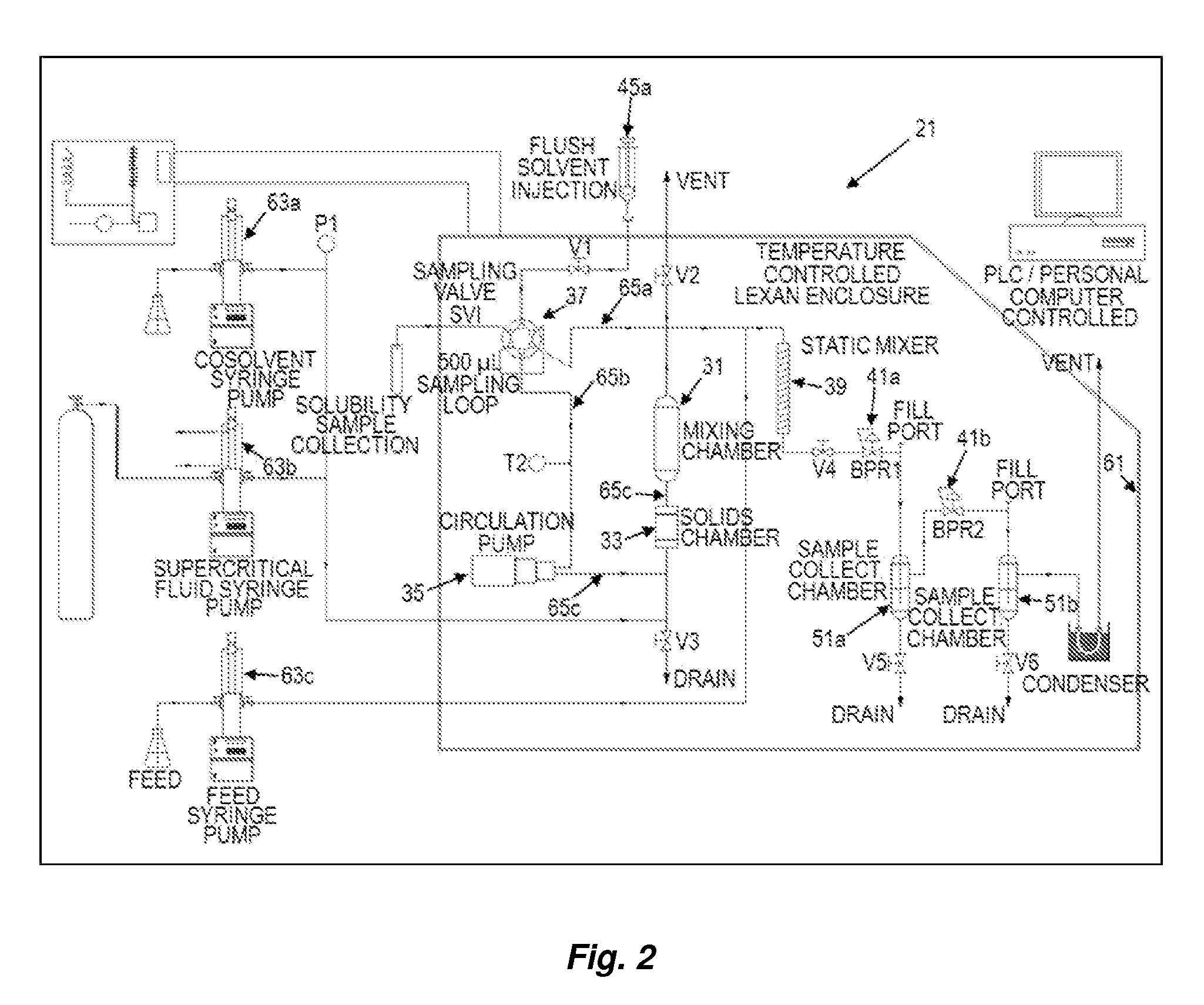
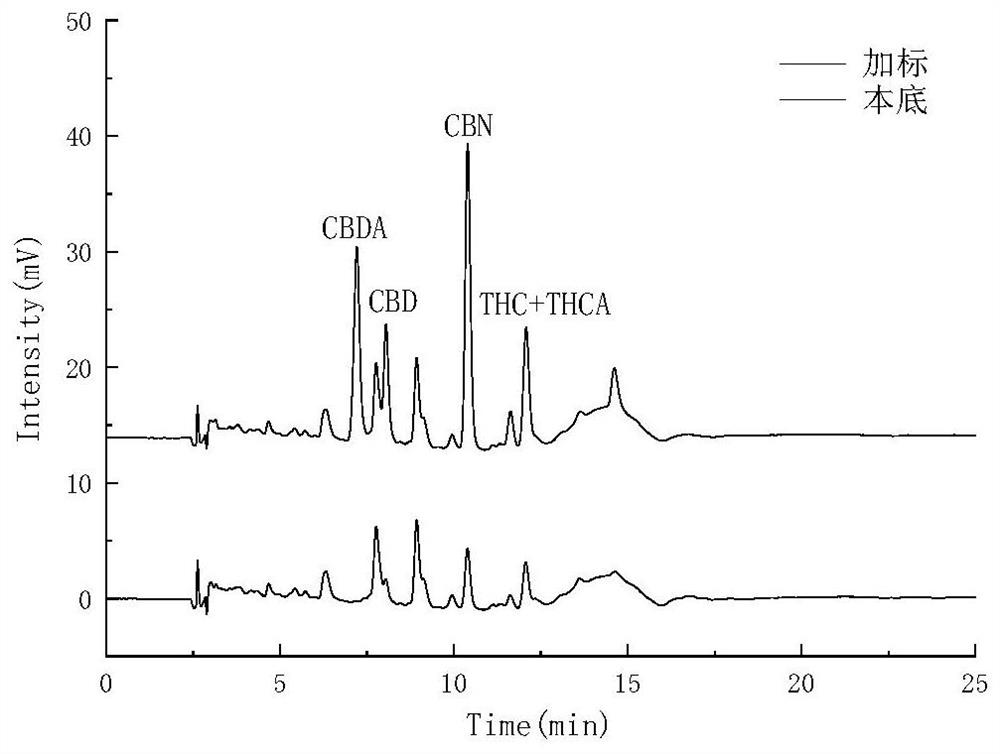
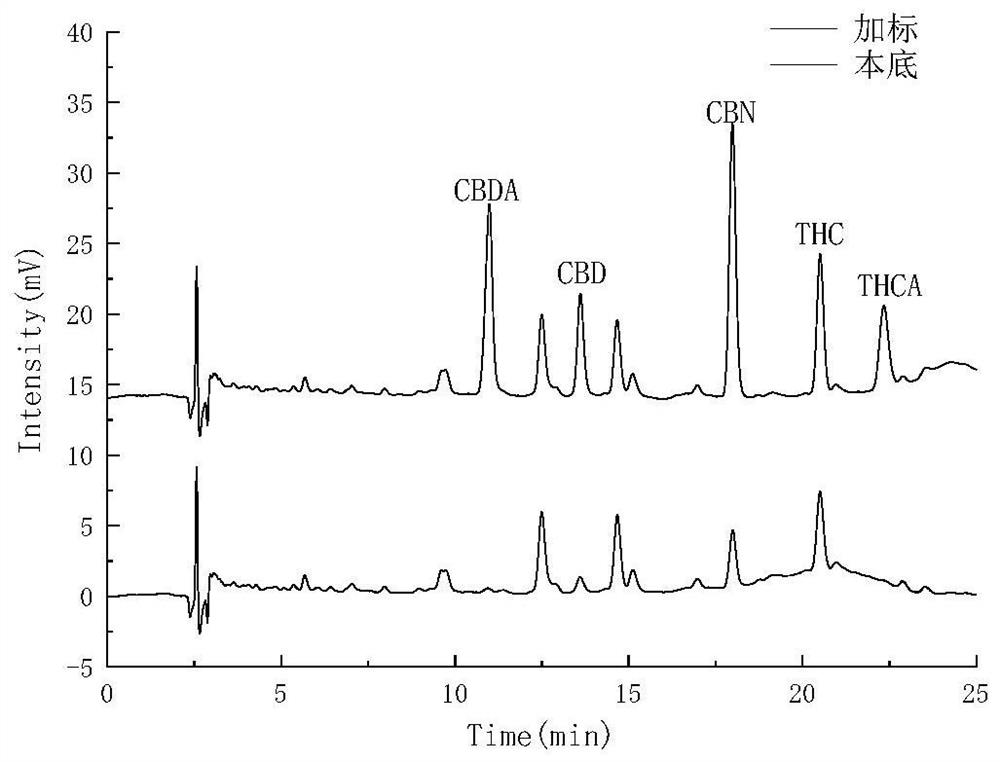
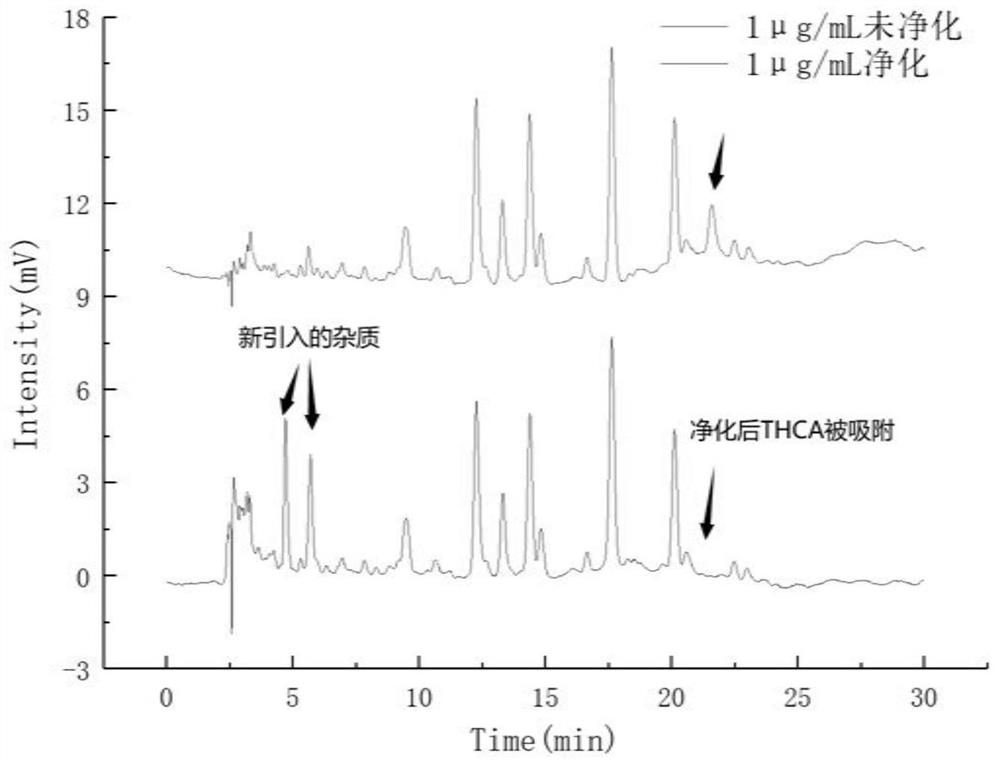
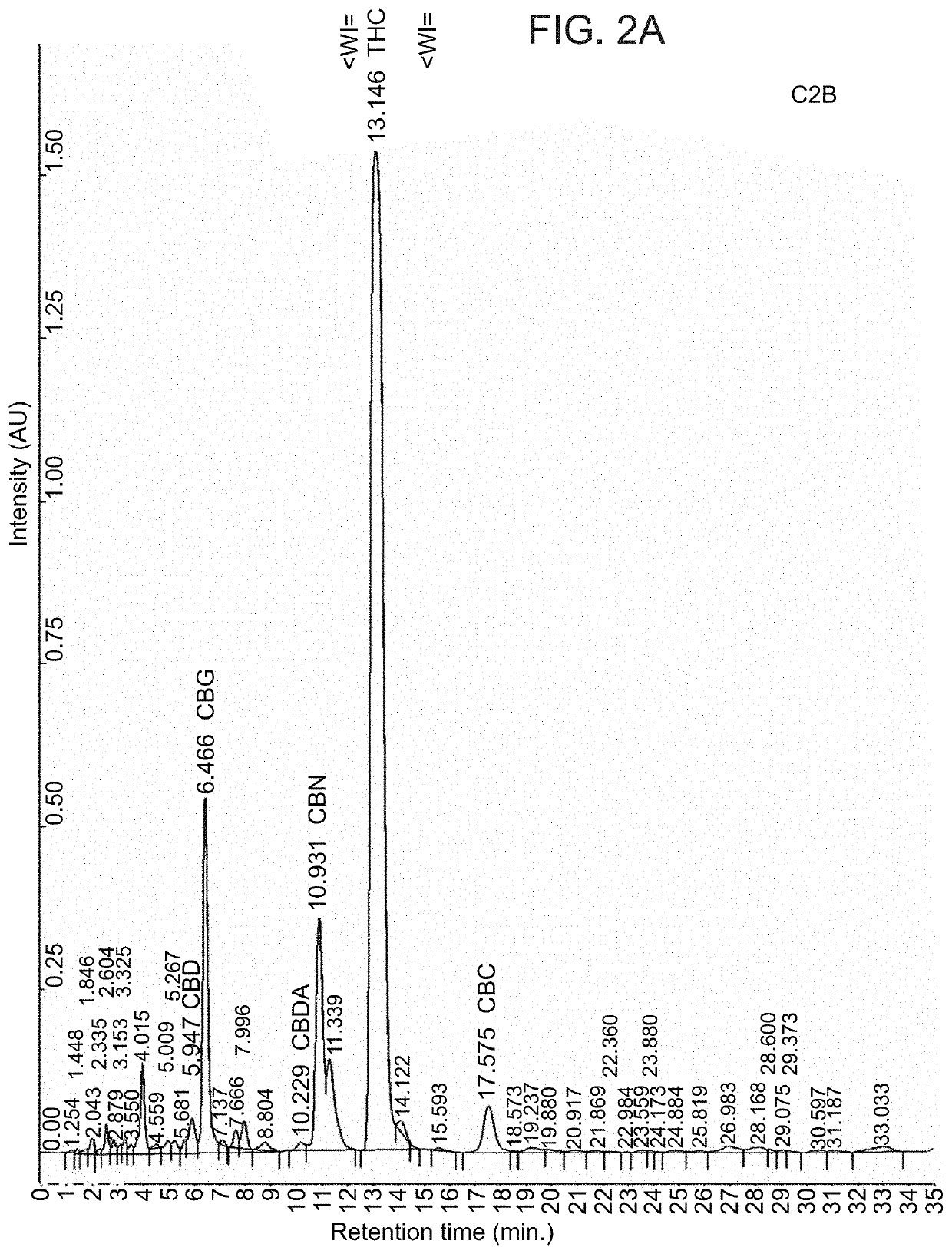
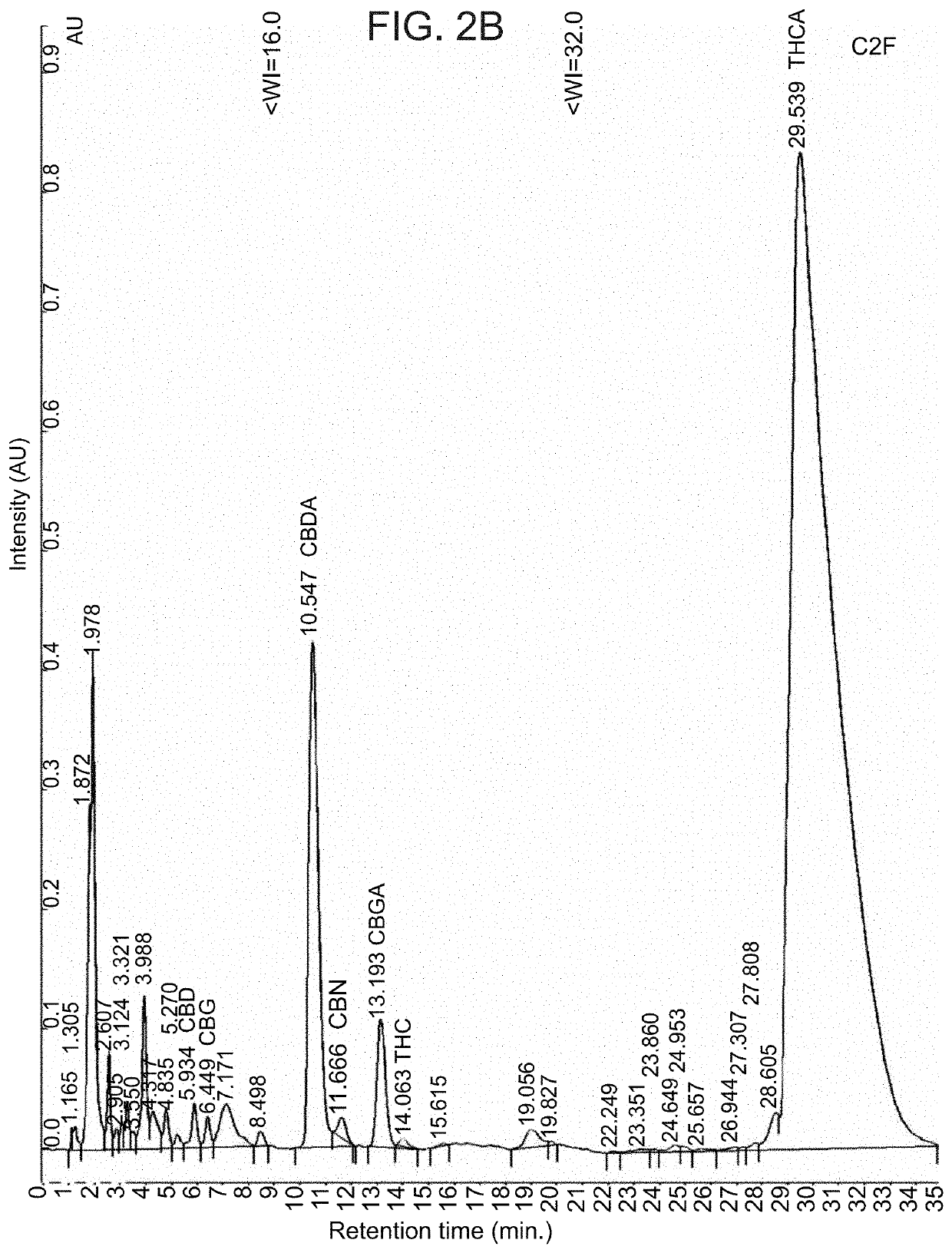
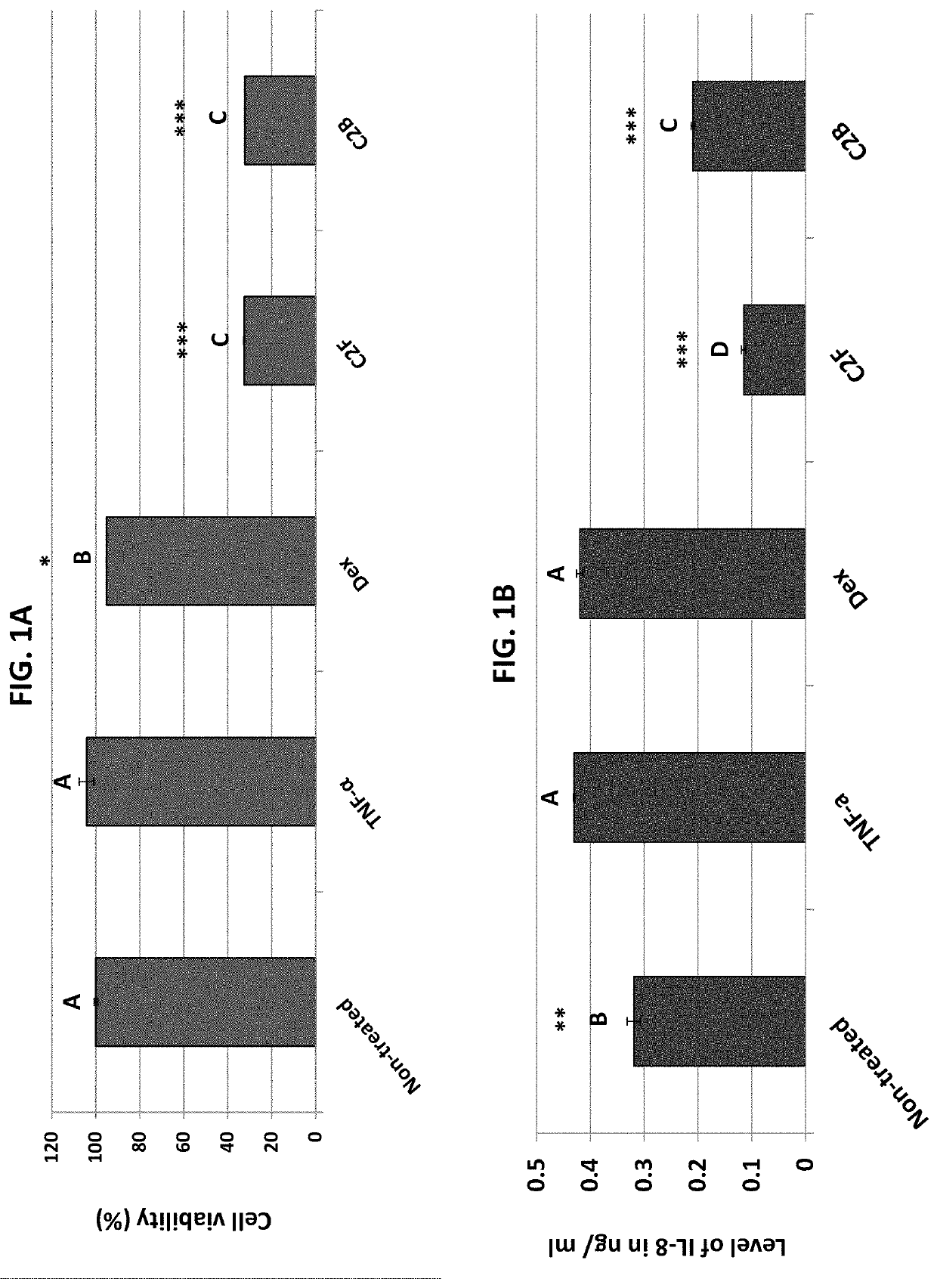
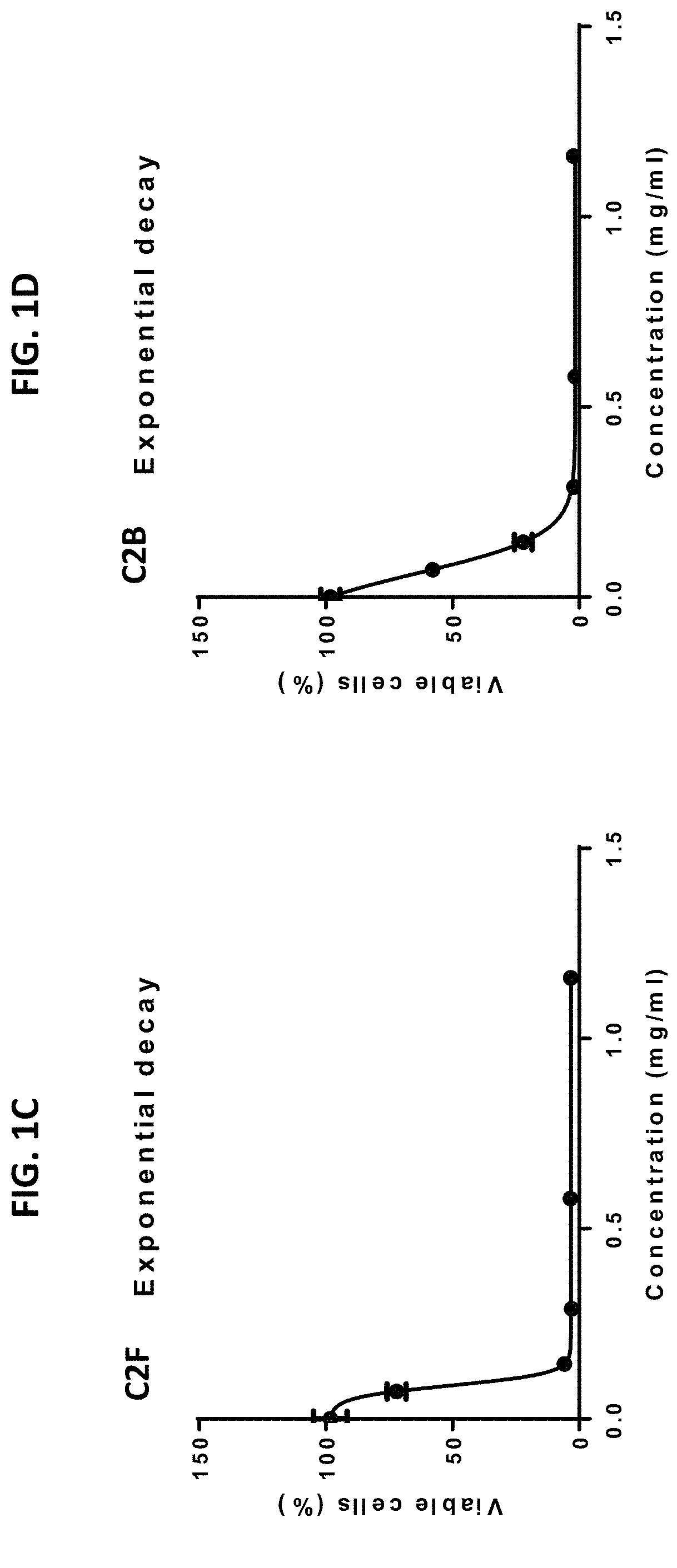
Patents
Literature
42 results about "Tetrahydrocannabinolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
