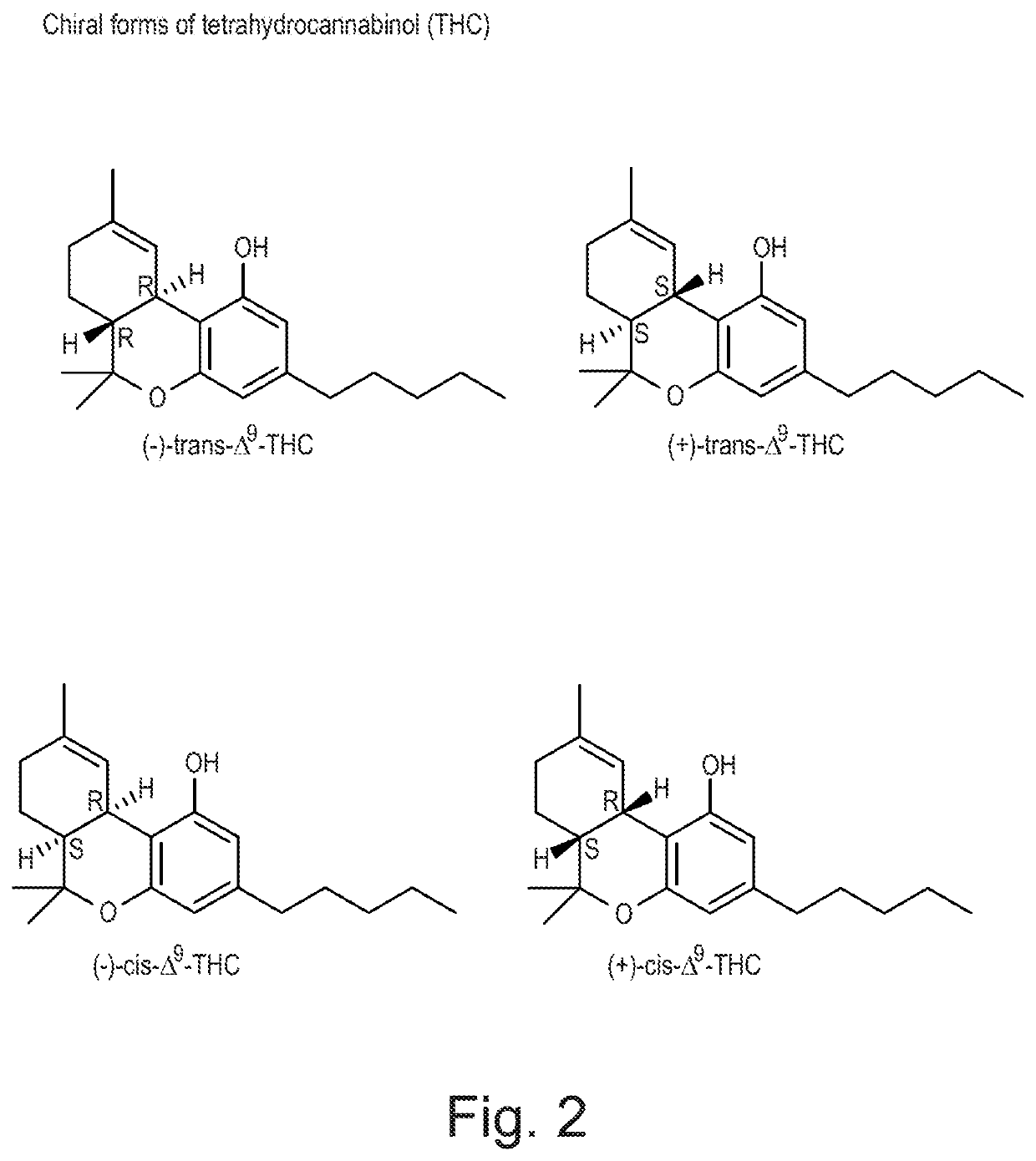
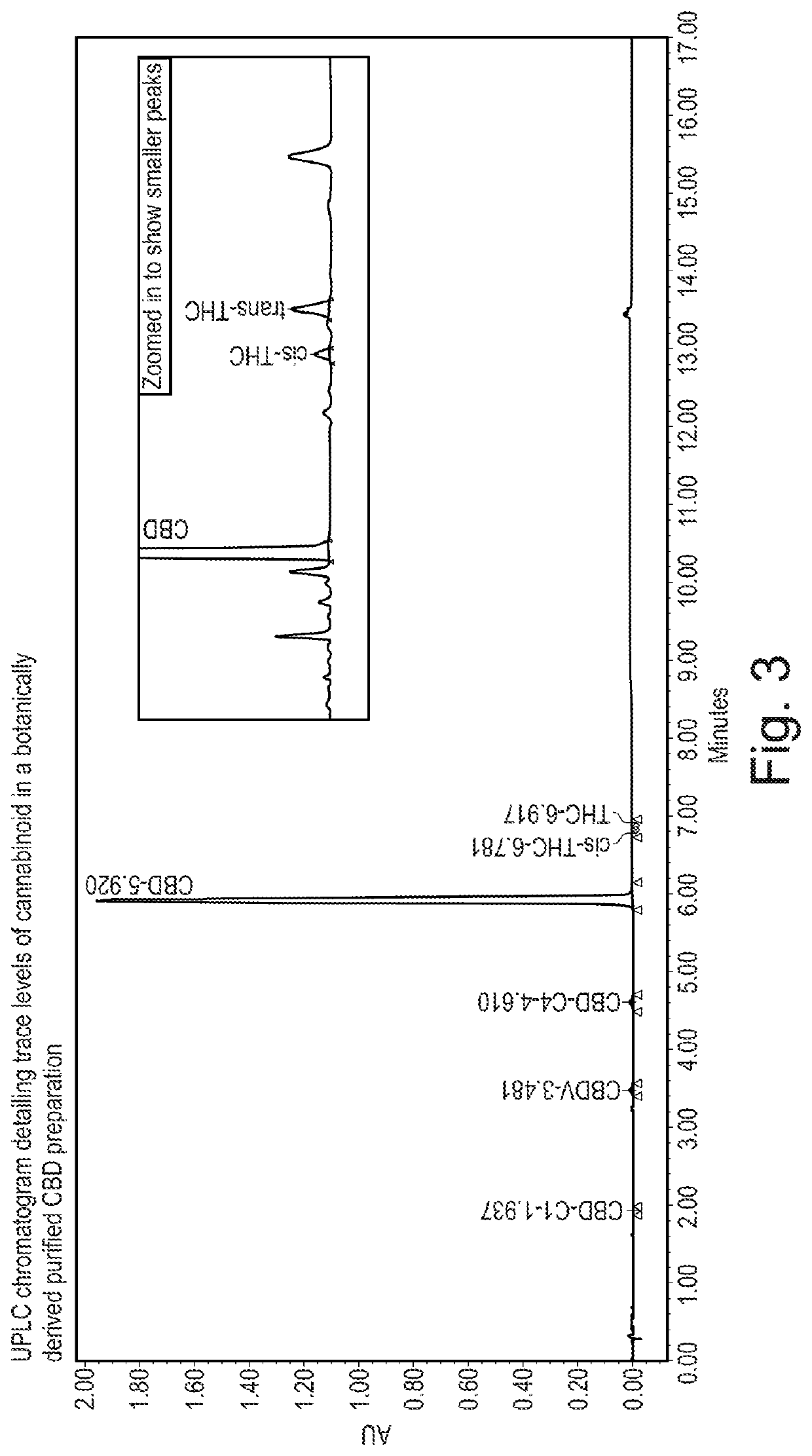
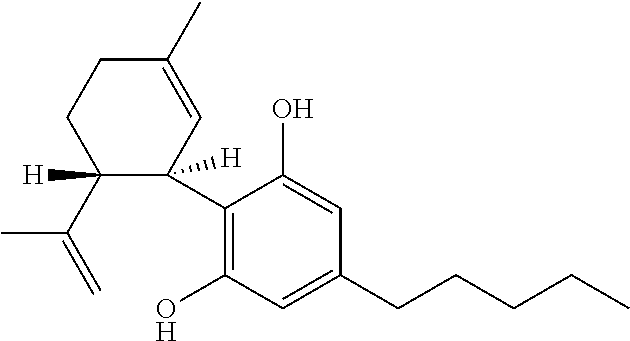
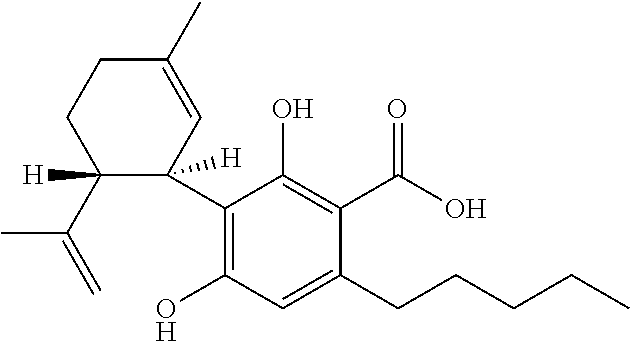
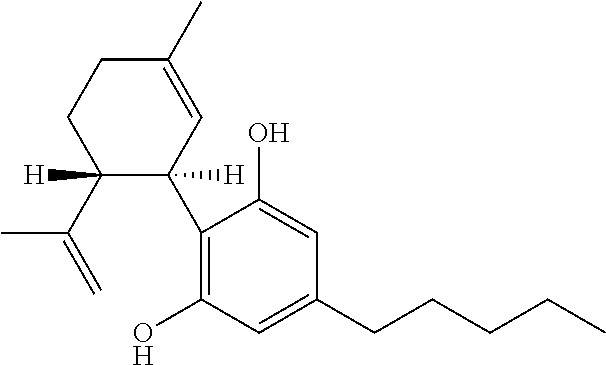
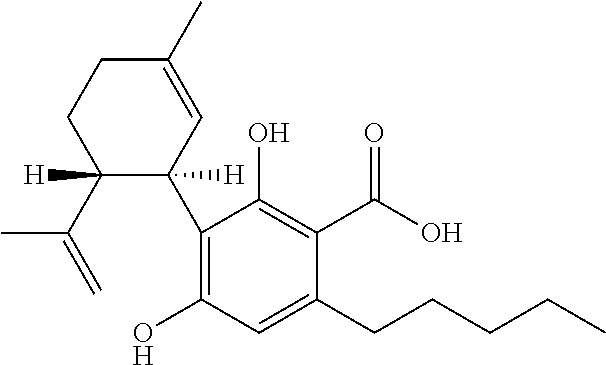
Cannabivarin (CBV), also known as cannabivarol, is a non-psychoactive cannabinoid found in minor amounts in the hemp plant Cannabis sativa. It is an analog of cannabinol (CBN) with the side chain shortened by two methylene bridges (-CH₂-). CBV is an oxidation product of tetrahydrocannabivarin (THCV, THV).
A pharmaceutical composition comprising the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD)
This invention relates to a pharmaceutical composition comprising or consisting essentially of the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD). The composition is particularly safe and efficacious for use in the treatment of neurological conditions, characterized by hyper-excitability of the central nervous system, convulsions or seizures such as occur in epilepsy. Preferably the CBDV and the CBD are present with at least one non-cannabinoid component of cannabis such as one or more terpenes or a terpene fraction. More particularly the composition further comprises one or more cannabichromene type compounds. Particularly cannabichromene propyl variant (CBCV) and / or cannabichromene (CBC). More particularly still the composition is absent or substantially absent of other cannabinoids, including in particular tetrahydrocannabinol (THC) and tetrahydrocannabivarin (THCV), which would normally be present in significant amounts in cannabis chemotypes bred to contain a significant amount of CBDV and / or CBD.