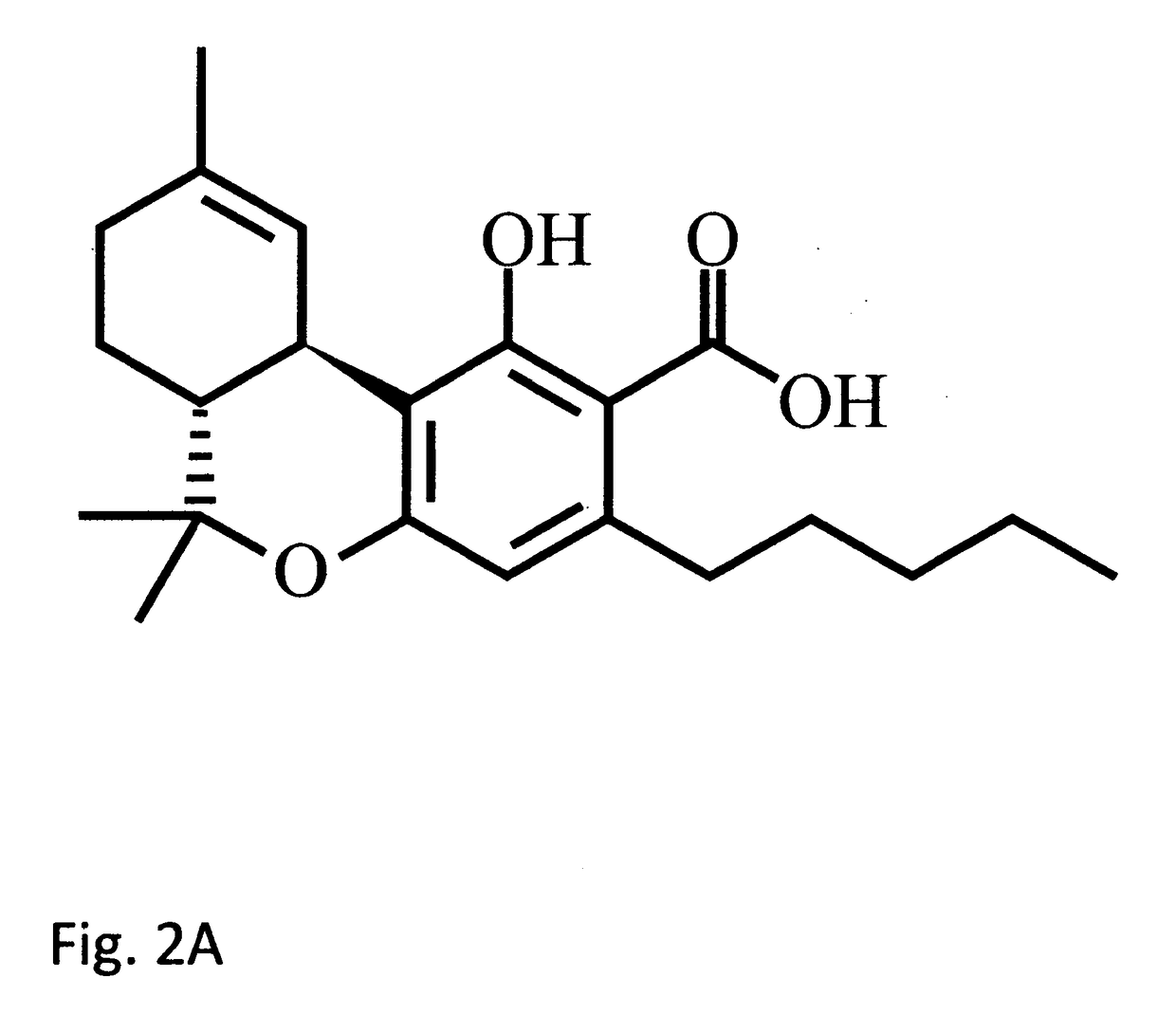
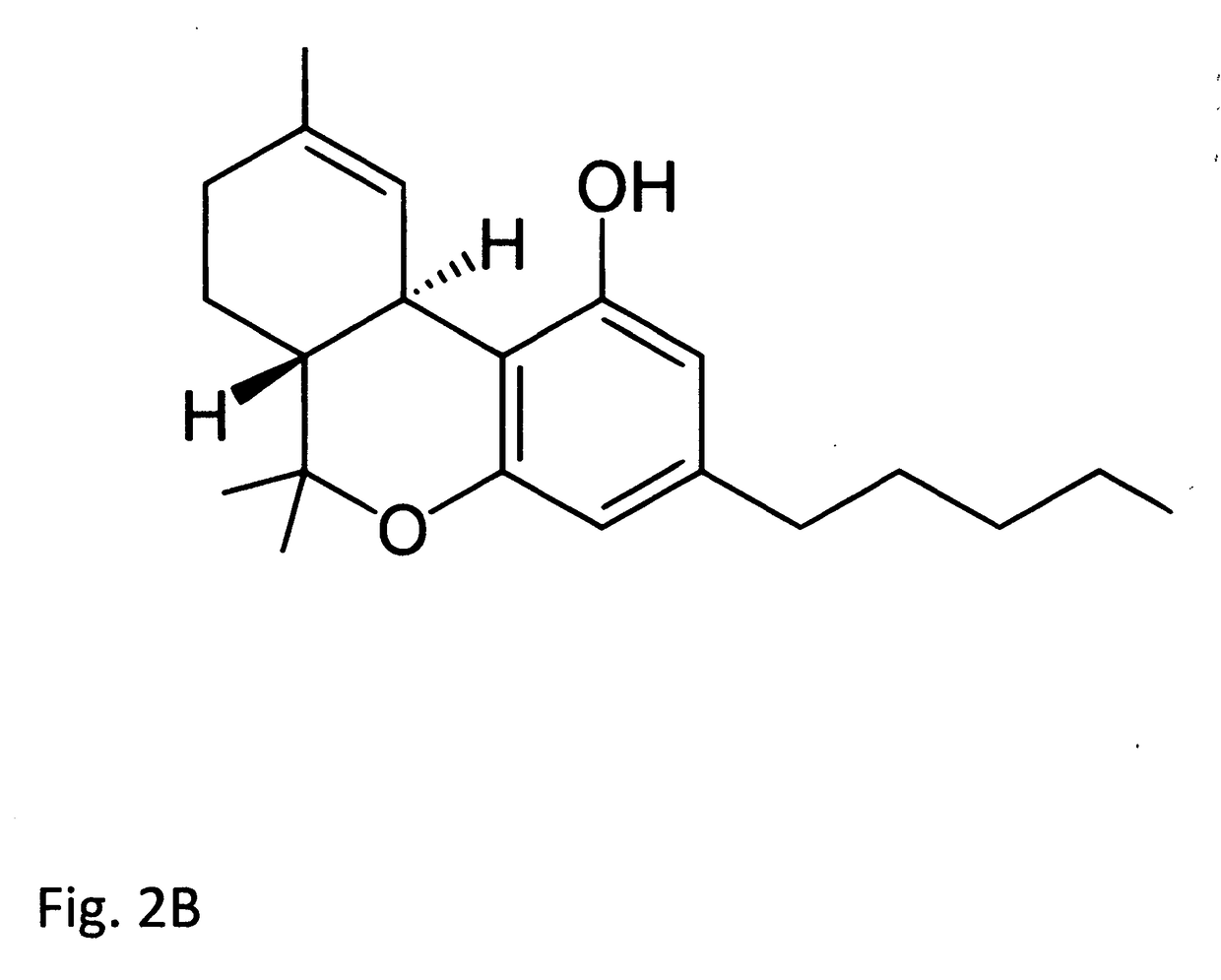
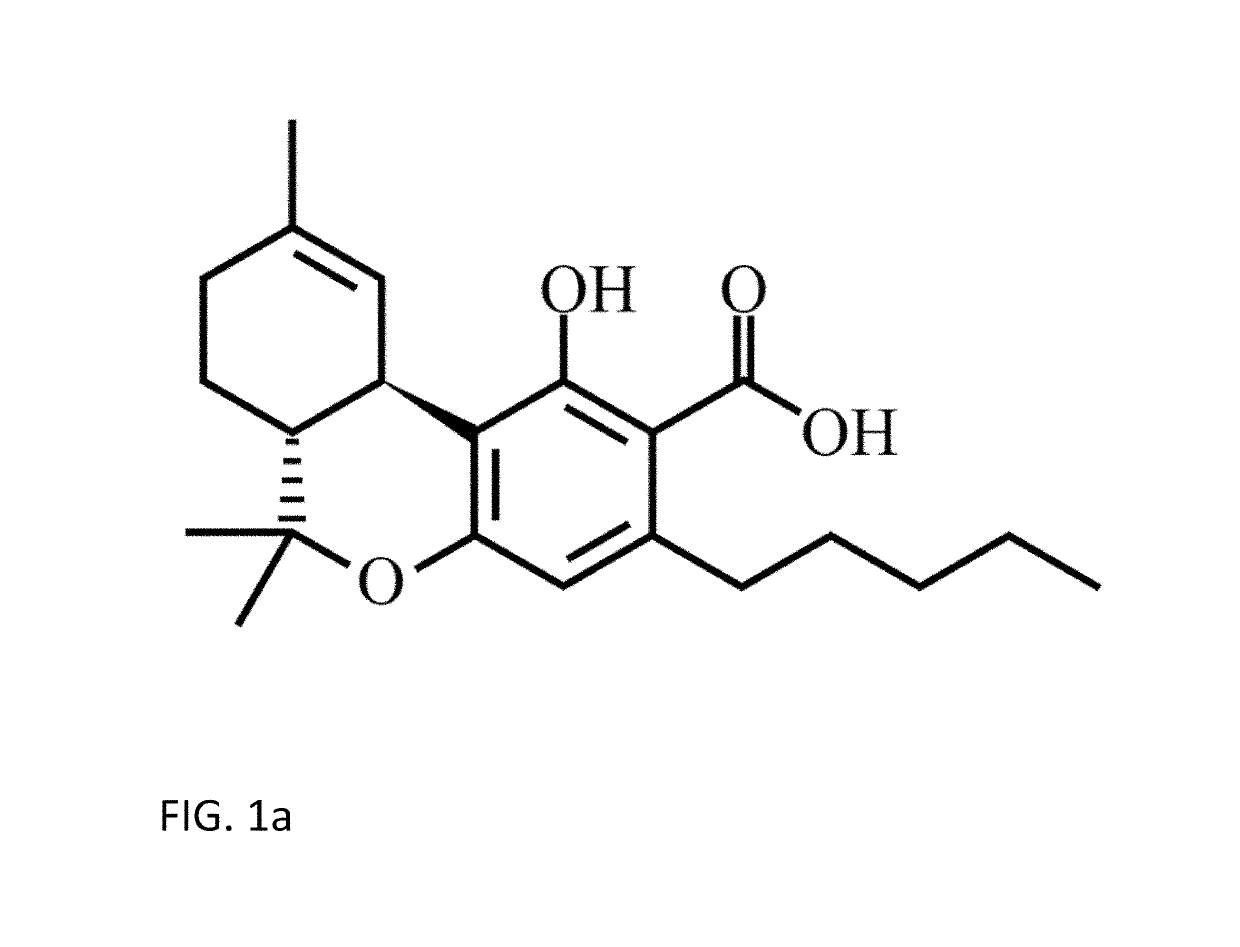
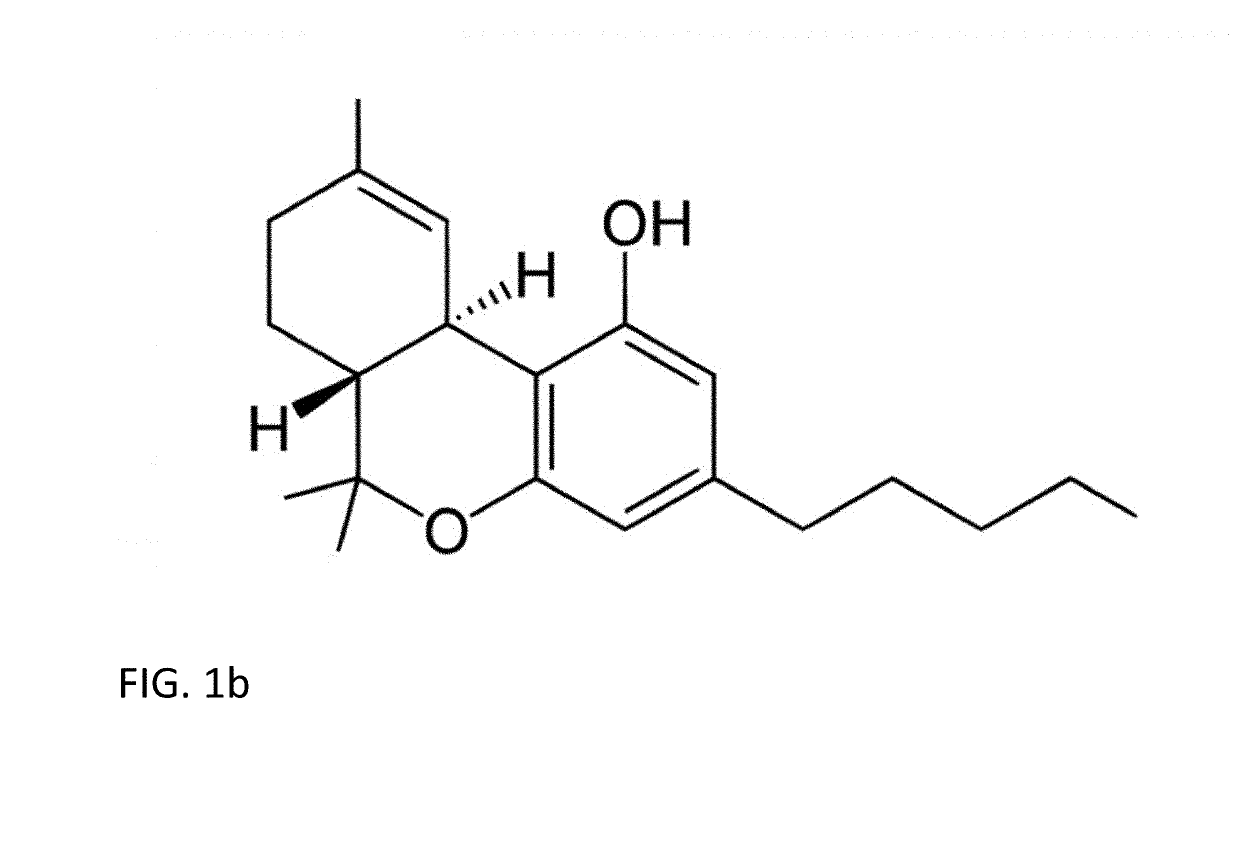
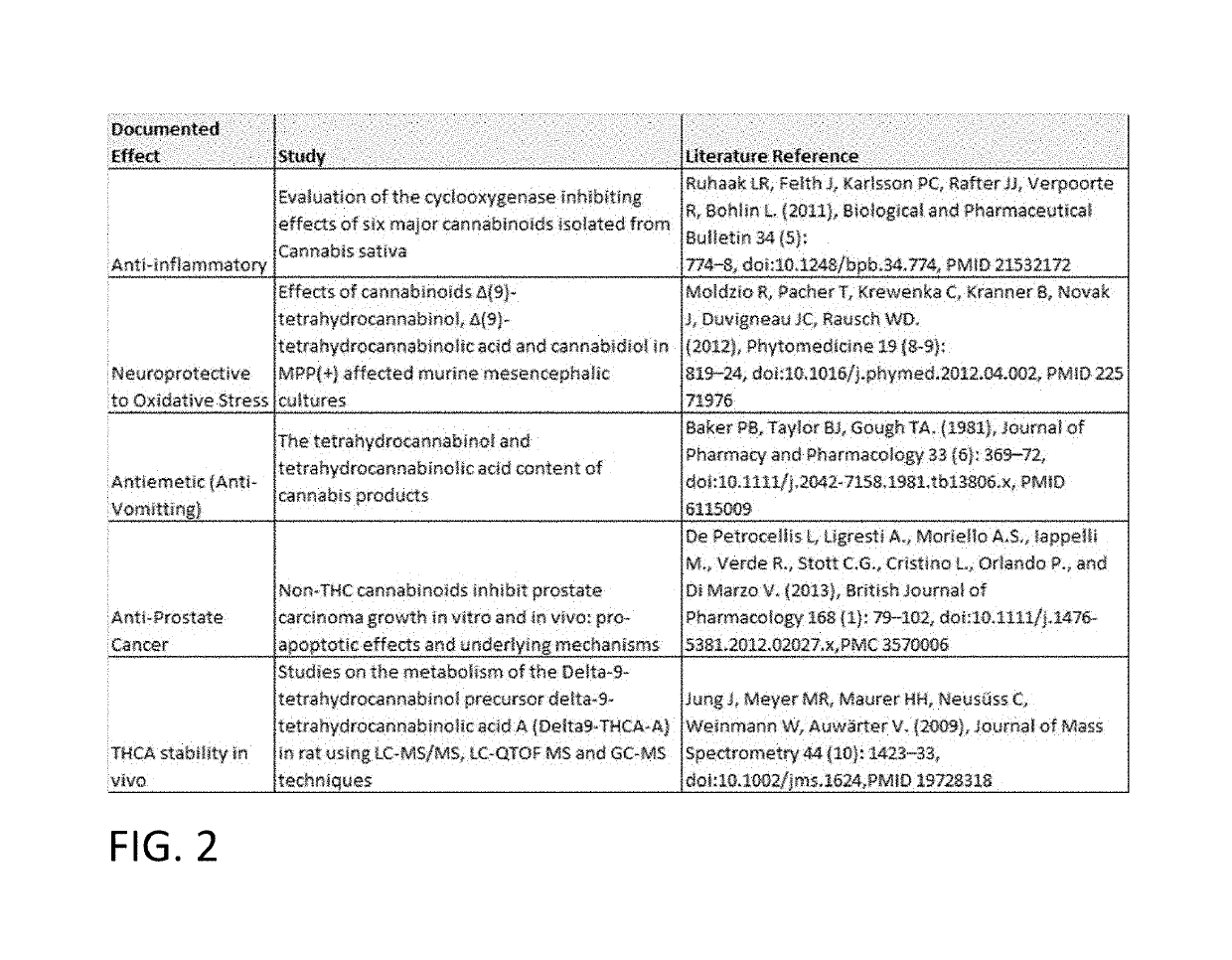
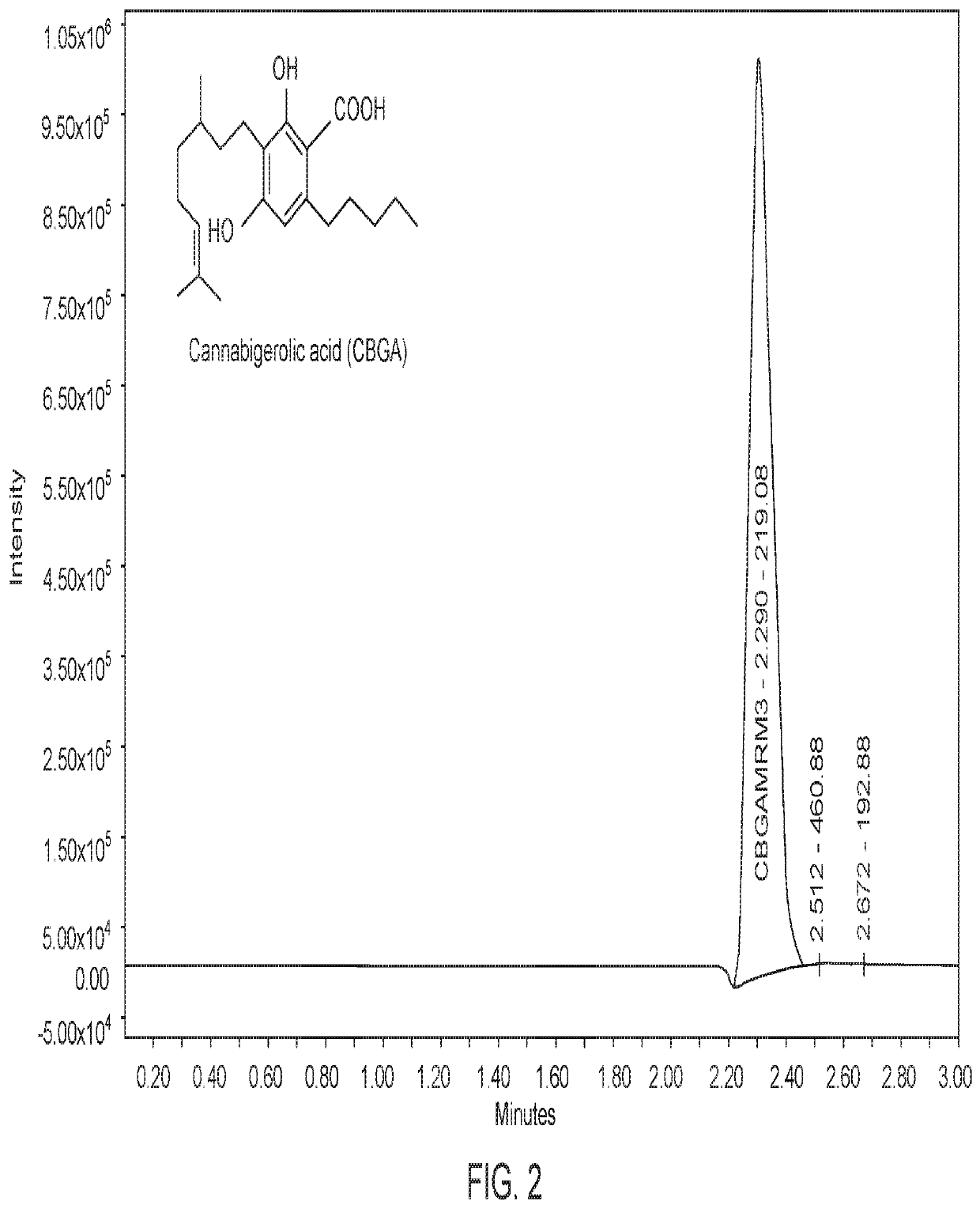
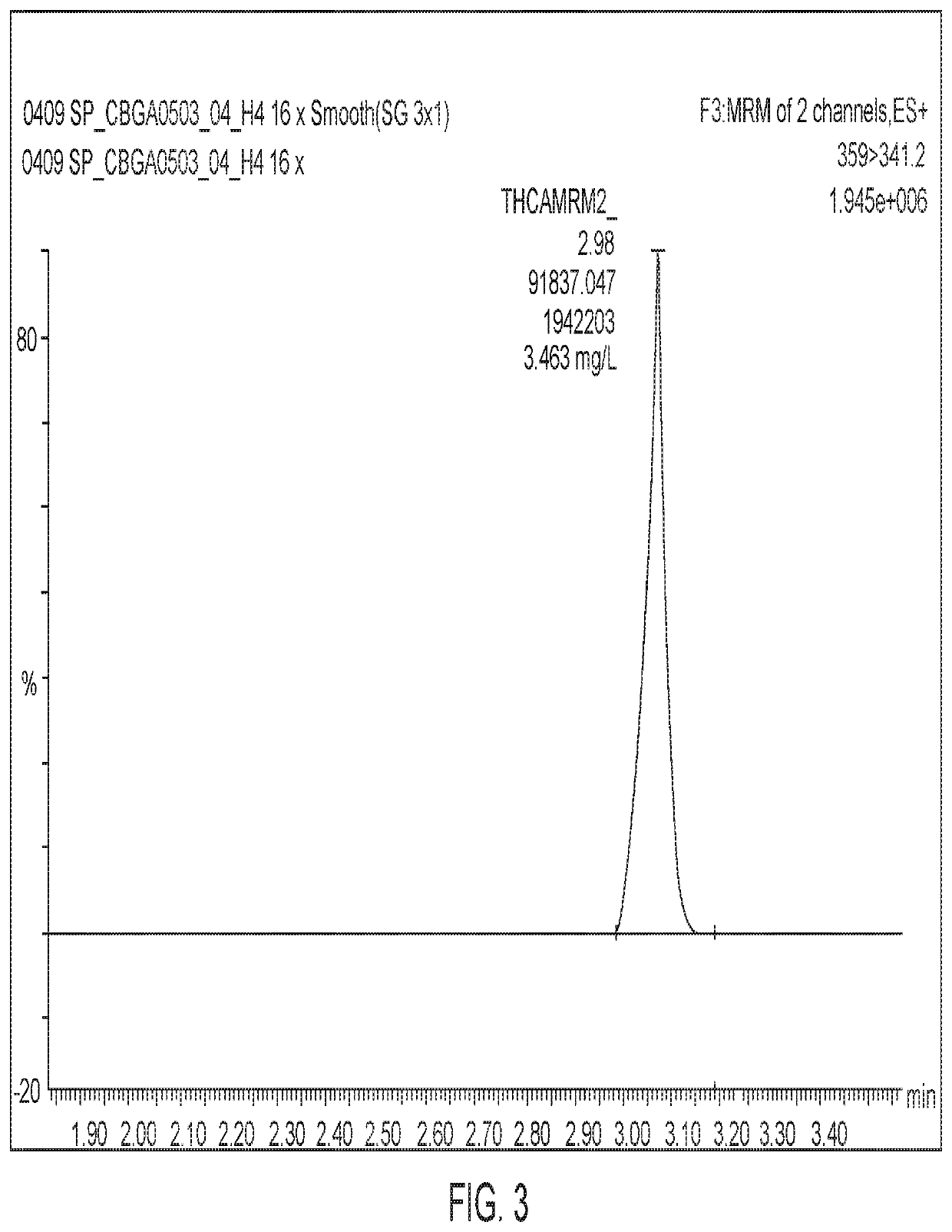
Tetrahydrocannabinolic acid (THCA, 2-COOH-THC; conjugate base tetrahydrocannabinolate) is a precursor of tetrahydrocannabinol (THC), the active component of cannabis. THCA is found in variable quantities in fresh, undried cannabis, but is progressively decarboxylated to THC with drying, and especially under intense heating such as when cannabis is smoked or cooked into cannabis edibles. THCA is often the majority constituent in cannabis resin concentrates, such as hashish and hash oil, when prepared from high-THC cannabis plant material, frequently comprising between 50% and 90% by weight.