Owner:GW PHARMA LTD
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Cannabinoid active pharmaceutical ingredient for improved dosage forms
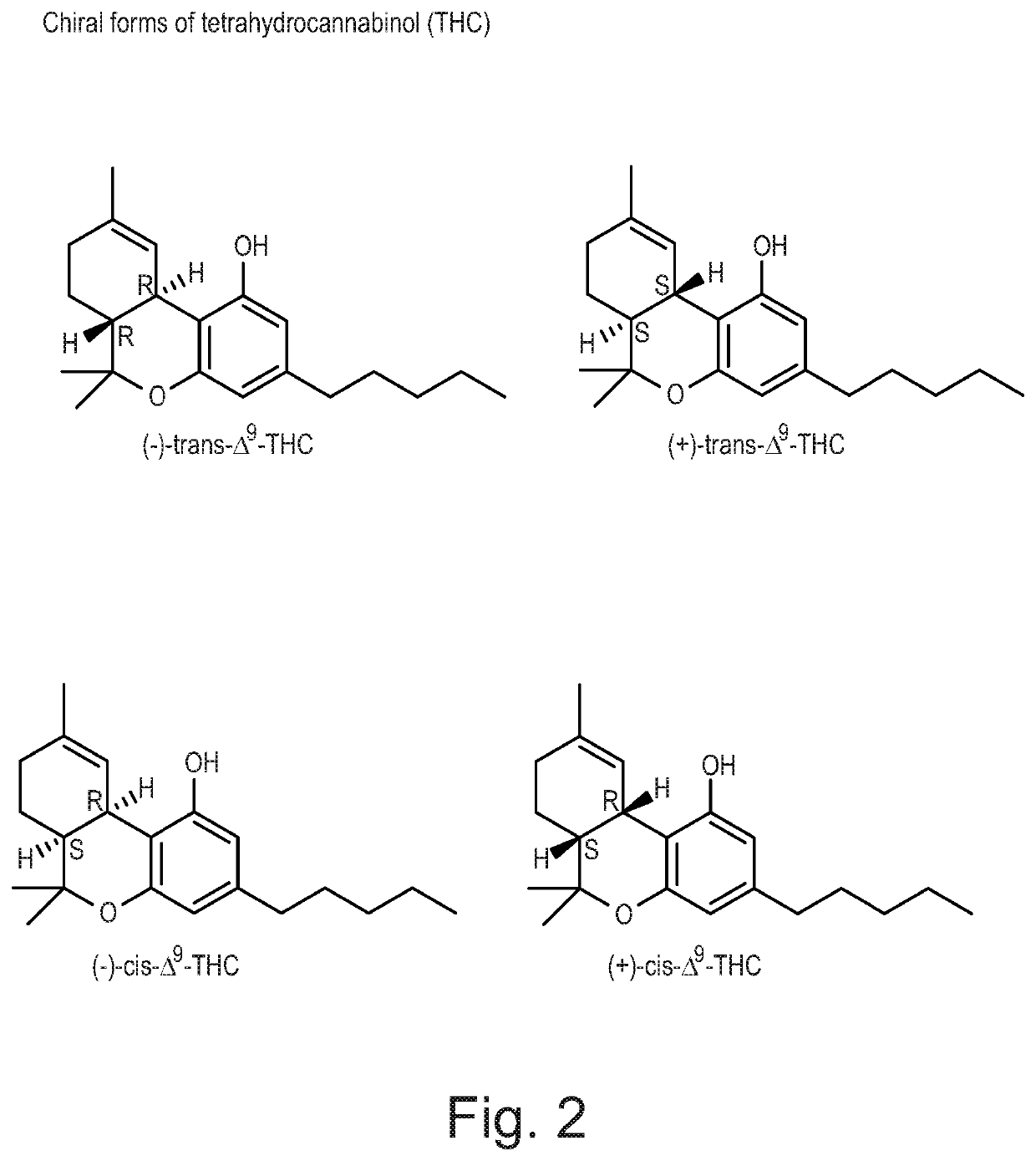
Pharmaceutical compositions comprising the cannabinoid active pharmaceutical ingredient, crystalline trans-(±)-Δ9-tetrahydrocannabinol, and formulations thereof are disclosed. The invention also relates to methods for treating or preventing a condition such as pain comprising administering to a patient in need thereof an effective amount of crystalline trans-(±)-Δ9-tetrahydrocannabinol. In specific embodiments, the crystalline trans-(±)-Δ9-tetrahydrocannabinol administered according to the methods for treating or preventing a condition such as pain can have a purity of at least about 98% based on the total weight of cannabinoids.
Owner:SVC PHARMA
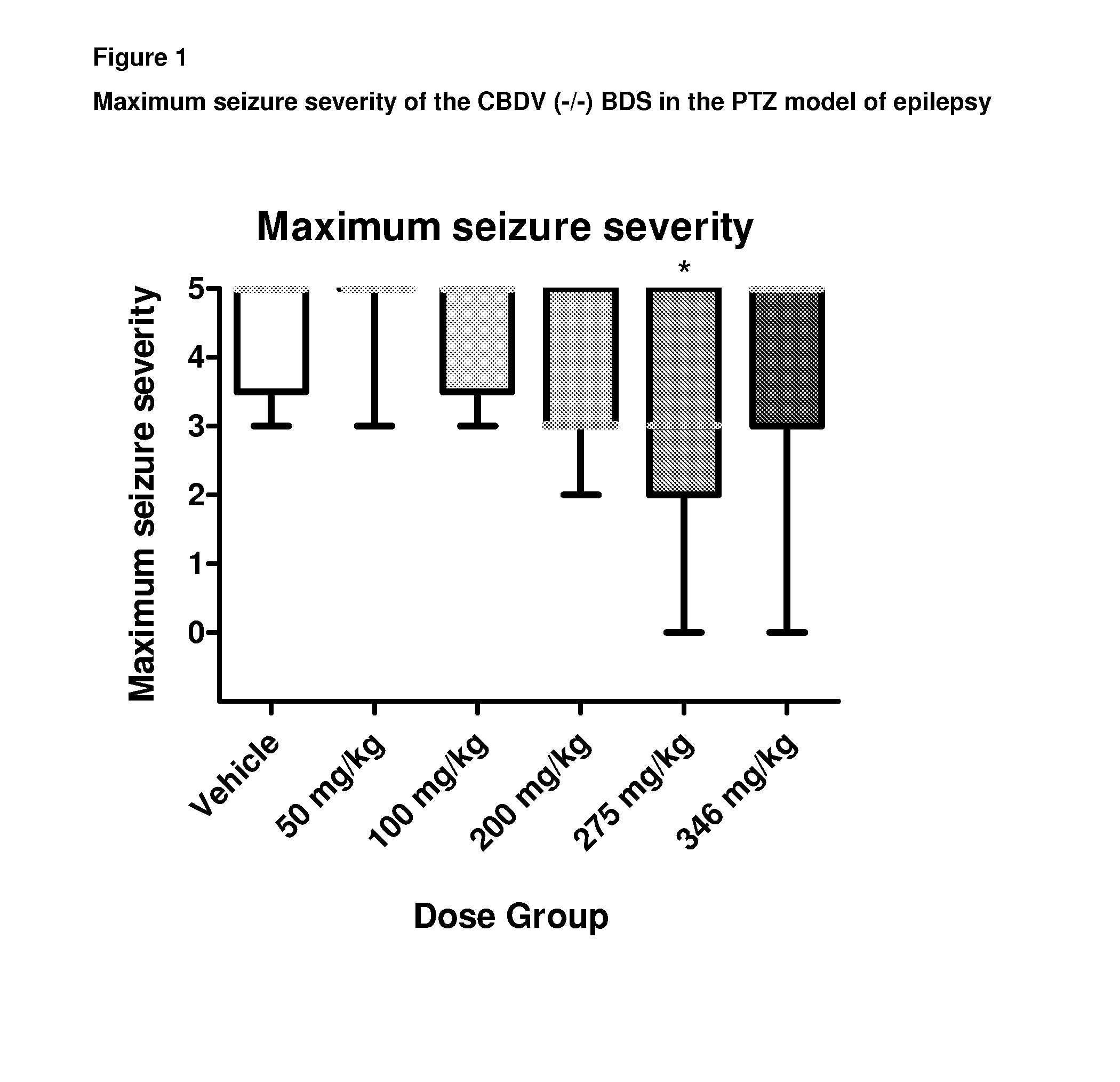
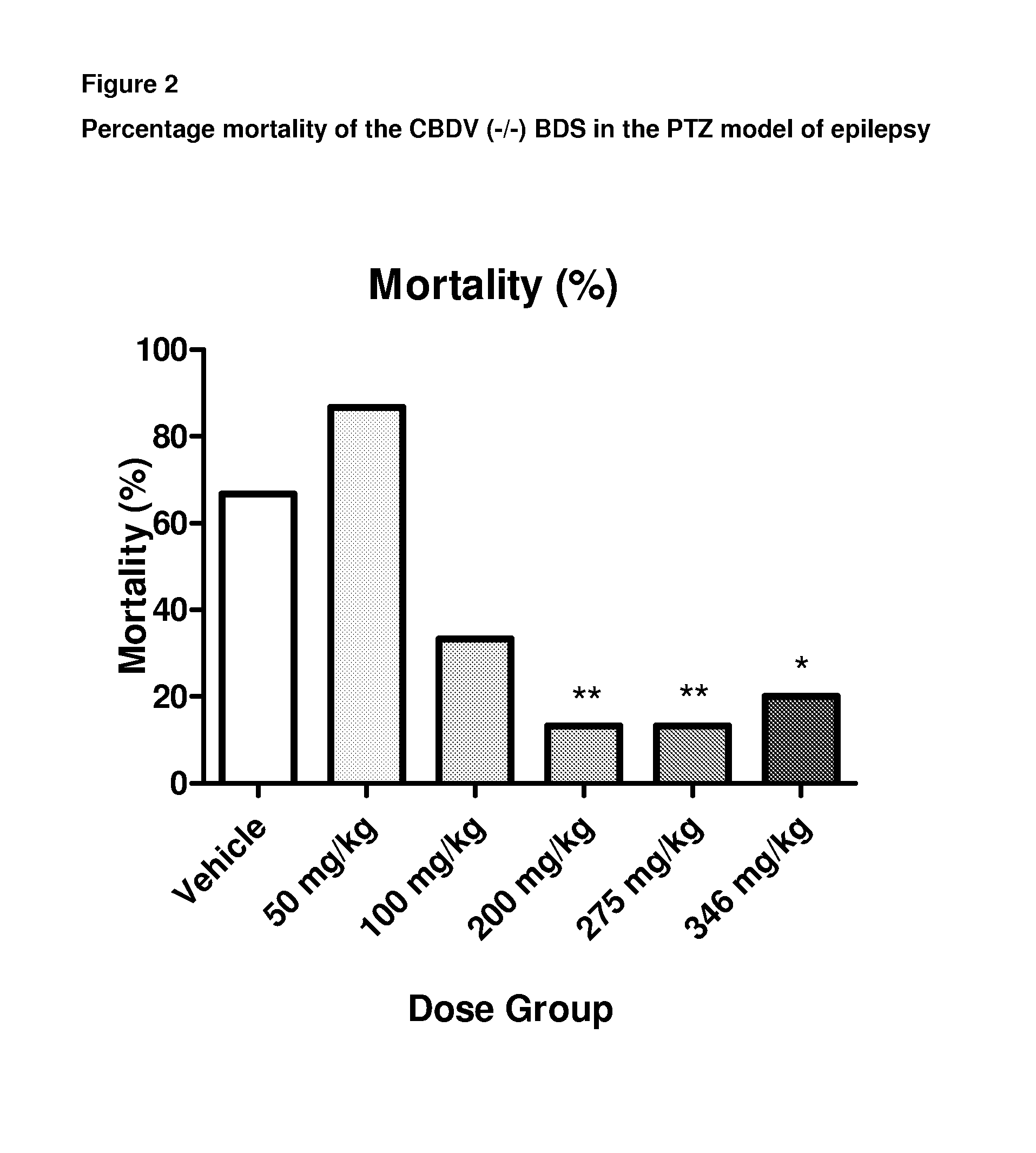
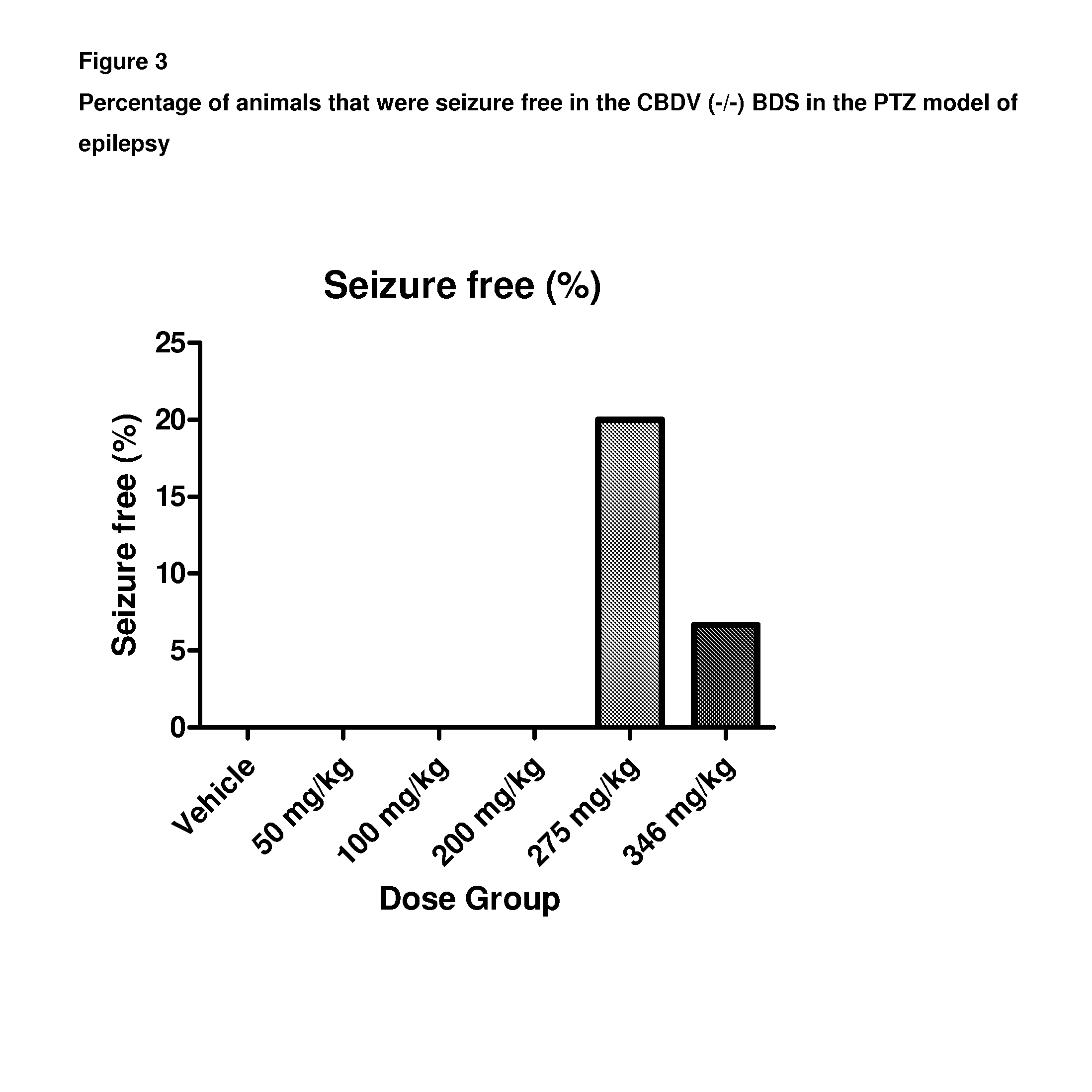
Use of the phytocannabinoid cannabidivarin (CBDV) in the treatment of epilepsy
ActiveUS20120004251A1Well side effect profileGrowth inhibitionBiocideNervous disorderPhenobarbitalDrug
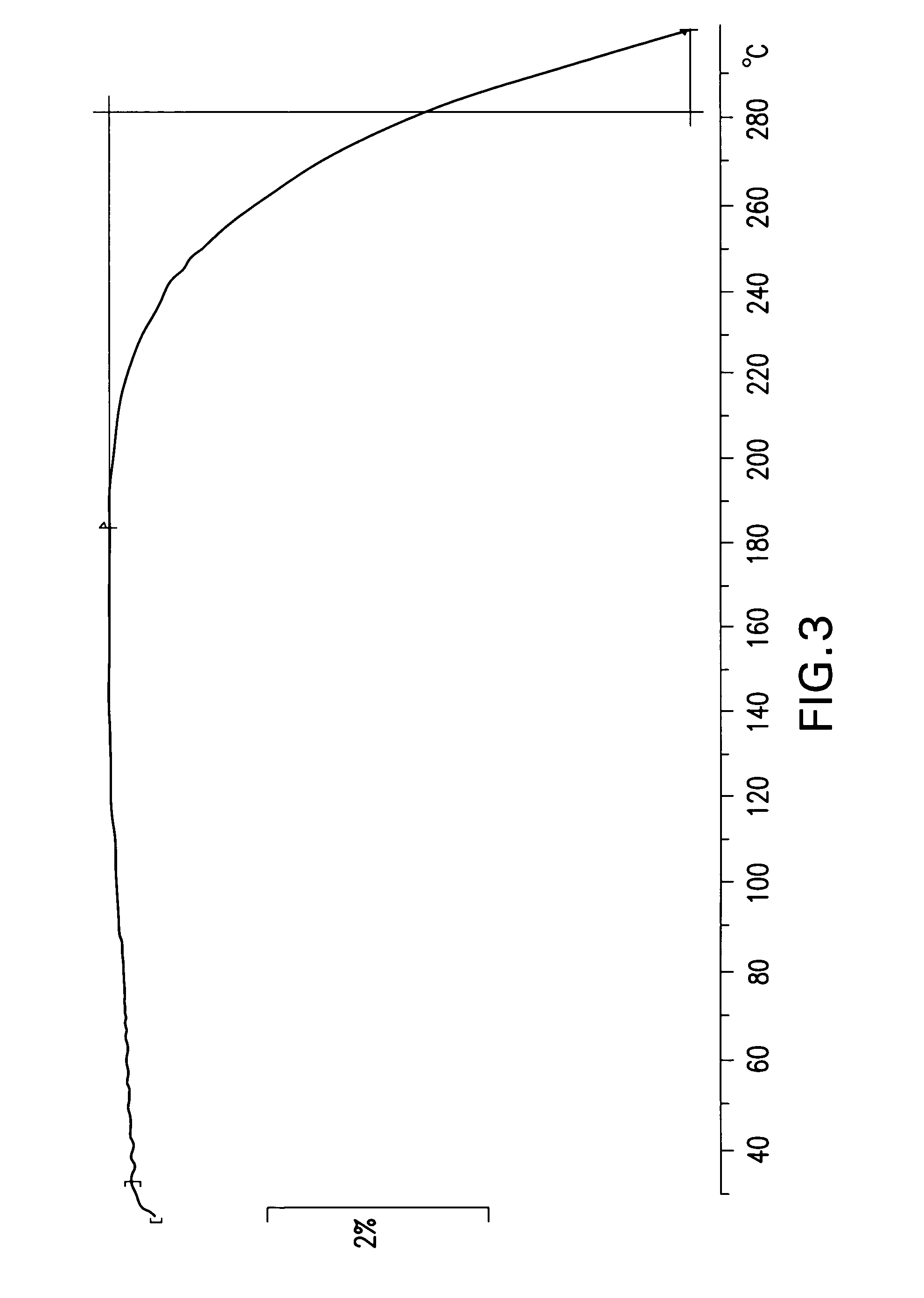
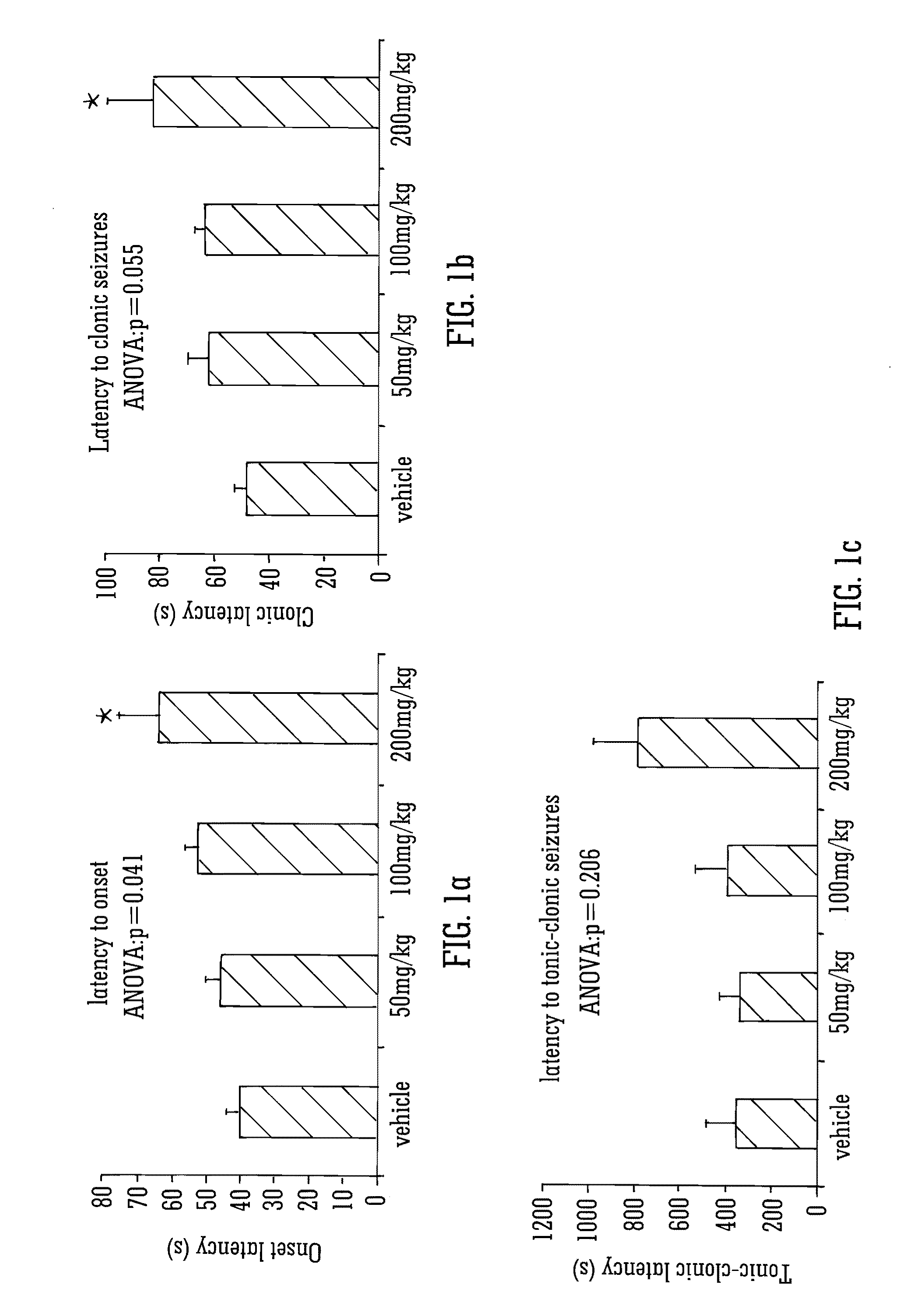
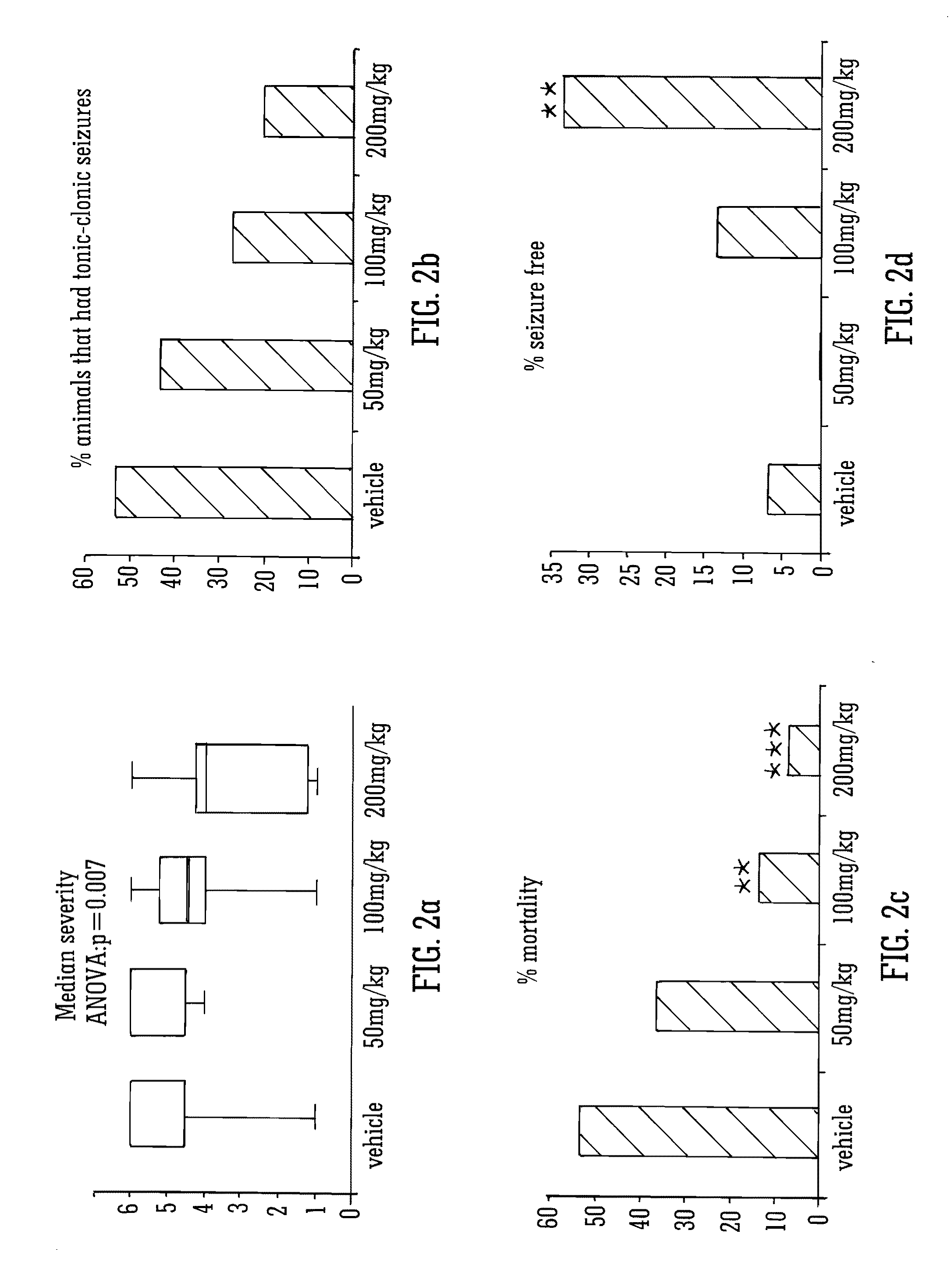
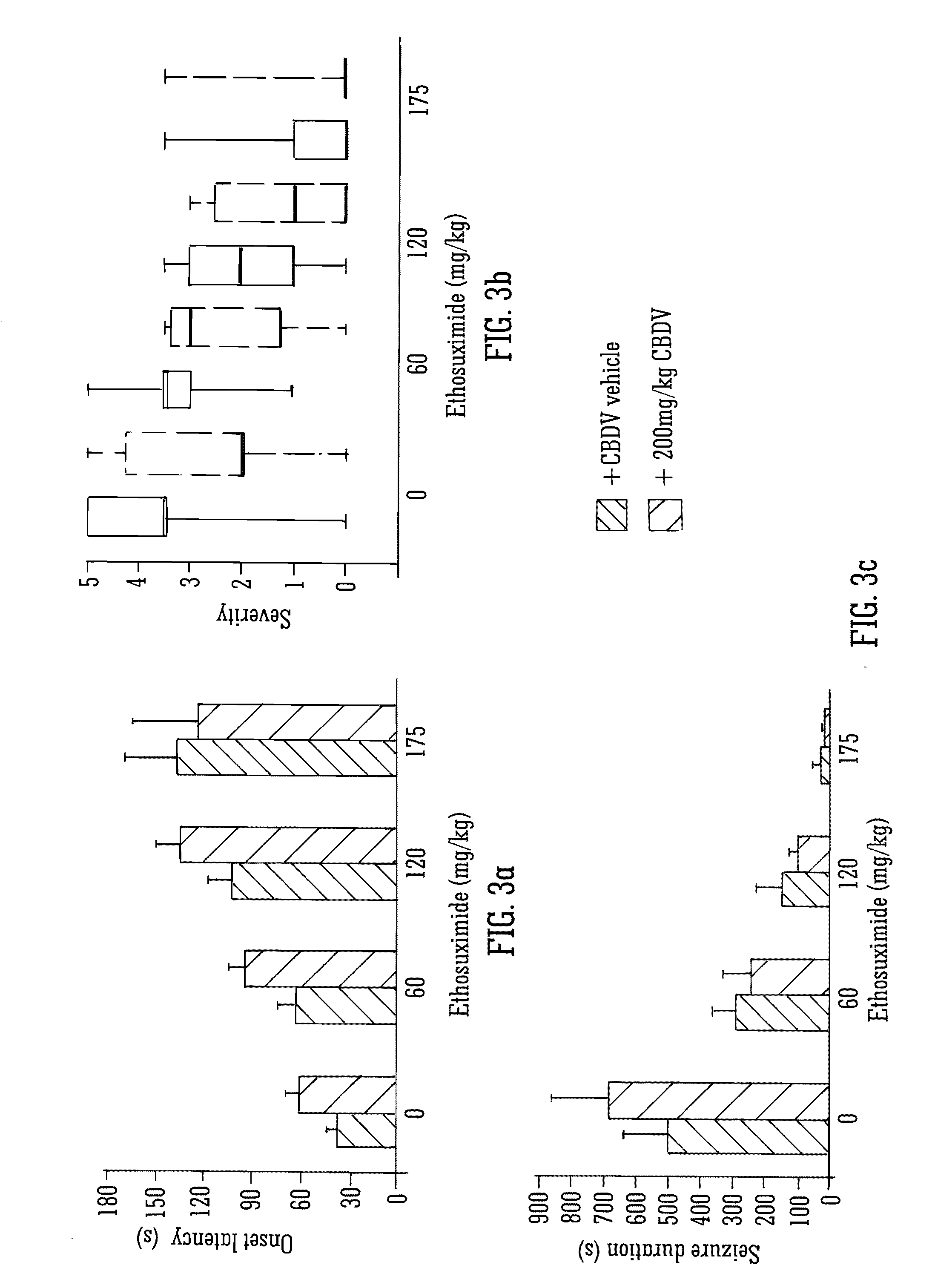