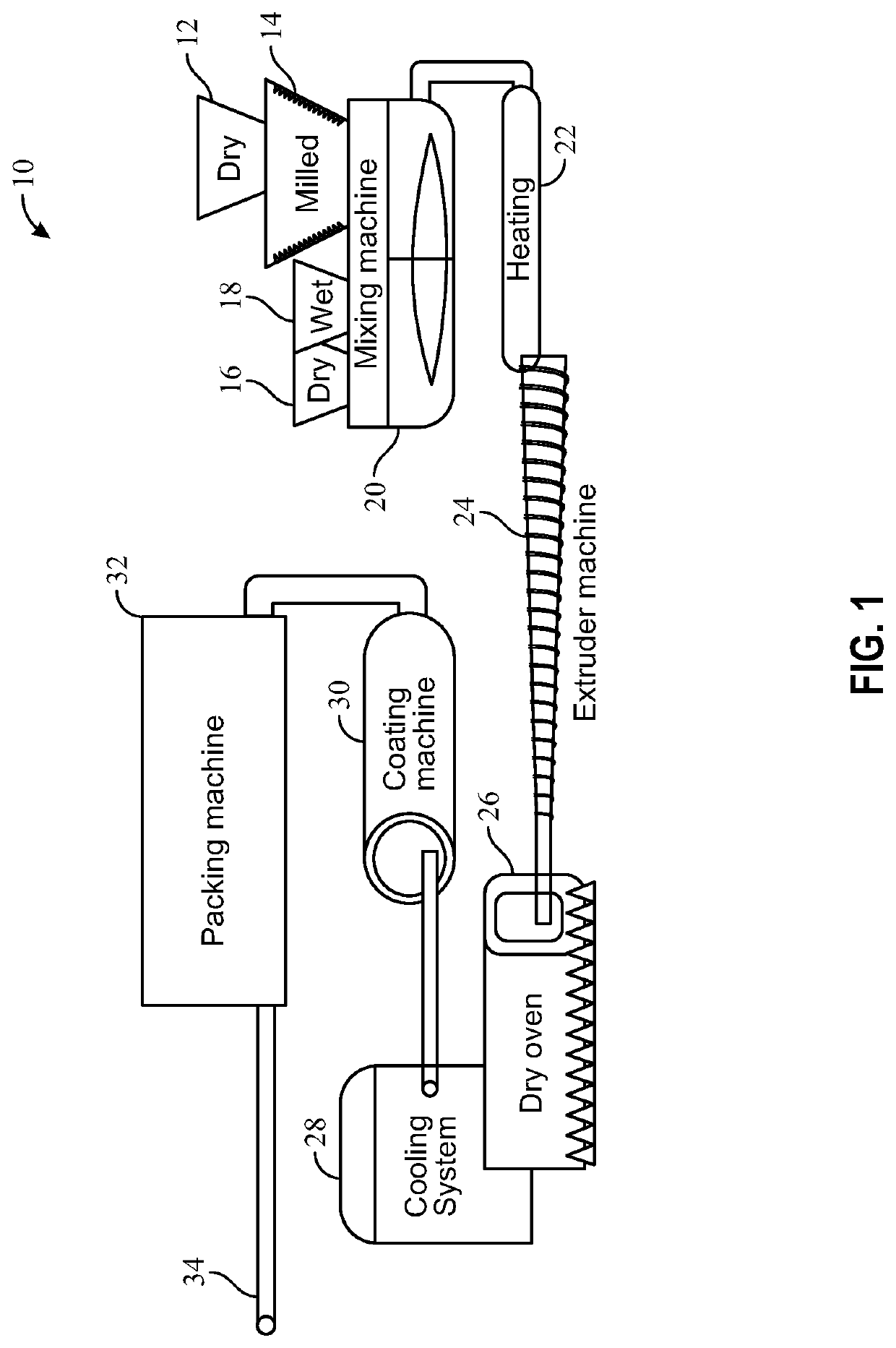
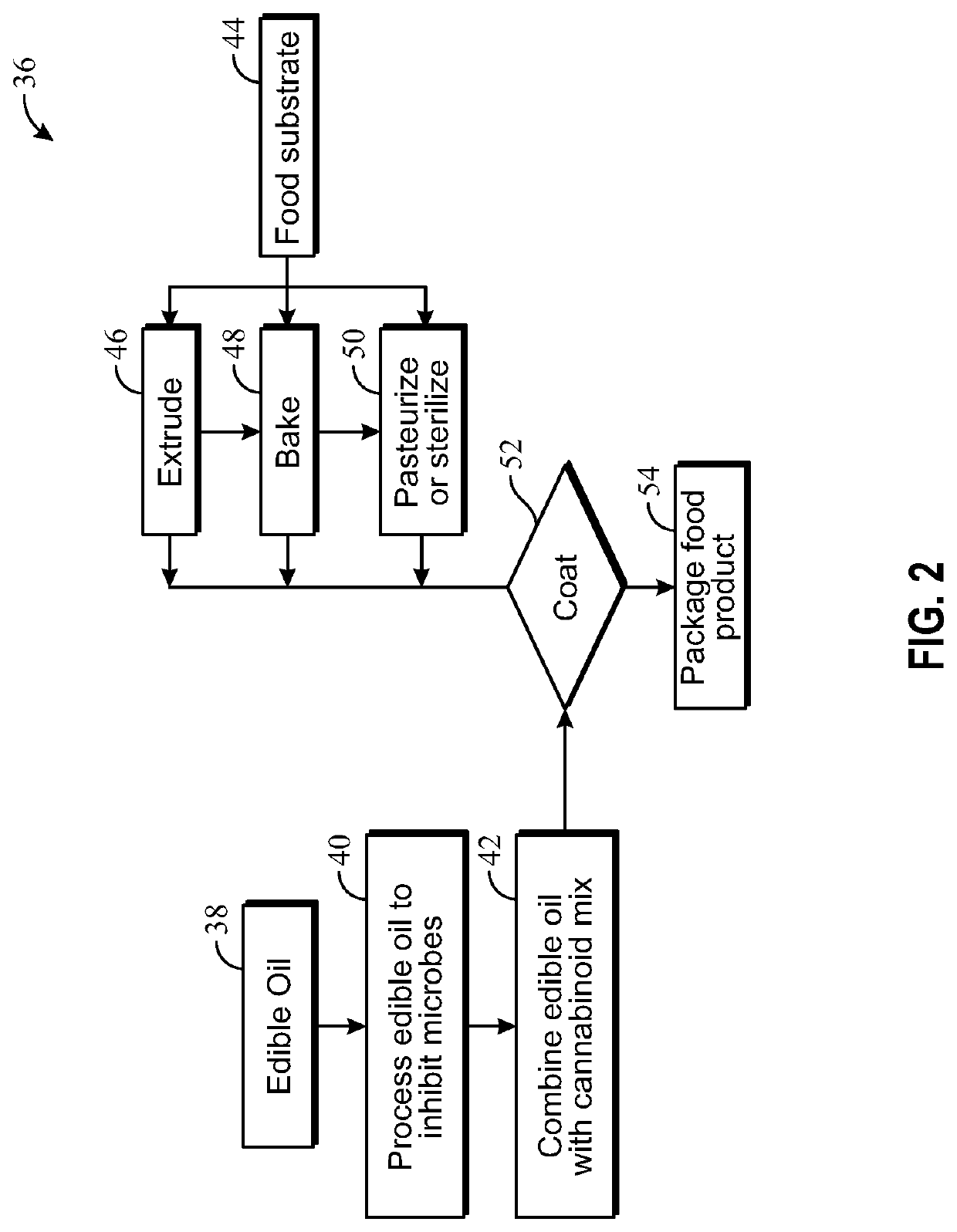
Cannabis plant isolate comprising delta-9-tetrahydrocannabinol and a method for preparing such an isolate
InactiveUS20150126754A1High purityHigh yieldNervous disorderOrganic chemistryDelta-9-tetrahydrocannabinolSolvent
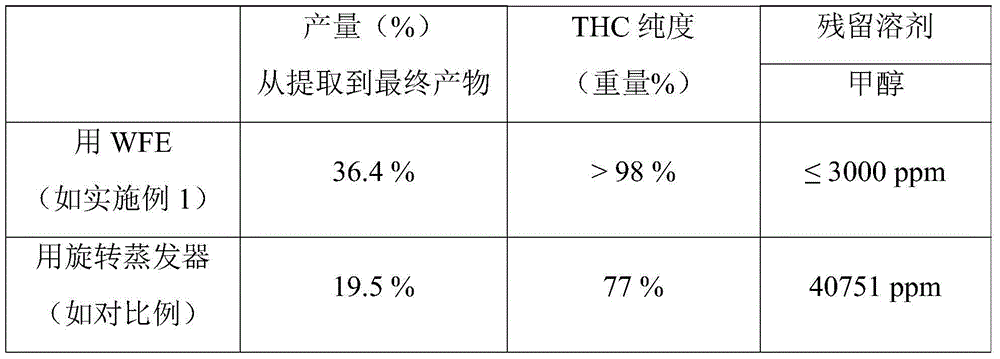
The present invention relates to a method for preparing a Cannabis plant Δ9-tetrahydrocannabinol isolate from a crude solvent extract of Cannabis plant material. The invention relates further to a Cannabis plant THC isolate comprising Δ9-tetrahydrocannabinol, Cannabinol (CBN) and / or Cannabidiol (CBD) and to a pharmaceutical composition comprising the Cannabis plant THC isolate.
Owner:ECHO PHARM BV (NL)
Oral Dosage Form Of Tetrahydrocannabinol And A Method Of Avoiding And/Or Suppressing Hepatic First Pass Metabolism Via Targeted Chylomicron/Lipoprotein Delivery
ActiveUS20110092583A1Easy to transportPromote lymphatic transportBiocideSenses disorderChylomicronCytochrome P450
Self-emulsifying drug delivery systems are provided to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron / lipoprotein delivery and optimal bioavailability through the mammalian intestinal tract. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY RAM B +1
Methods for making compositions and compositions for treating pain and cachexia
InactiveUS20080103193A1Avoiding high first metabolismHighly psychoactiveBiocideOrganic chemistryΔ9-tetrahydrocannabinolPharmacology
The present invention pertains to methods for making compositions and compositions for treating pain and cachexia or AIDS wasting. In particular, the instant invention employs methods for making Δ9-tetrahydrocannabinol (Δ9-THC) and Δ9-tetrahydrocannabinolic acid (Δ9-THCA) from Cannabis sativa, and compositions for the treatment of these diseases.