This invention relates to the use of the phytocannabinoid cannabidivarin (CBDV) and combinations of the phytocannabinoid CBDV with tetrahydrocannabivarin (THCV) and cannabidiol (CBD) in the treatment of epilepsy. The invention further relates to the use of the phytocannabinoid CBDV in combination with standard anti-epileptic drugs (SAEDs). Preferably the SAED is one of ethosuximide, valproate or phenobarbital.
Owner:GW PHARMA LTD +1
Aqueous dronabinol formulations
InactiveUS20080112895A1Stabilize cannabinoidEffective and stableBiocidePowder deliveryPolyethylene glycolRoom temperature
A room temperature stable aqueous cannabinoid formulation is disclosed. In preferred embodiments, the cannabinoid formulation comprises dronabinol in a mixture of buffer solution, and organic cosolvents such as ethanol, propylene glycol and polyethylene glycol.
Owner:INSYS THERAPEUTICS
Stable cannabinoid formulations
The present invention is generally directed to substantially pure cannabidiol, stable cannabinoid pharmaceutical formulations, and methods of their use.
Owner:RADIUS PHARMA INC
Oral Dosage Form Of Tetrahydrocannabinol And A Method Of Avoiding And/Or Suppressing Hepatic First Pass Metabolism Via Targeted Chylomicron/Lipoprotein Delivery
ActiveUS20110092583A1Easy to transportPromote lymphatic transportBiocideSenses disorderChylomicronCytochrome P450
Self-emulsifying drug delivery systems are provided to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron / lipoprotein delivery and optimal bioavailability through the mammalian intestinal tract. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY RAM B +1
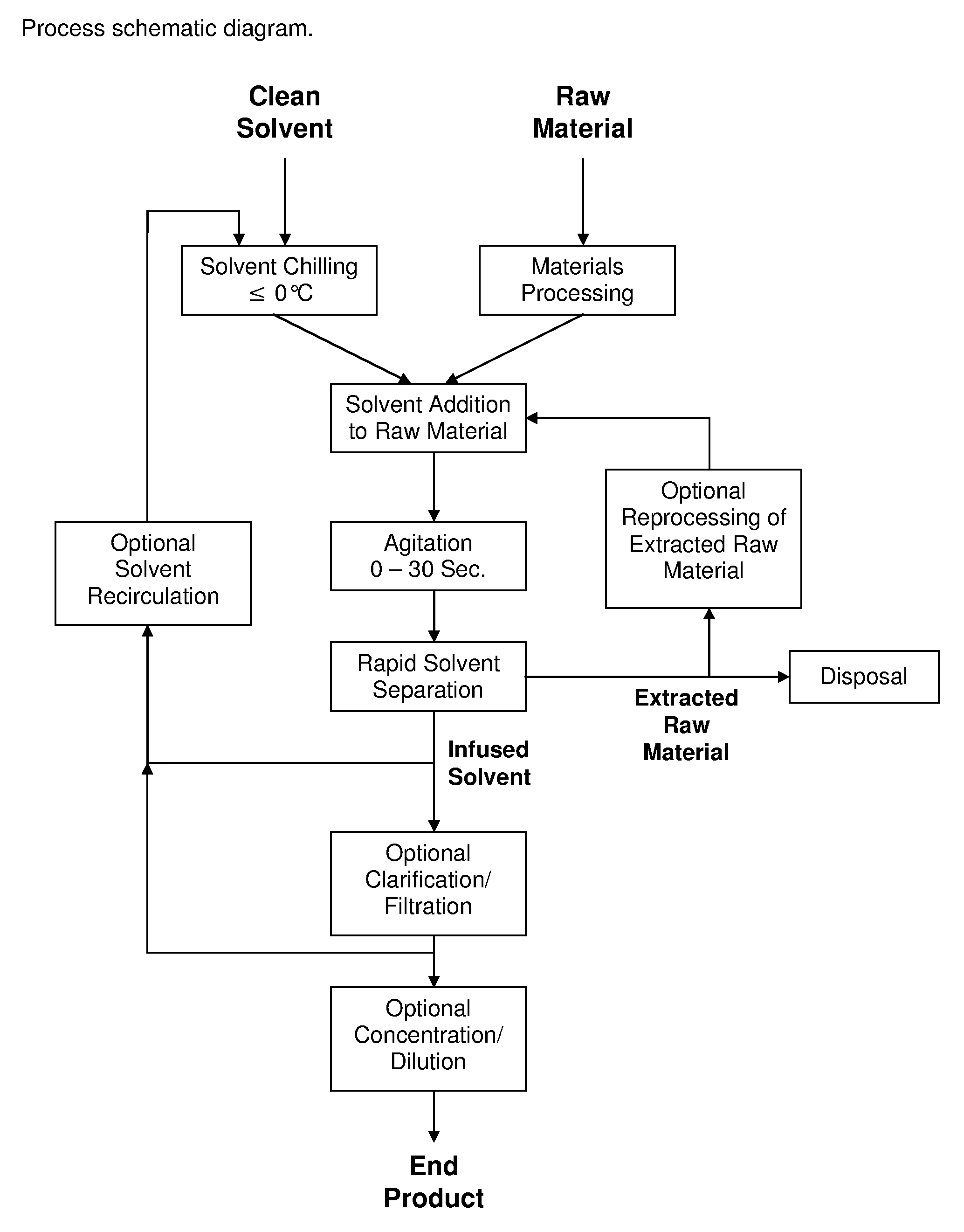
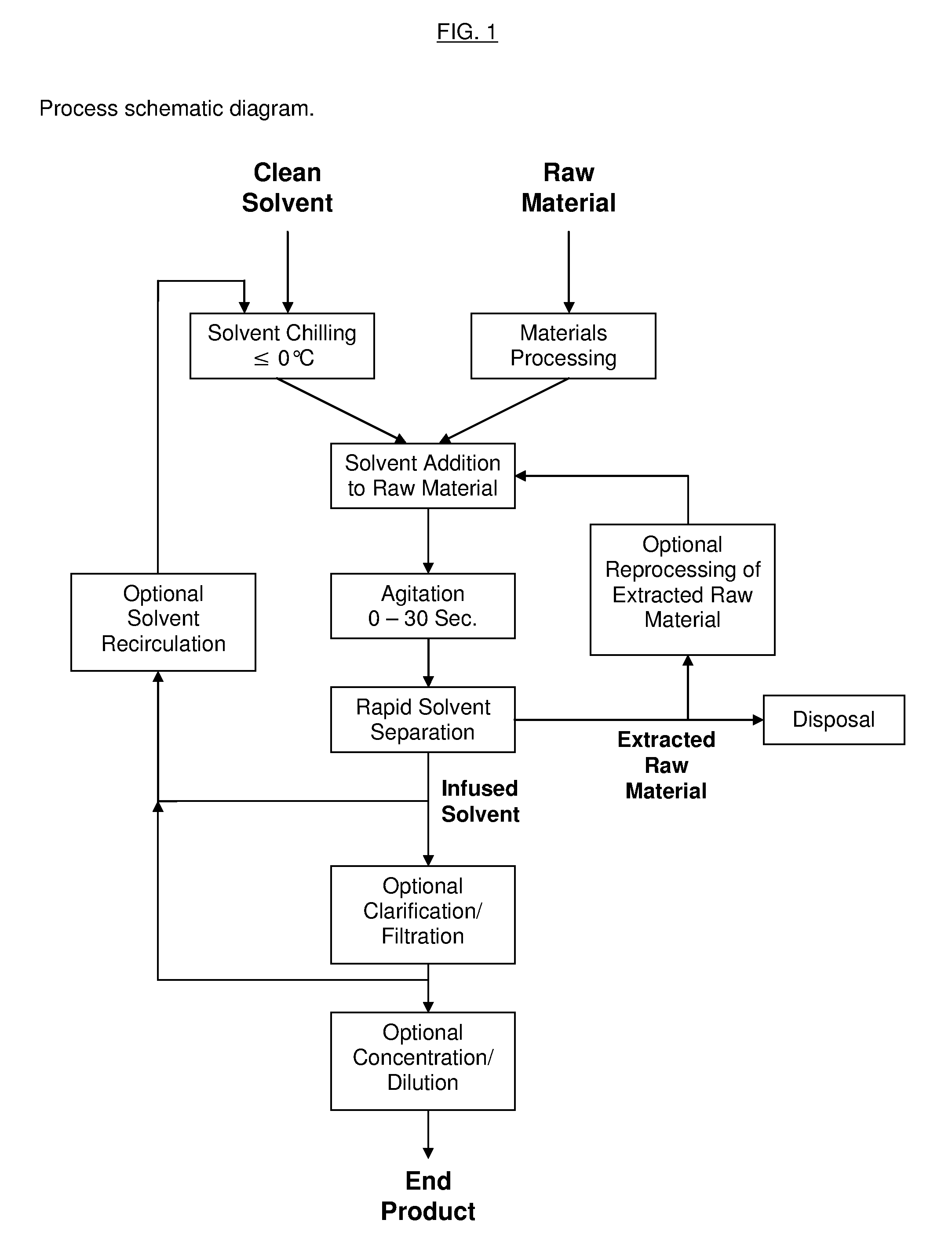
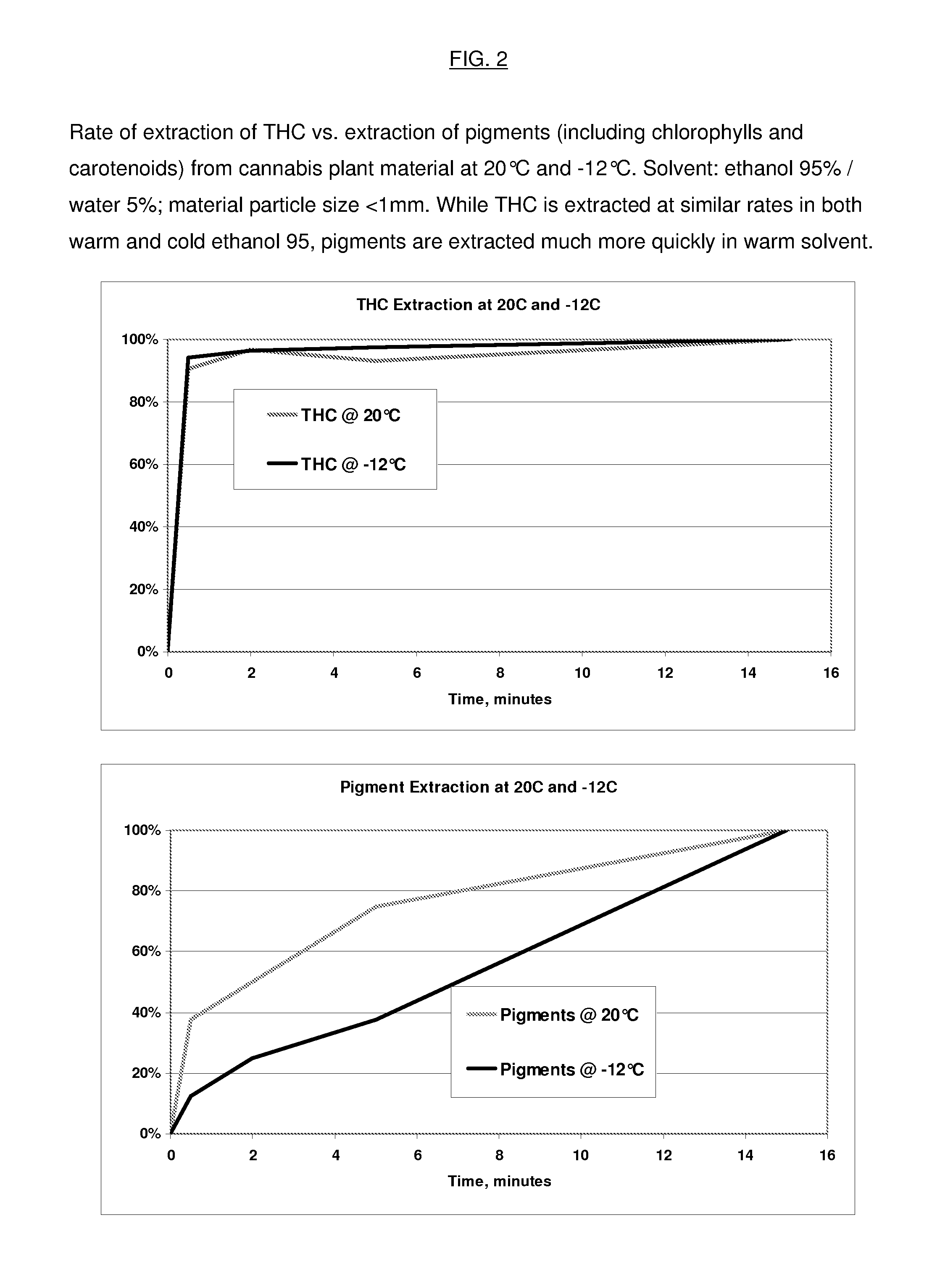
Process for the Rapid Extraction of Active Ingredients from Herbal Materials
InactiveUS20130079531A1Reduce solubilityRapid extractionOrganic chemistryLiquid solutions solvent extractionSolventCannabis
The invention is a process for the rapid extraction of active ingredients from herbal materials using a cold solvent and a very short mixing period in order to yield commercially desirable extracts. In particular, the process can be applied to the rapid extraction of cannabinoids from cannabis. The claimed invention also includes any equipment or machine, or assemblage of equipments or machines, designed or employed to utilize this process.
Owner:RM3 LABS
Pharmaceutical compositions for the treatment of pain
The present invention relates to treatment of cancer related pain and constipation. Preferably the subject in need is administered a combination of the cannabinoids cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). More preferably the cannabinoids are in a predefined ratio by weight of approximately 1:1 of CBD to THC.
Owner:GW RES LTD
Pharmaceutical Compositions For the Treatment of Disease and/or Symptoms in Arthritis
InactiveUS20080139667A1Increase flexibilityLess side effectsPowder deliveryBiocideDrugDisease modification