Owner:CASTOR TREVOR PERCIVAL +3
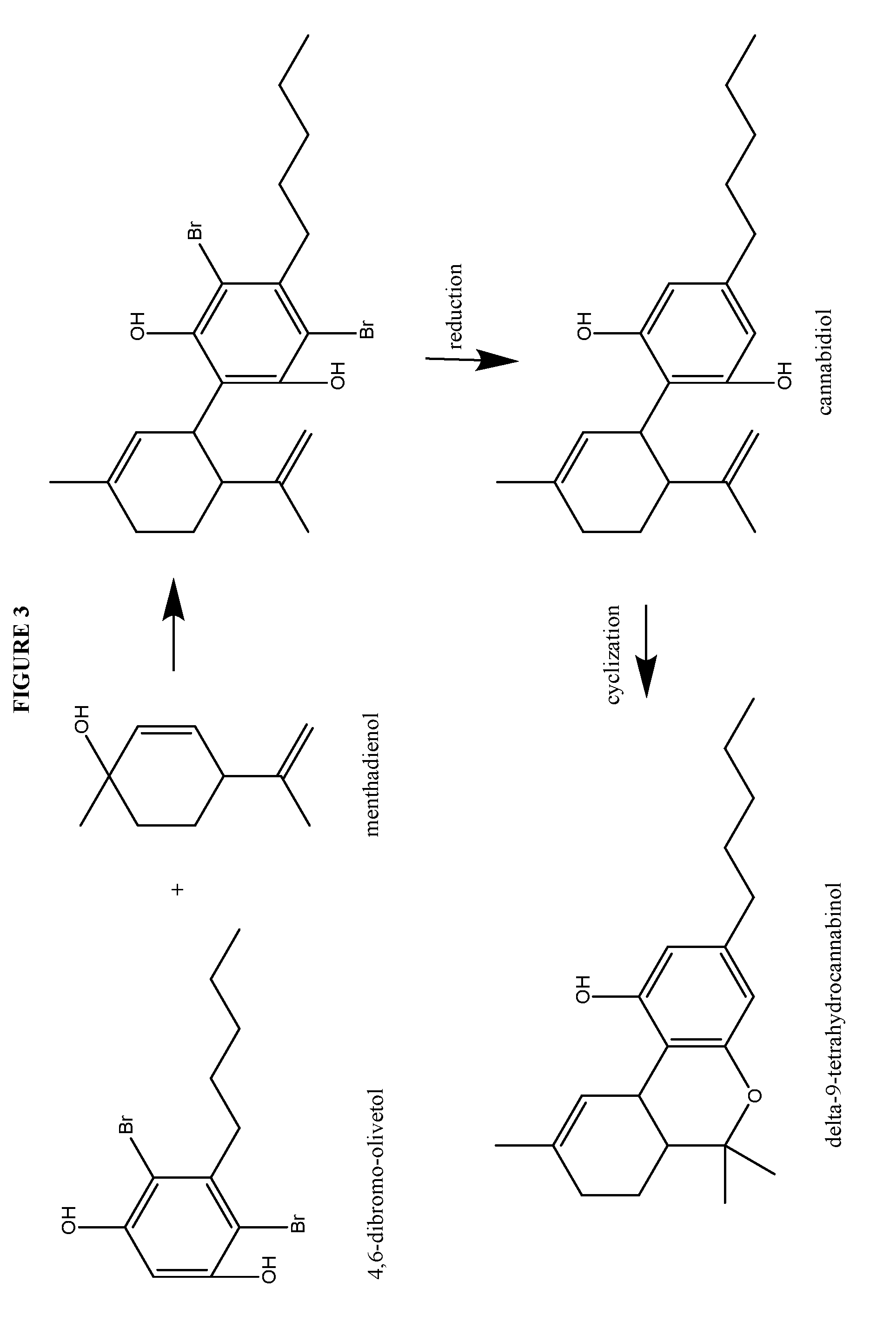
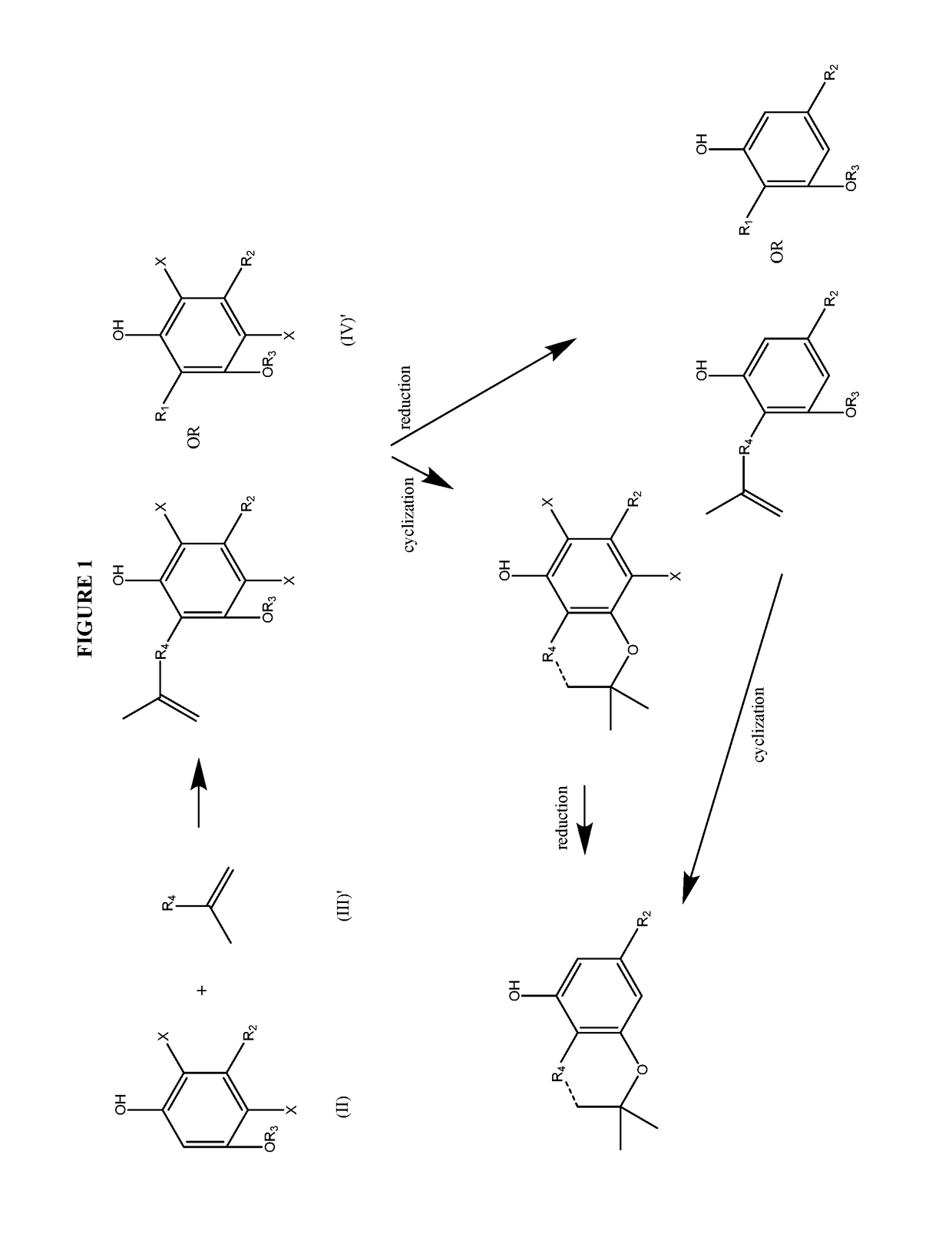
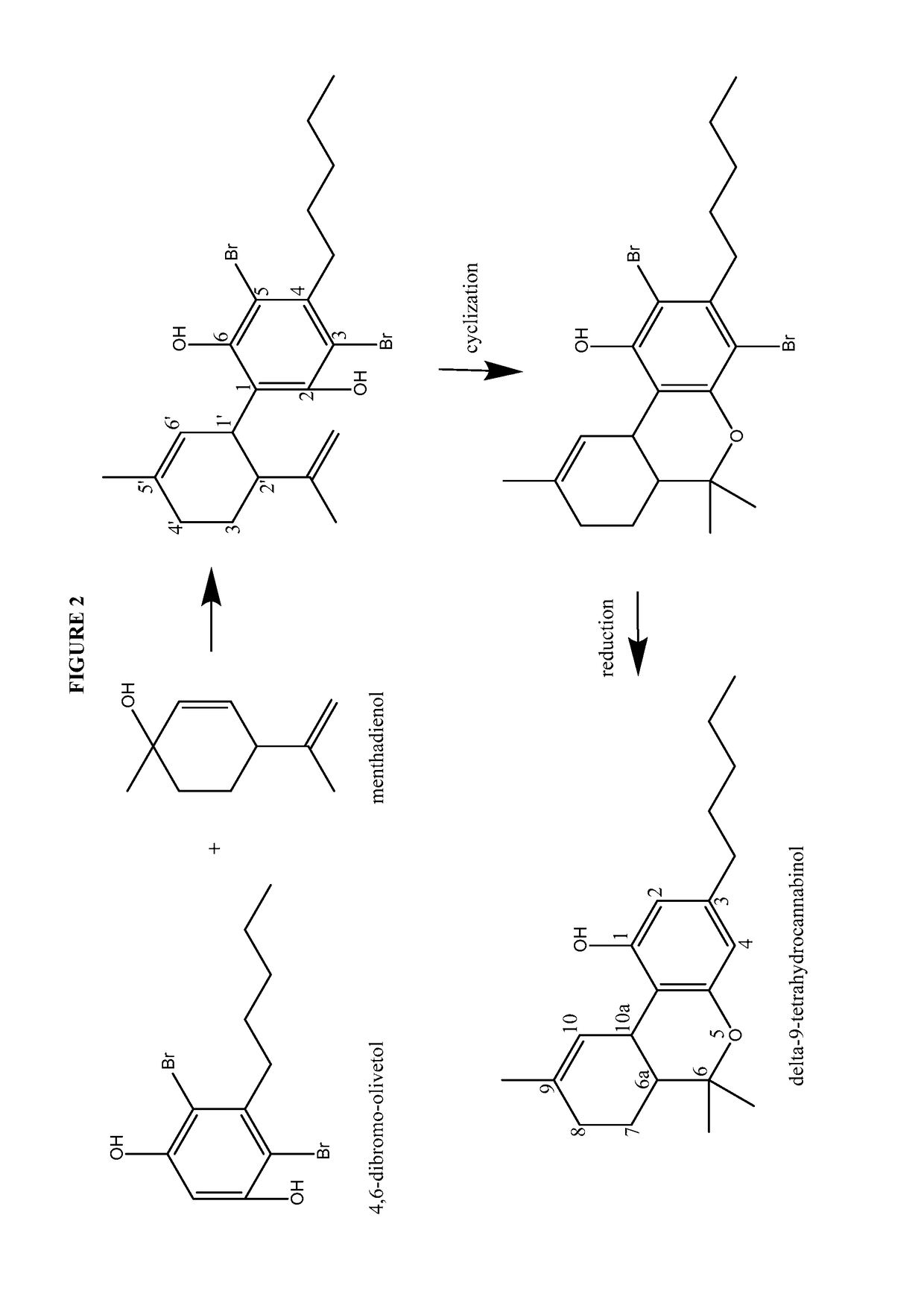
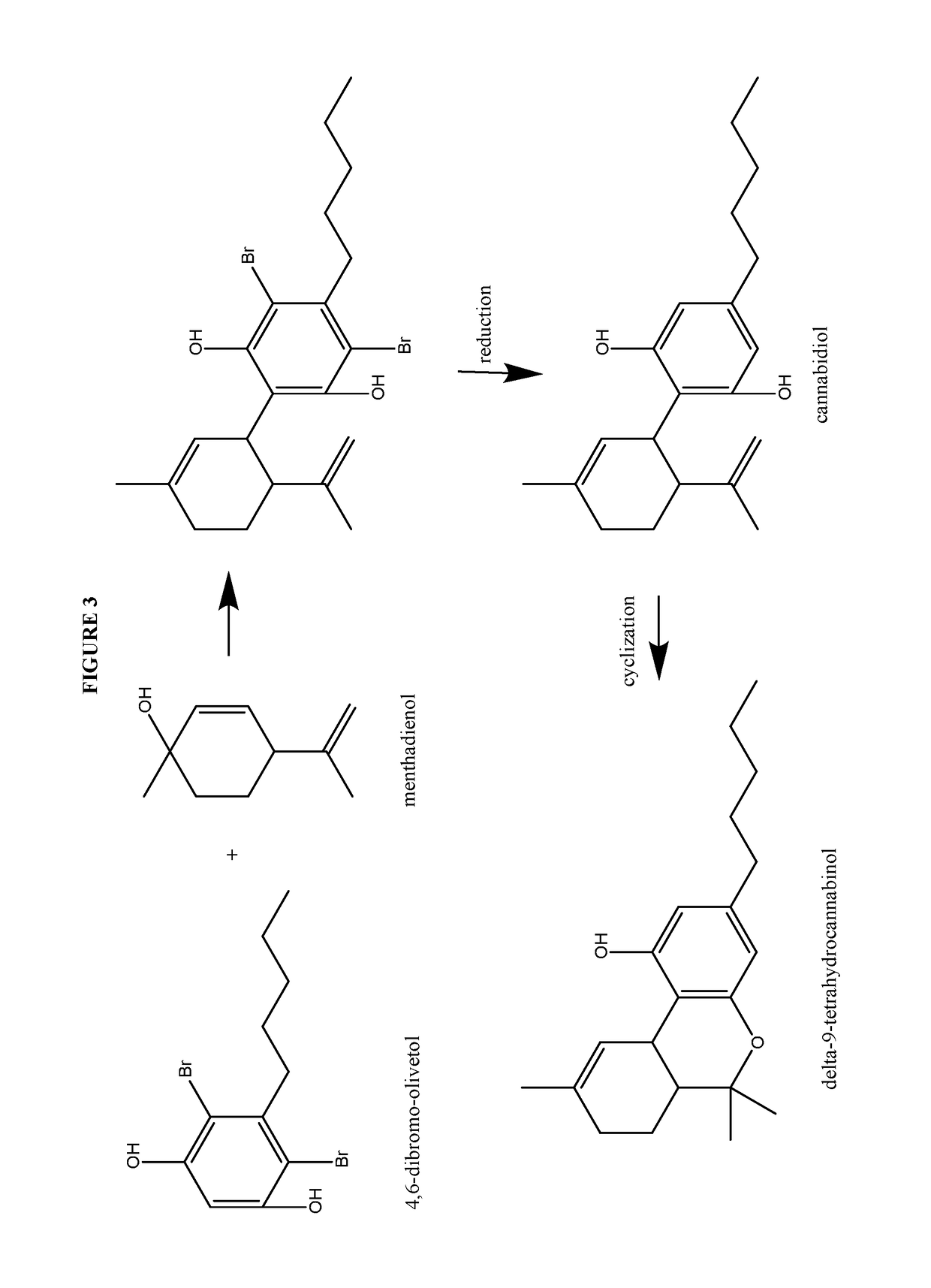
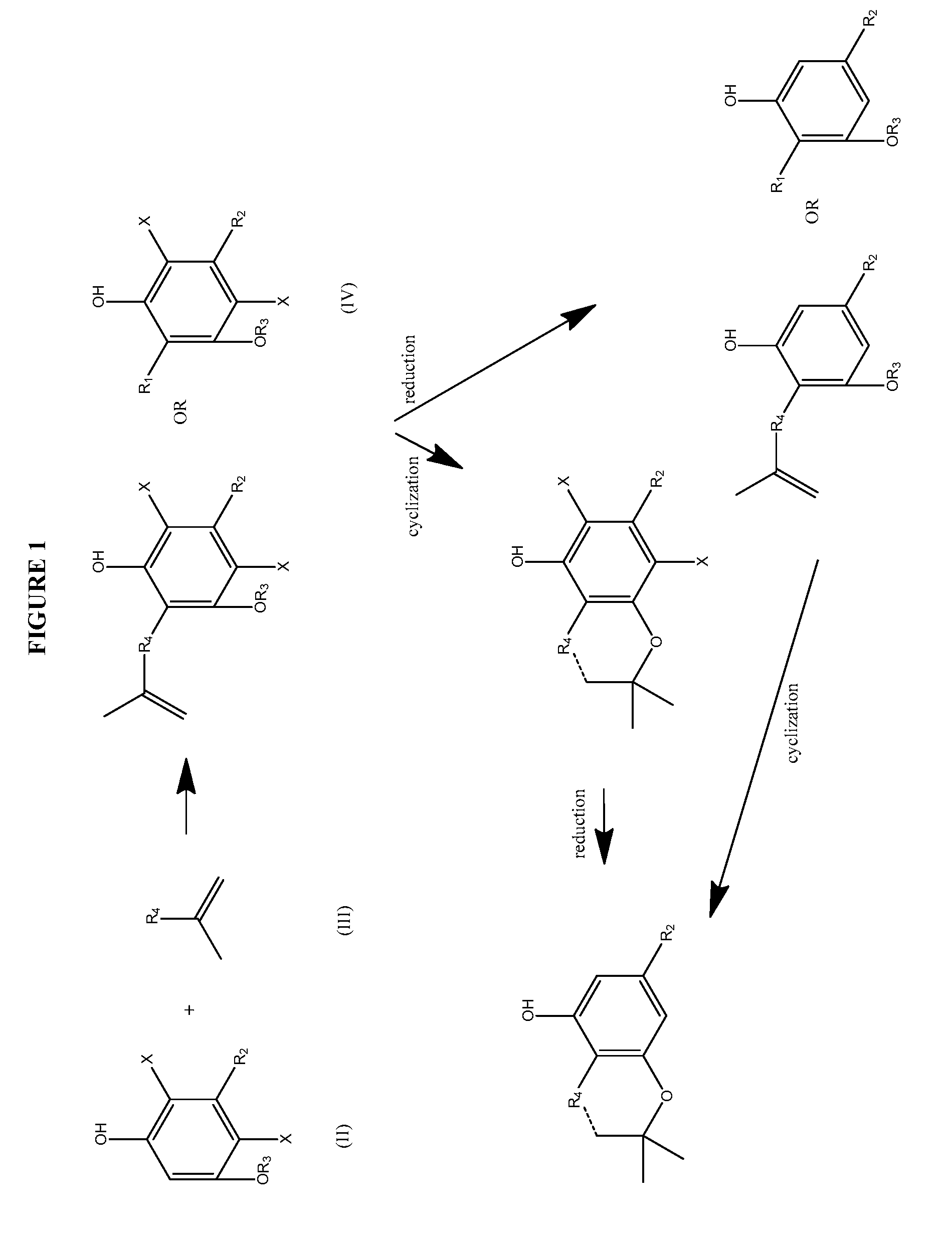
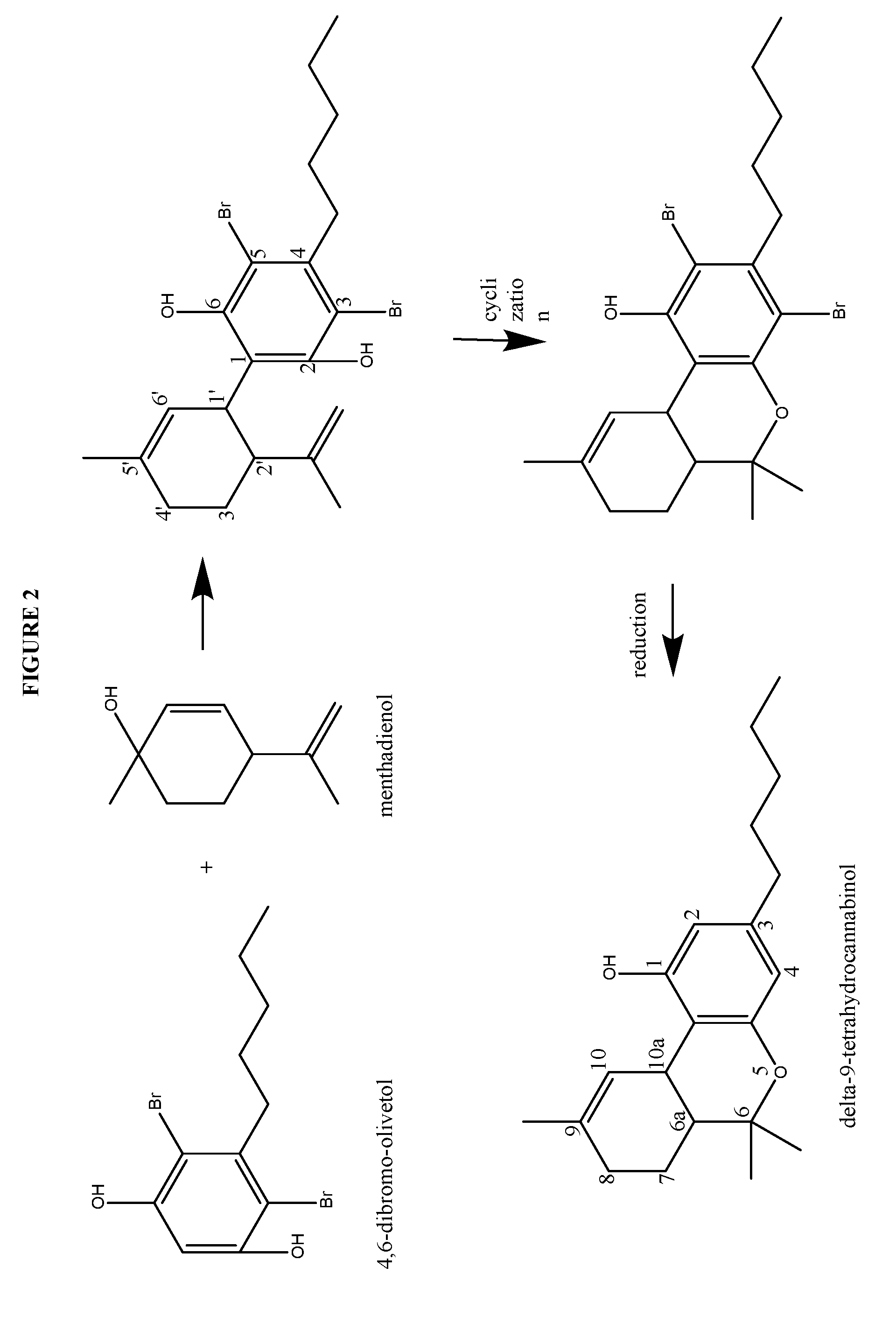
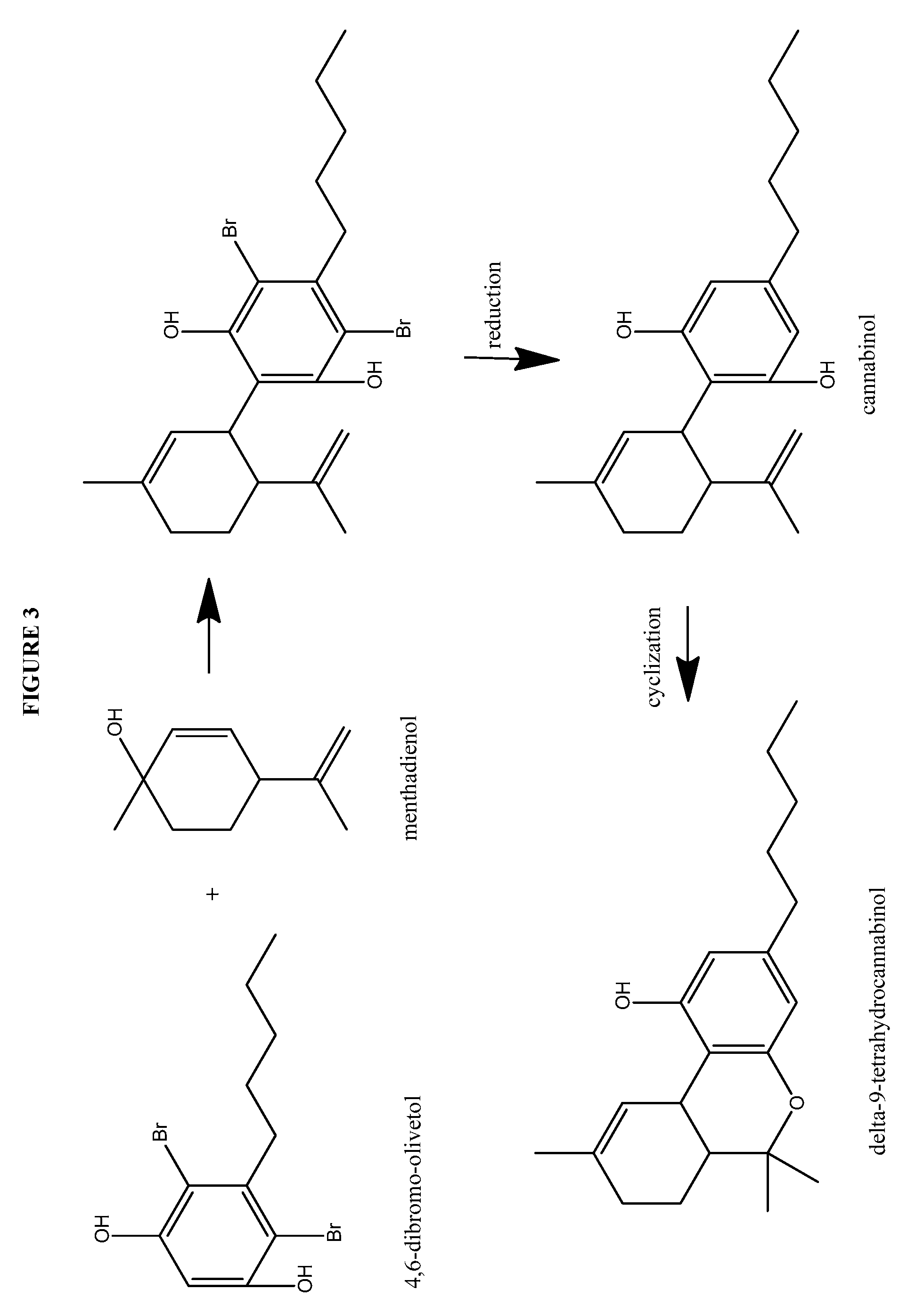
Process for the production of cannabidiol and delta-9-tetrahydrocannabinol
ActiveUS20170008868A1Low selectivityLow yieldNervous disorderOrganic chemistryDelta-9-tetrahydrocannabinolAcid catalyzed
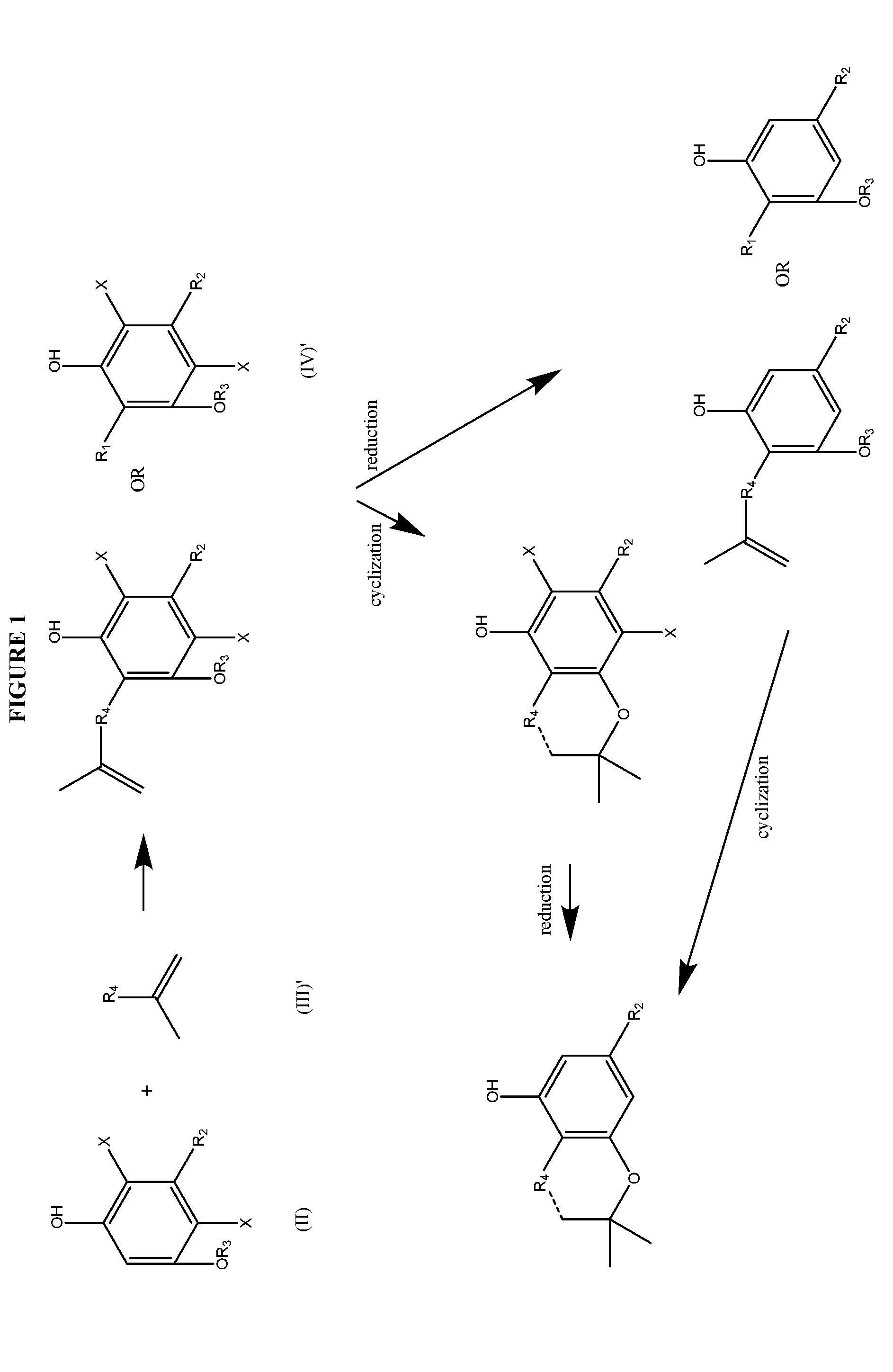
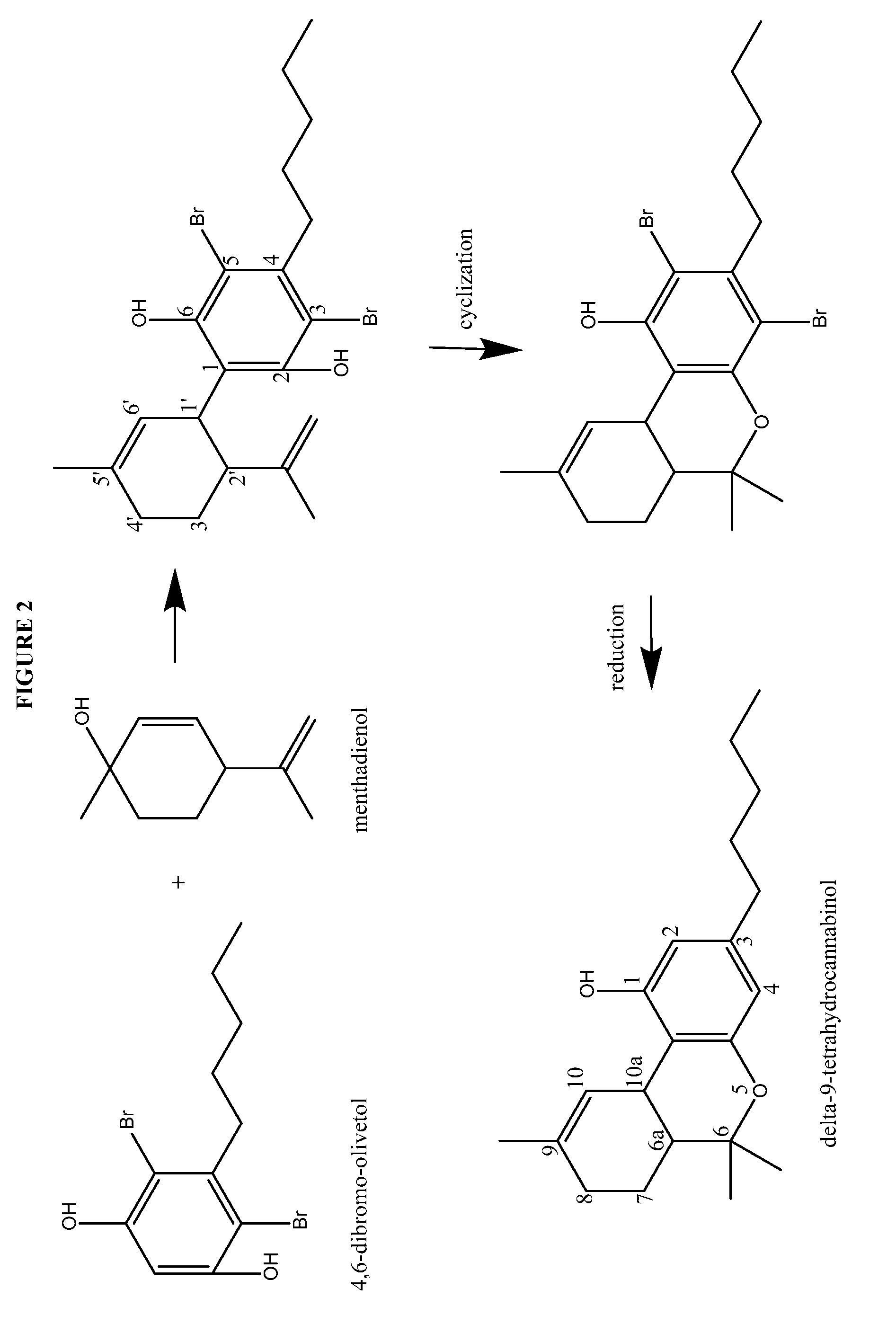
The present disclosure relates to the preparation of a cannabidiol compound or a derivative thereof. The cannabidiol compound or derivatives thereof can be prepared by an acid-catalyzed reaction of a suitably selected and substituted di-halo-olivetol or derivative thereof with a suitably selected and substituted cyclic alkene to produce a dihalo-cannabidiol compound or derivative thereof. The dihalo-cannabidiol compound or derivative thereof can be produced in high yield, high stereospecificity, or both. It can then be converted under reducing conditions to a cannabidiol compound or derivatives thereof.