The invention relates to the use of a combination of cannabinoids for the treatment of pain, inflammation and / or disease modification in arthritis. Preferably the cannabinoids are selected from cannabidiol (CBD) or cannabidivarin (CBDV) and delta-9-tetrahydrocannabinol (THC) or tetrahydrocannabinovarin (THCV). More preferably the cannabinoids are in a predefined ratio by weight of less than or equal to 19:1 of CBD or CBDV to THC or THCV.
Owner:GW PHARMA LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20190083418A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeMedicine
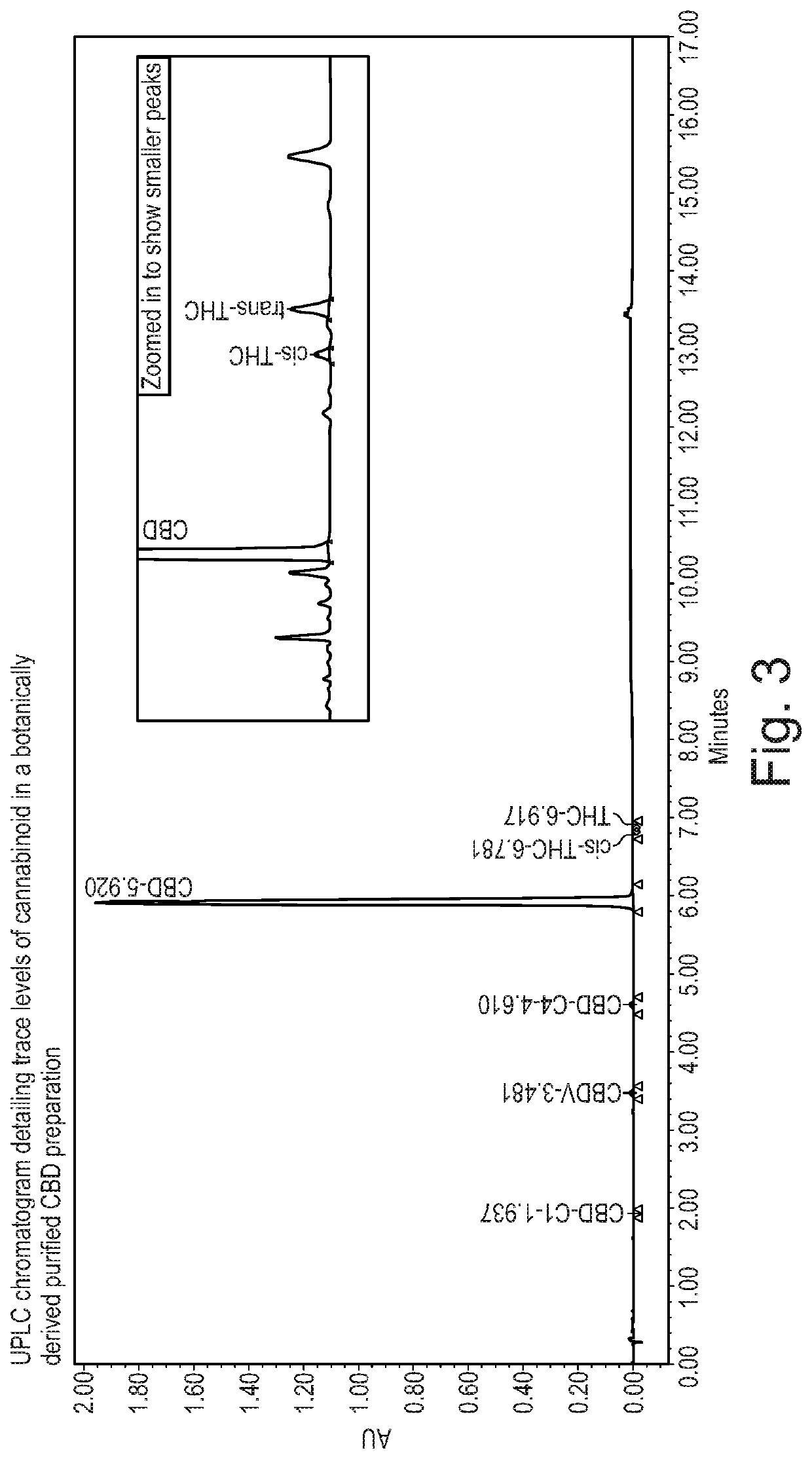
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
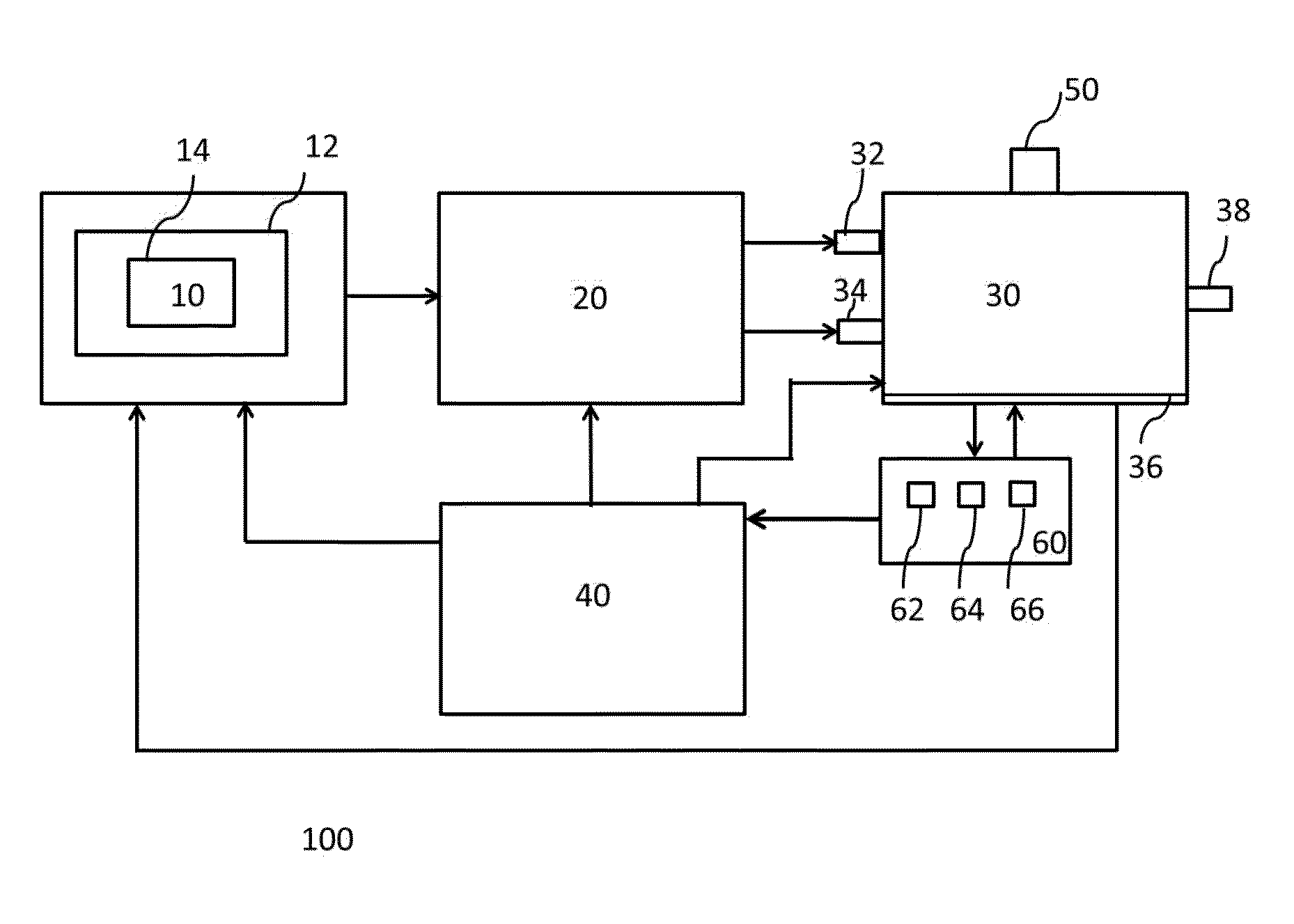
Apparatus and methods for the simultaneous production of compounds
ActiveUS9394510B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCannabielsoinEngineering
The present invention provides an apparatus for simultaneously producing different cannabinoids in different ratios. Specifically, the apparatus according to the invention produces tetrahydrocannabinolic acid (THCA) and cannabichormenic acid (CBCA) or cannabidiolic acid (CBDA) in different ratios, and it comprises a bioreactor that comprises a first automated supply system configured to deliver a substrate and a cannabinoid acid synthase, and a second automated system to cease the reaction; an extractor configured to recover the cannabionoids so produced; and a controller configured to modify at least on property of the reaction.
Owner:TEEWINOT TECH LTD
Pharmaceutical compositions for the treatment of pain
ActiveUS20060247304A1Small doseBiocideNervous disorderCancer-Related PainDelta-9-tetrahydrocannabinol
The present invention relates to treatment of cancer related pain and constipation. Preferably the subject in need is administered a combination of the cannabinoids cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). More preferably the cannabinoids are in a predefined ratio by weight of approximately 1:1 of CBD to THC.
Owner:GW RES LTD
Medicinal cannabis added in food
The invention is a product and a process wherein cannabinoids such as Medicinal Δ9-THC and / or other substances associated with medicinal cannabis, including yet not necessarily limited to cannbidiols, cannabigerol are added to a foodstuff where the medicinal cannabis is not evenly distributed throughout the foodstuff where the food stuff contains a known weight of medicinal cannabis. Another provision of the invention is providing controlled amounts or ratios of Δ9-THC as compared to CBD in or on a foodstuff.
Owner:HOSPODOR ANDREW DAVID
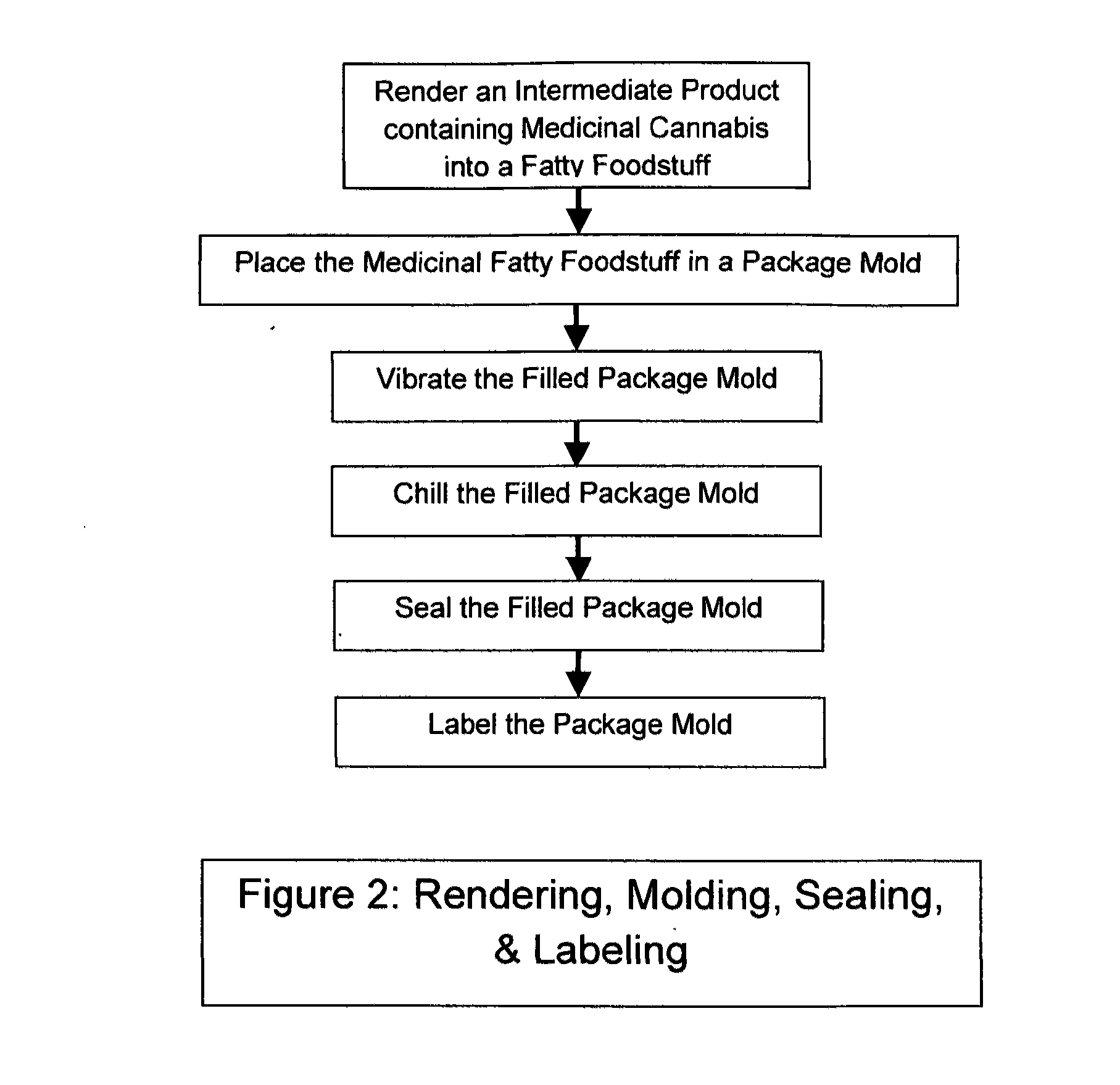
Medicinal cannabis fatty foodstuff
InactiveUS20120043242A1Organic active ingredientsNervous disorderDelta-9-tetrahydrocannabinolMedicine
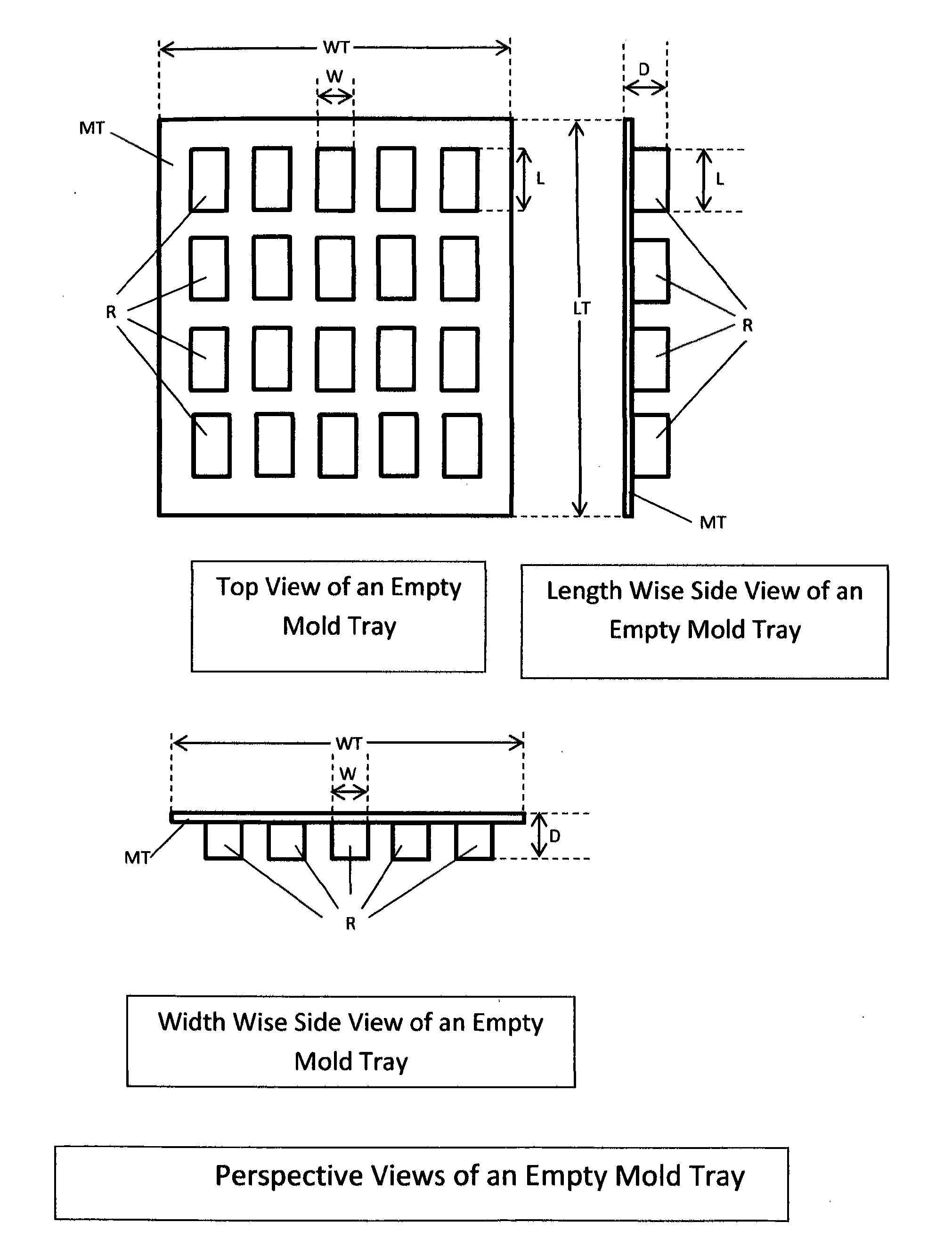
The invention is a product and a process wherein Medicinal Delta-9 tetrahydrocannabinol (Δ9-THC) and potentially other cannabinoids (medicinal cannabis substances) associated with decarboxylated cannabis, including yet not necessarily limited to cannbidiols, and cannabigerol are rendered into a fatty foodstuff and then molded into a mold that also acts as a package. The best mode of the invention is a blister pack containing a plurality of voids or receptacles of desired sizes. A product that is characterized by a controlled amount of medicinal cannabis per unit volume of a fatty foodstuff base material is inserted into the mold, then cooled, and finally sealed. Each void or receptacle contains a known amount of medicinal cannabis that are independently dispensable.