Owner:PURISYS LLC
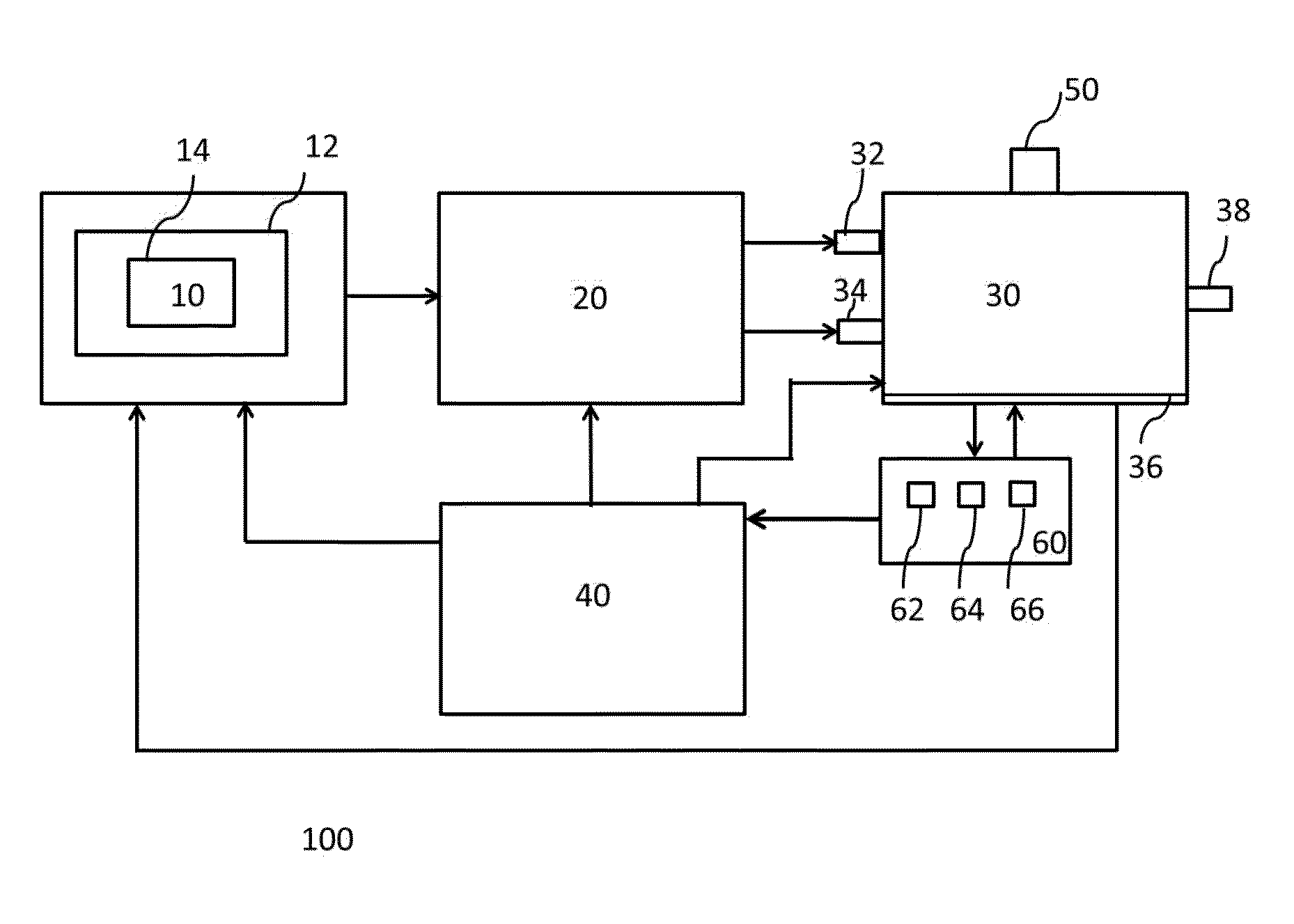
Apparatus and methods for the simultaneous production of compounds
ActiveUS9394510B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCannabielsoinEngineering
The present invention provides an apparatus for simultaneously producing different cannabinoids in different ratios. Specifically, the apparatus according to the invention produces tetrahydrocannabinolic acid (THCA) and cannabichormenic acid (CBCA) or cannabidiolic acid (CBDA) in different ratios, and it comprises a bioreactor that comprises a first automated supply system configured to deliver a substrate and a cannabinoid acid synthase, and a second automated system to cease the reaction; an extractor configured to recover the cannabionoids so produced; and a controller configured to modify at least on property of the reaction.
Owner:TEEWINOT TECH LTD
Parenteral formulations
ActiveUS20190314296A1Improve solubilityLow toxicityHydroxy compound active ingredientsPharmaceutical delivery mechanismSURFACTANT BLENDPharmacology
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
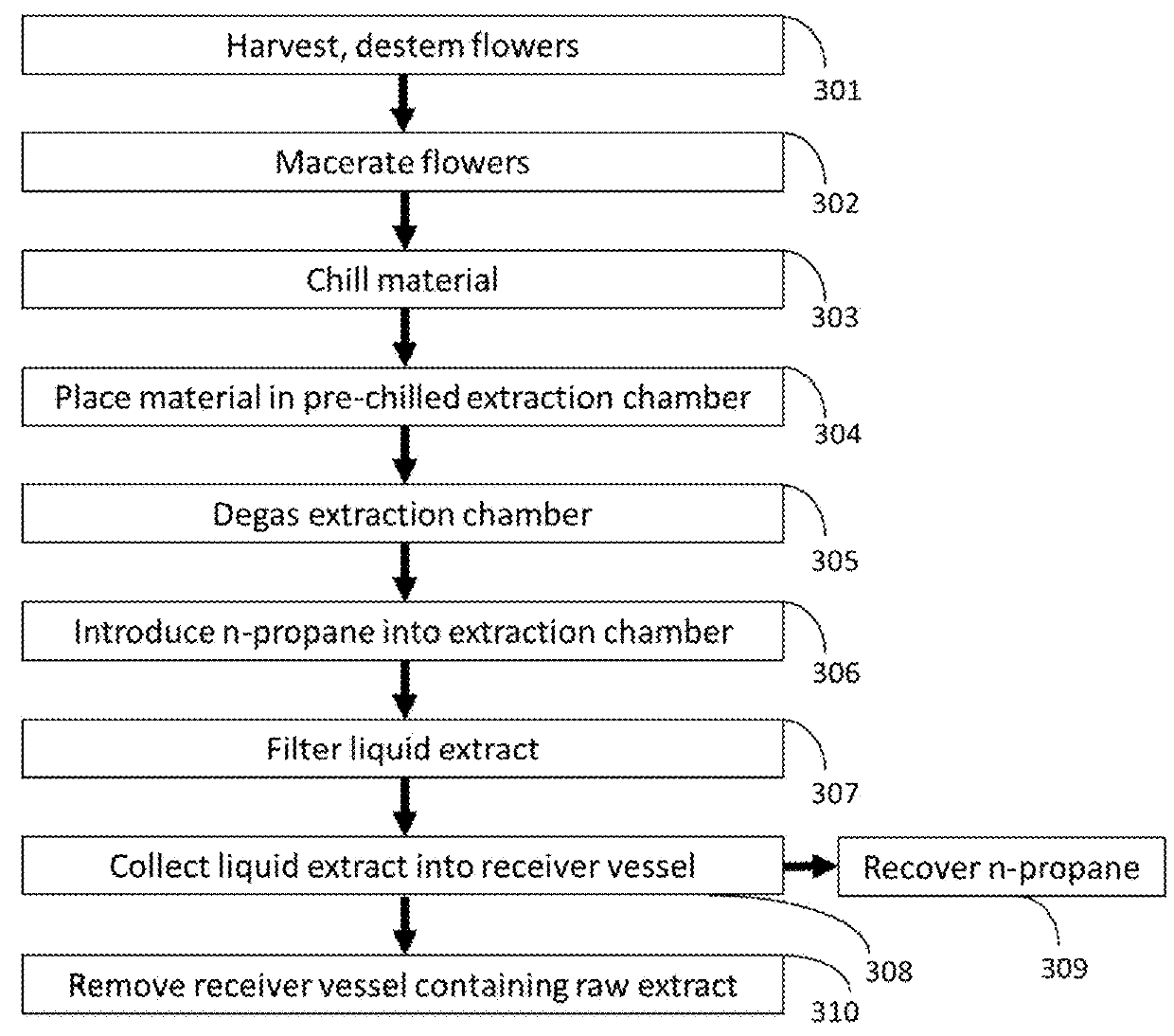
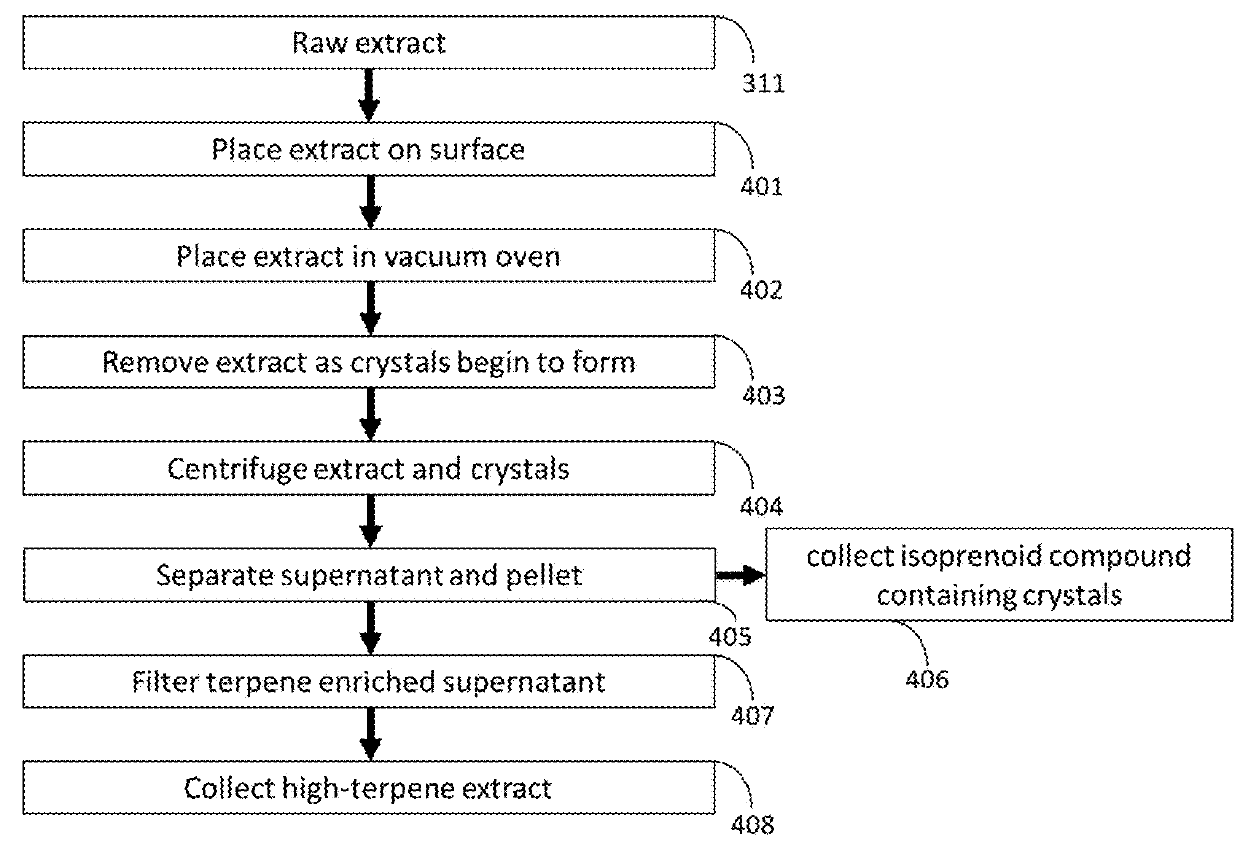
Methods for Extraction and Isolation of Isoprenoid and Terpene Compounds from Biological Extracts
ActiveUS20180344785A1Rapidly cost-effectively extractedOrganic active ingredientsMagnoliophyta medical ingredientsCannabisPrenylation
A method for the extraction and isolation of the terpene and isoprenoid compounds from plant material, followed by a centrifugal force induced selective crystallization of isoprenoids resulting in a separation of terpene and isoprenoid fractions. This this method is suitable for the extraction of cannabinoids from Cannabis and the enrichment tetrahydrocannabinolic acid and reduction of tetrahydrocannabinol in an extract.
Owner:CONCENTRATED CONSULTING GRP LLC
Cannabis plants having modified expression of thca synthase
ActiveUS20180258439A1Increased cannabidiol (CBD) contentOrganic active ingredientsSenses disorderDelta-9-tetrahydrocannabinolPolynucleotide
The invention relates to novel genetically modified plants and methods or materials, such as polynucleotides, expression cassettes, or vectors for producing the same. Moreover, the invention relates to altering the content of cannabinoids in plants and to medical compositions derived from such plants. In particular embodiments, the present invention relates to cannabis plants having modified expression of tetrahydrocannabinolic acid (THCA) synthase and methods of modifying the amount of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in cannabis by modifying expression of THCA synthase.
Owner:CANOPY GROWTH CORP
Process for the production of cannabidiol and delta-9-tetrahydrocannabinol
ActiveUS10059683B2High yieldHigh stereospecificityNervous disorderOrganic chemistryDelta-9-tetrahydrocannabinolAcid catalyzed
The present disclosure relates to the preparation of a cannabidiol compound or a derivative thereof. The cannabidiol compound or derivatives thereof can be prepared by an acid-catalyzed reaction of a suitably selected and substituted di-halo-olivetol or derivative thereof with a suitably selected and substituted cyclic alkene to produce a dihalo-cannabidiol compound or derivative thereof. The dihalo-cannabidiol compound or derivative thereof can be produced in high yield, high stereospecificity, or both. It can then be converted under reducing conditions to a cannabidiol compound or derivatives thereof.