Owner:HOSPODOR ANDREW DAVID
Aminoacid derivatives containing a disulfanyl group in the form of mixed disulfanyl and aminopeptidase N inhibitors
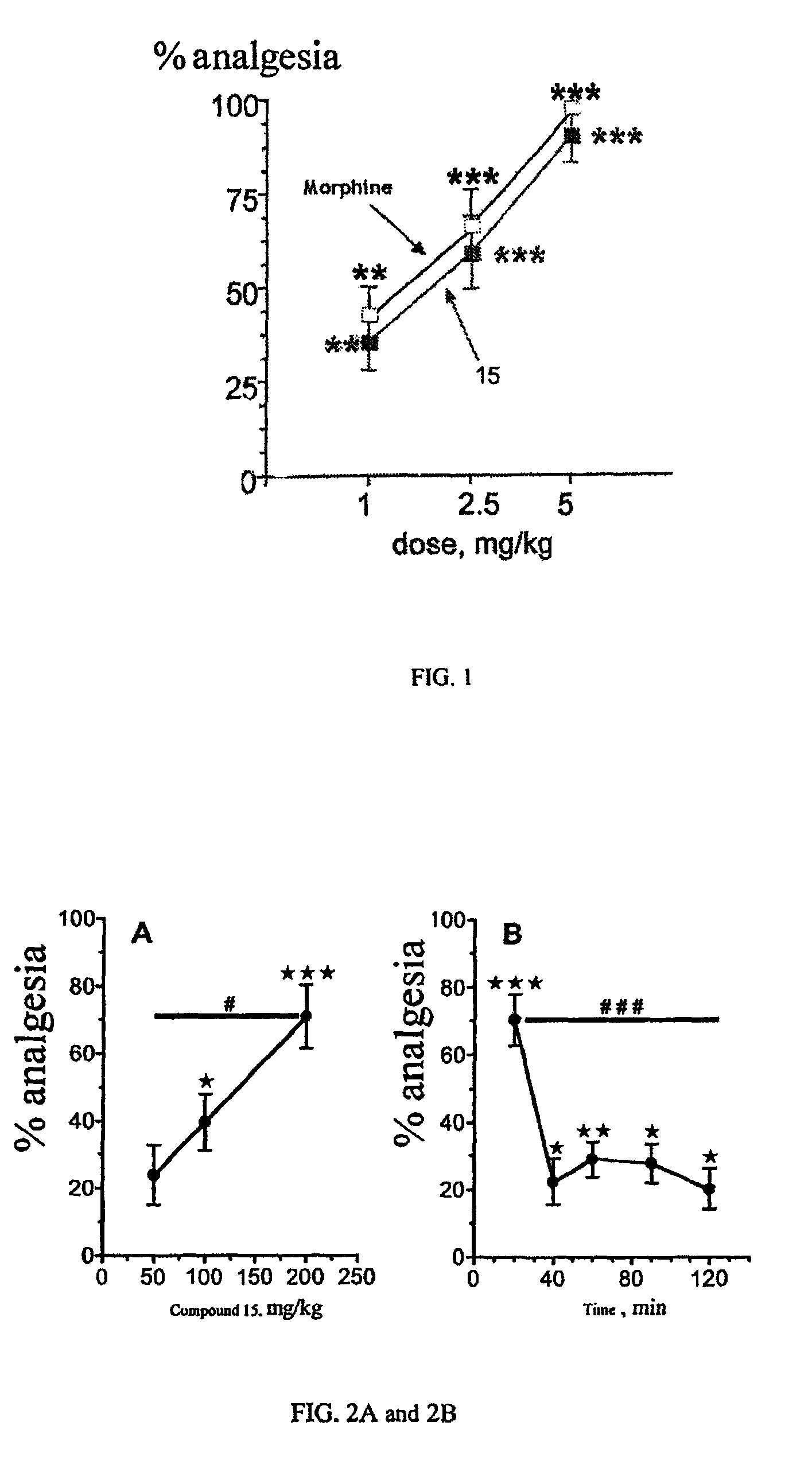
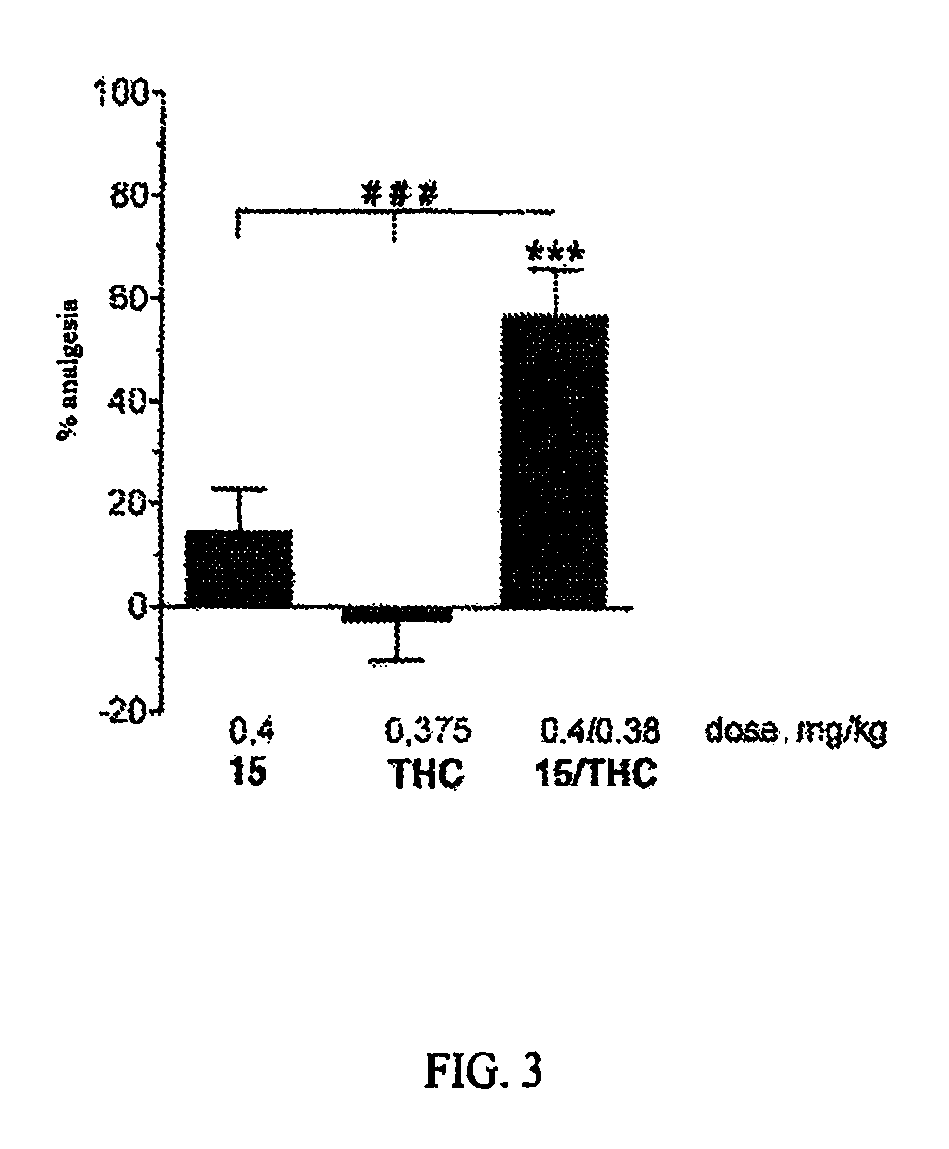
The invention relates to novel compounds of formula (I): H2N—CH(R1)—CH2—S—S—CH2—CH(R2)—CONH—R5, wherein R1 is a hydrocarbon chain, phenyl or benzyl radical, methylene radical substituted by a 5 or 6 atom heterocycle; R2 is a phenyl or benzyl radical, a 5 or 6 atom aromatic heterocycle, methylene group substituted by a 5 or 6 atom heterocycle; R5 is a CH(R3)—COOR4 radical, wherein R3 is hydrogen, an OH or OR group, a saturated hydrocarbon group, a phenyl or benzyl radical and OR4 is hydrophile ester, or 5 or 6 membered heterocycle comprising several heteroatoms selected from a group consisting of nitrogen, sulphur and oxygen, with at least two nitrogene atoms, wherein said heterocycle is substitutable by an alkyl C1-C6, phenyl or benzyl radical. The use of the inventive compounds in the form of drugs, a pharmaceutical composition comprising said compounds, a pharmaceutically acceptable excipient, the use in conjunction of at least one type of cannabinoid derivative for potentiating the analgesic and antidepressant effect of the novel compounds of formula (I) and / or morphine or the derivatives thereof are also disclosed.
Owner:KOS THERAPEUTICS INC
Cannabinoid formulations
The present invention provides stable, fast-acting liposomal and micelle formulations of cannabinoids that are suitable for pharmaceutical and nutraceutical applications.
Owner:TEEWINOT TECH LTD
Stable cannabinoid formulations
PendingUS20170224634A1Dispersion deliveryHydroxy compound active ingredientsCannabidiolPharmaceutical formulation
The present invention is generally directed to substantially pure cannabidiol, stable cannabinoid pharmaceutical formulations, and methods of their use.
Owner:RADIUS PHARMA INC
Recombinant production systems for prenylated polyketides of the cannabinoid family
The present invention relates generally to production methods, enzymes and recombinant yeast strains for the biosynthesis of clinically important prenylated polyketides of the cannabinoid family. Using readily available starting materials, heterologous enzymes are used to direct cannabinoid biosynthesis in yeast.
Owner:BAYMEDICA INC
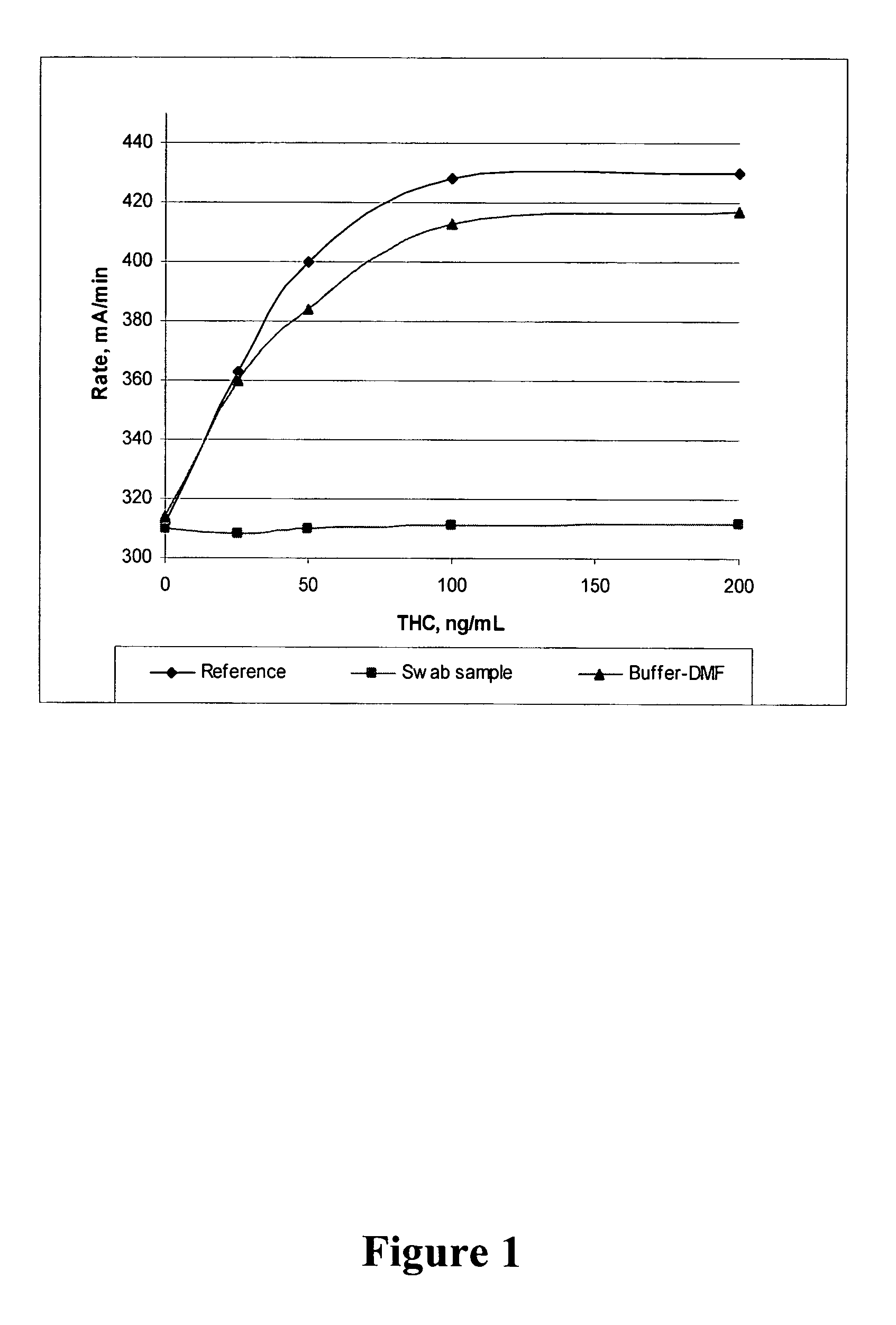
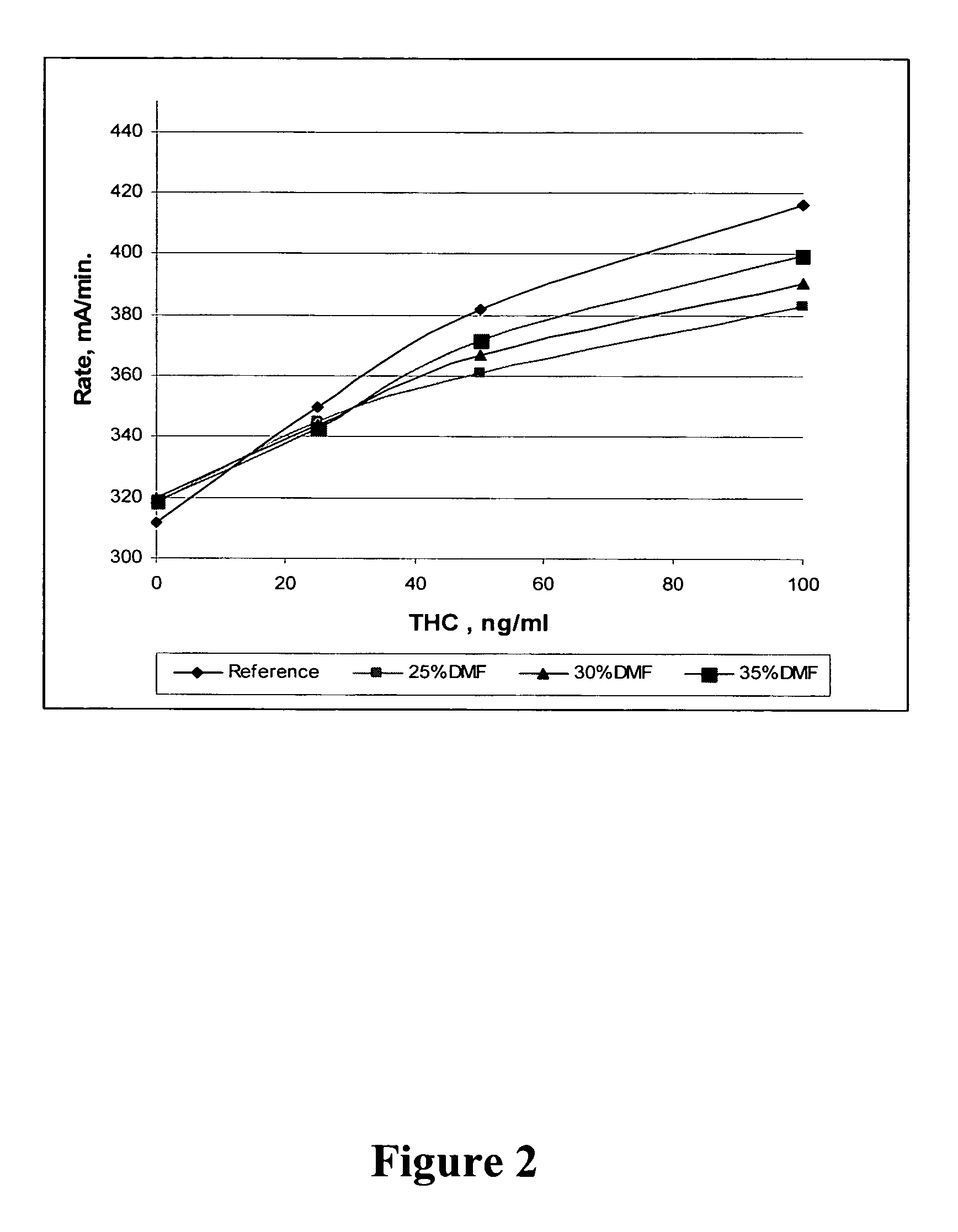
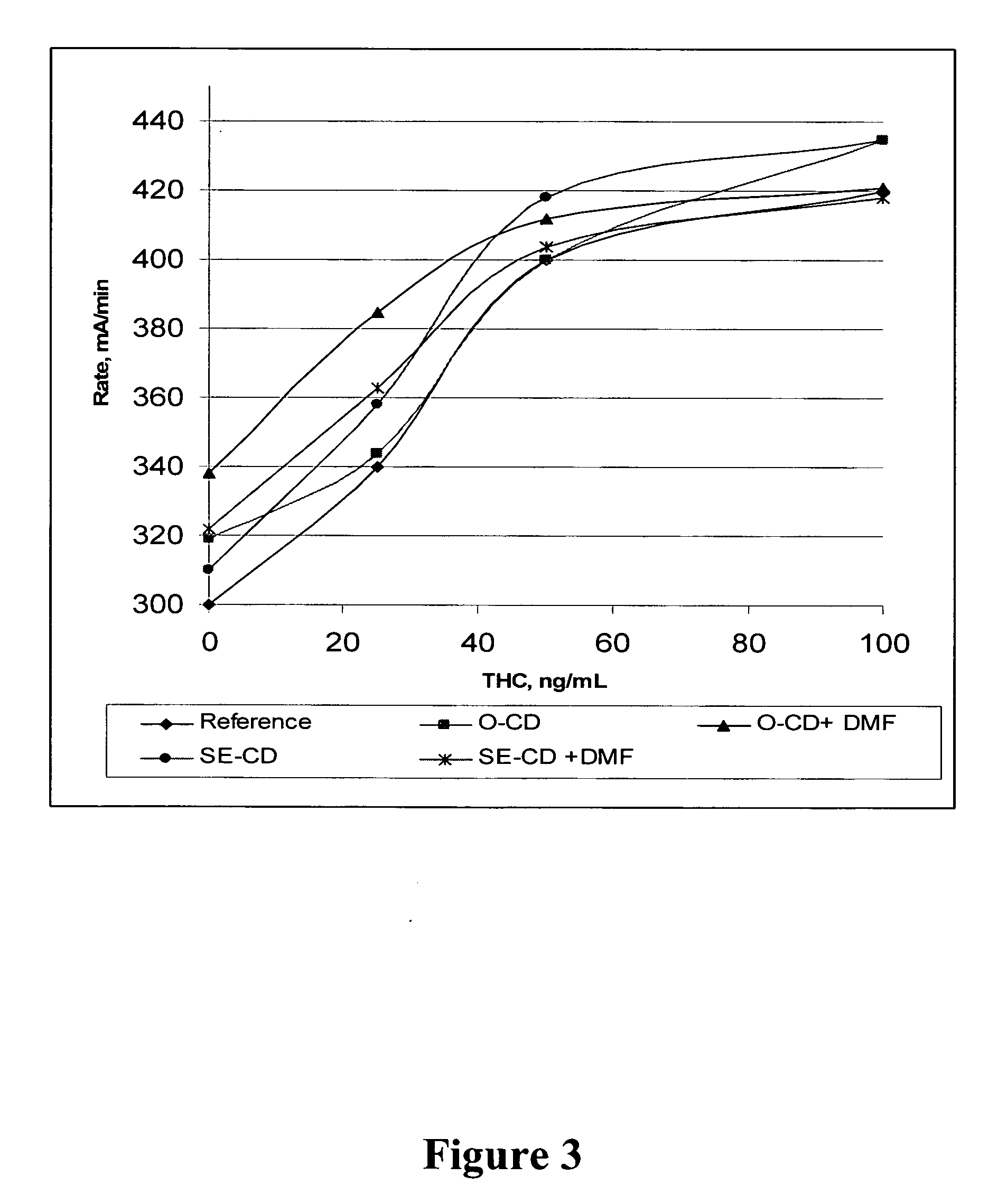
Method for retrieving delta9-THC from oral fluid
The present invention discloses methods and kits for retrieving a cannabinoid from an oral fluid collection device having adsorbed the cannabinoid and to prepare an oral fluid specimen for the determination of Δ9-tetrahydrocaninabinol (Δ9-THC) concentration qualitatively or quantitatively by an enzyme immunoassay. The methods and kits are particularly useful in preserving the Δ9-THC quantitatively from oral fluid samples during the process of collection and preparation.
Owner:LIN ZHI INT
Cannabidiol preparations and its uses
Owner:GW RES LTD
Stable cannabinoid compositions and methods for making and storing them
Owner:MACFARLAN SMITH
Use of cannabinoids in the treatment of epilepsy
InactiveUS20200138738A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeEpilepsy seizure
The present invention relates in the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Surge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Stable cannabinoid formulations
PendingUS20160367496A1Dispersion deliveryHydroxy compound active ingredientsPharmaceutical formulationCannabinoid
The present invention is generally directed to substantially pure cannabidiol, stable cannabinoid pharmaceutical formulations, and methods of their use.
Owner:RADIUS PHARMA INC
Stable cannabinoid compositions and methods for making and storing them
A composition comprising a high purity cannabinoid, an acid, and a pharmaceutically-acceptable solvent achieves room temperature stability for over 24 months. The acid improves the stability of the composition and the solvent enhances the solubility of the acid, thereby allowing the acid to have an improved stabilizing effect on the highly pure cannabinoid. Preferably, the solvent is an alcohol and, more preferably, the composition contains an oil. A method for making the composition includes combining the cannabinoid and the solvent and evaporating a portion of the solvent, along with adding an acid to the composition, before, during, or after the evaporating step. A method for making and storing the composition includes storing the composition in a manner adapted to maintain its stability. Pharmaceutical dosage forms include a formulated composition, such as having the oil. A method of treating a subject comprises administering to the subject the dosage form.
Owner:MACFARLAN SMITH
Oral pharmaceutical formulation comprising cannabinoids and poloxamer
PendingUS20210059976A1Improve bioavailabilityTotalPowder deliveryNervous disorderPharmaceutical formulationCannabidiol
The present invention relates to a novel cannabinoid oral pharmaceutical dosage form, based on a Type IV or Type IV-like formulation, as classified using the Lipid Formulation Classification System. The formulation comprises a combination of at least two cannabinoids. The first cannabinoid is selected from the group consisting of tetrahydrocannabinol (THC) and analogues thereof; and the second cannabinoid is selected from the group consisting of cannabidiol (CBD) and analogues thereof.
Owner:GW RES LTD
Cannabidiol preparations and its uses
ActiveUS20210015789A1Low back painGood blood pressurePowder deliveryNervous disorderCannabis sativa plantDisease
Cannabidiol (CBD) is a cannabinoid designated chemically as 2-[(1R,6R)-3-Methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol. Its empirical formula is C21H30O2 and its molecular weight is 314.46. CBD is a cannabinoid that naturally occurs in the Cannabis sativa L. plant. CBD is a white to pale yellow crystalline solid which is insoluble in water and soluble in organic solvents. The present invention encompasses the surprising recognition that certain CBD preparations which are prepared from a botanical origin are more effective in treating diseases or disorders than preparations of CBD which are synthetic or purified to the extent no other impurities in the form of other cannabinoids are present. Prior CBD compositions have been prepared such that no psychoactive components, e.g., tetrahydrocannabinol (THC), remain in the final CBD preparation. Surprisingly, the absence of such minor impurities reduces the efficacy of CBD treatment. Such CBD preparations are characterized by chemical components and / or funtional properties that distinguish them from prior CBD compositions. One or more components of the preparations described herein provide an unexpectedly synergistic effect when utilized in combination.
Owner:GW RES LTD
Use of cannabinoids in the treatment of epilepsy
InactiveUS20200352878A1Reduce seizuresPoor response rateNervous disorderHydroxy compound active ingredientsAntiepileptic AgentsStiripentol
The present invention relates to the use of cannabidiol (CBD) in the treatment of patients with childhood-onset epilepsy who are concurrently taking one or more antiepileptic drugs that works via GABA receptor agonism. Preferably the AED is stiripentol. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Method for extracting and separating high-purity cannabidiol from low-content industrial hemp floral leaves
ActiveCN111470953AIncrease contentHigh purityOrganic chemistryOrganic compound preparationCannabielsoinDigestion
The invention discloses a method for extracting and separating high-purity cannabidiol (CBD) from low-content industrial hemp floral leaves. The method adopts the modes of digestion, macroporous resinadsorption and desorption, reverse chromatography and crystallization to finally obtain a cannabidiol pure product with the purity of 99% or above. In order to separate other cannabinoids, extractionand decoloration processes can be added between the digestion process and the macroporous resin adsorption and desorption process, and a polymer reversed-phase filler is used for reversed-phase chromatography, so that cannabidivarin (CBDV), cannabinol (CBN) and tetrahydrocannabinol (THC) in the floral leaves can be separated. The method disclosed by the invention is high in digestion, extractionand decoloration process yield and high in impurity removal rate; the macroporous resin and reversed-phase column chromatography have the advantages of high loading capacity and favorable separation effect. Requirements of development of a plurality of industrial hemp products are met, and the method has great application advantages in the pharmaceutical field and is suitable for industrial popularization.
Owner:SUNRESIN NEW METERIALS CO LTD XIAN
Use of cannabinoids in the treatment of epilepsy
ActiveUS10583096B2Nervous disorderHydroxy compound active ingredientsSturge–Weber syndromeCannabielsoin
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com