Owner:PURISYS LLC
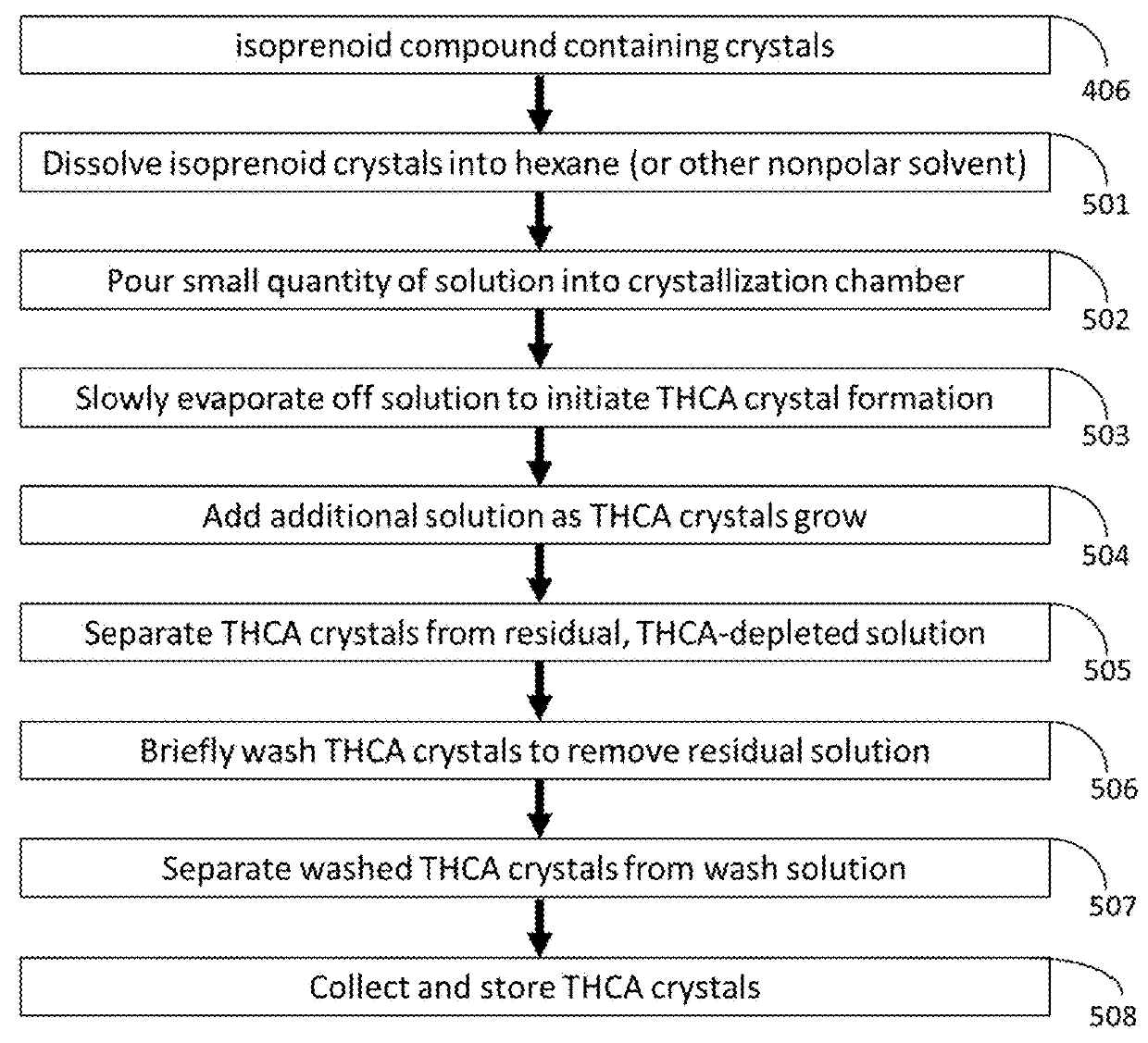
Methods for Purification of Non-Psychoactive Isoprenoid Compounds from Biological Extracts
ActiveUS20180273501A1Improve stabilityPrevent degradationOrganic chemistry methodsCentrifugal force sediment separationCannabisPrenylation
A method for the extraction and isolation of the terpene and isoprenoid compounds from plant material, followed by a centrifugal force induced selective crystallization of isoprenoids resulting in a separation of terpene and isoprenoid fractions. This this method is suitable for the extraction of cannabinoids from Cannabis and the enrichment tetrahydrocannabinolic acid and reduction of tetrahydrocannabinol in an extract. The purity of tetrahydrocannabinolic acid resulting from centrifugal crystallization is such that dissolution and selective recrystallization of tetrahydrocannabinolic acid is possible resulting in >99.9% pure tetrahydrocannabinolic acid, w / w.
Owner:CONCENTRATED CONSULTING GRP LLC
Process for the production of cannabidiol and delta-9-tetrahydrocannabinol
InactiveUS20170008869A1High yieldHigh stereospecificityNervous disorderOrganic chemistryDelta-9-tetrahydrocannabinolAcid catalyzed
The present disclosure relates to the preparation of a cannabidiol compound or a derivative thereof. The cannabidiol compound or derivatives thereof can be prepared by an acid-catalyzed reaction of a suitably selected and substituted di-halo-olivetol or derivative thereof with a suitably selected and substituted cyclic alkene to produce a dihalo-cannabidiol compound or derivative thereof. The dihalo-cannabidiol compound or derivative thereof can be produced in high yield, high stereospecificity, or both. It can then be converted under reducing conditions to a cannabidiol compound or derivatives thereof.
Owner:NORAMCO INC
Cannabis plant isolate comprising /\9-tetrahydrocannabinol and a method for preparing such an isolate
InactiveCN104619318AGood yieldHighly repeatableNervous disorderOrganic chemistrySolventΔ9-tetrahydrocannabinol
The present invention relates to a method for preparing a Cannabis plant Δ9-tetrahydrocannabinol isolate from a crude solvent extract of Cannabis plant material. The invention relates further to a Cannabis plant THC isolate comprising Δ9-tetrahydrocannabinol, Cannabinol (CBN) and / or Cannabidiol (CBD) and to a pharmaceutical composition comprising the Cannabis plant THC isolate.
Owner:ECHO PHARM BV (NL)
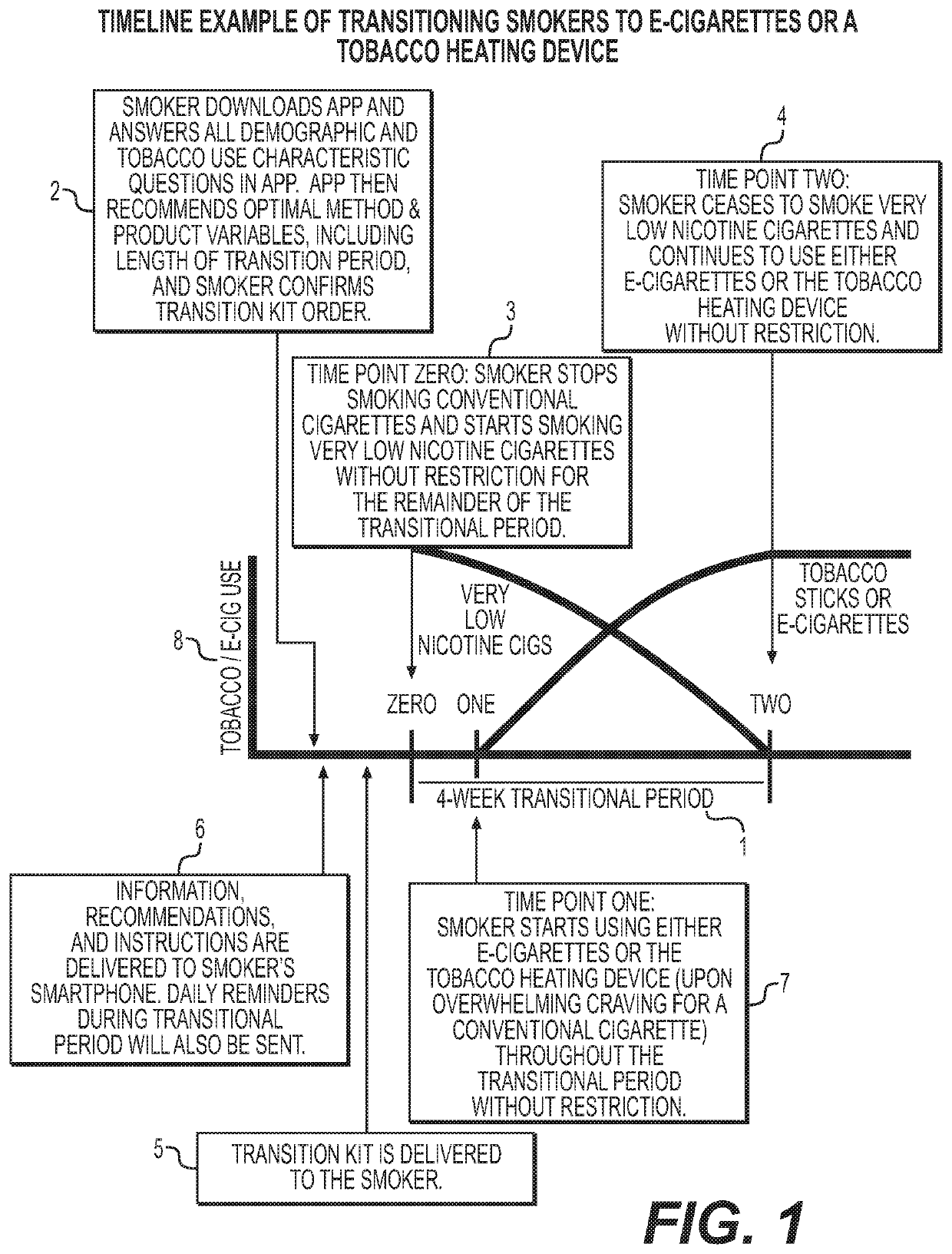
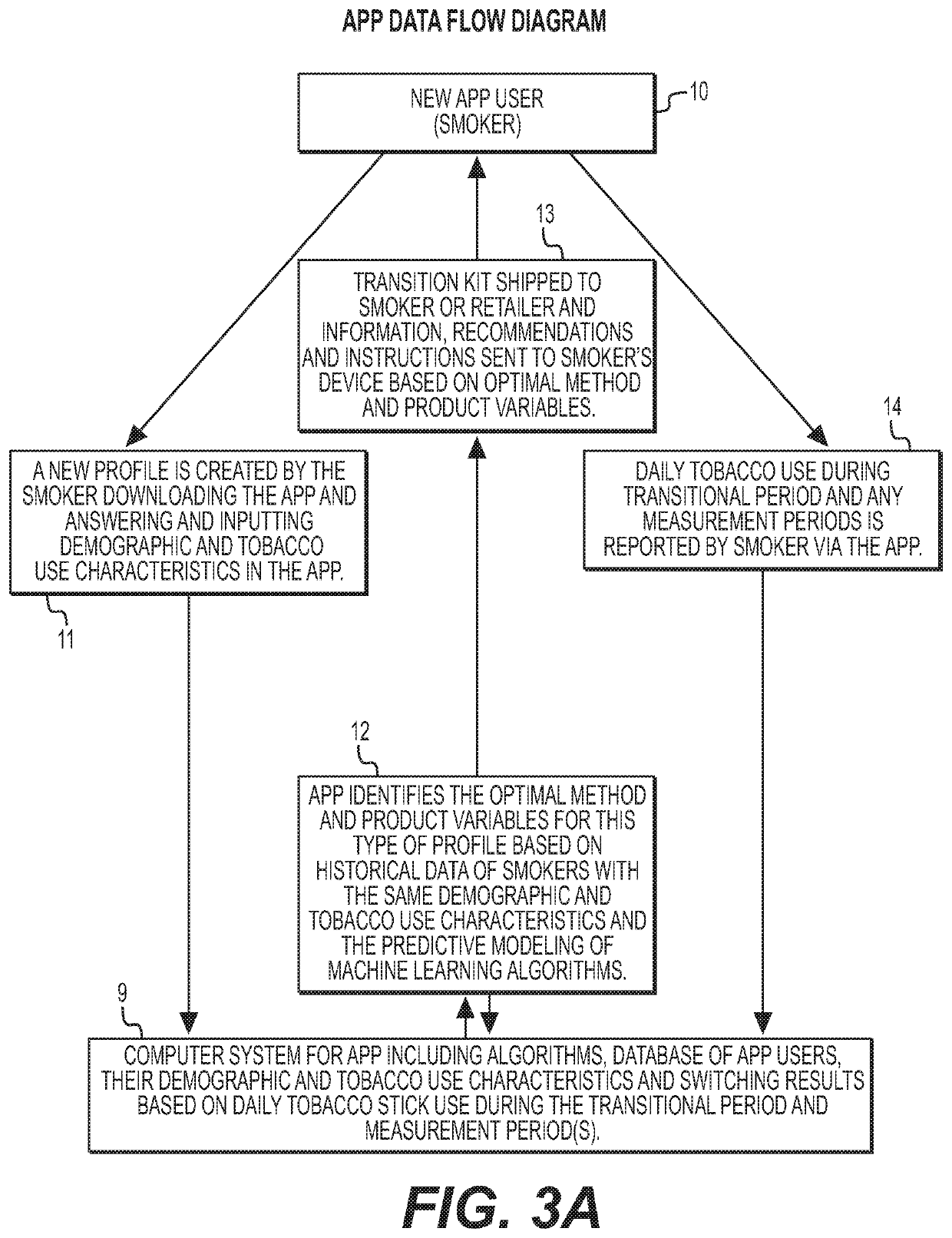
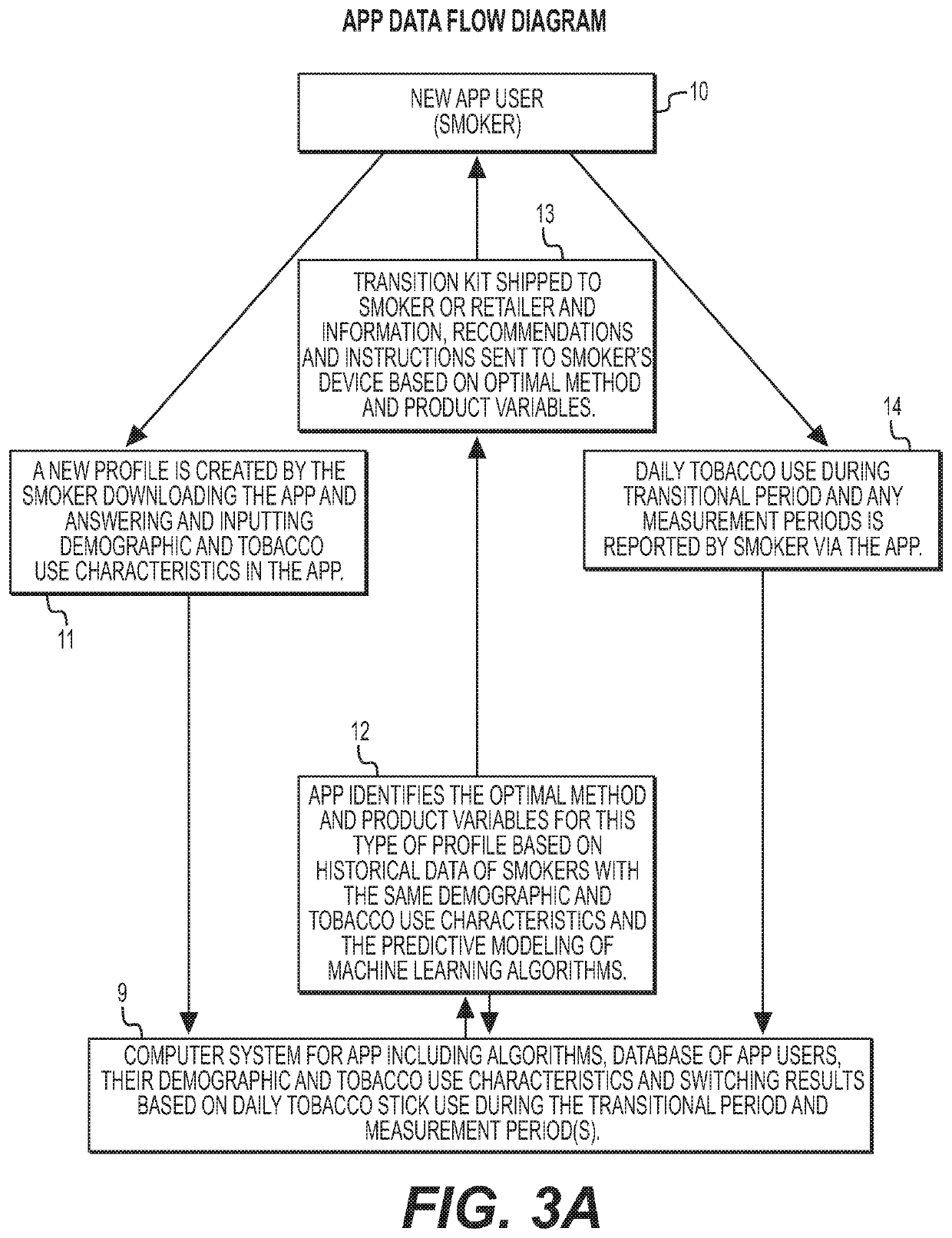
Methods and products to facilitate smokers switching to a tobacco heating product or e-cigarettes
The present disclosure relates to tobacco products, including methodology, devices and compositions, for assisting a smoker to transition from conventional tobacco cigarettes to either e-cigarettes or a tobacco heating device. Provided herein are methodology for switching, along with kits, products and apparatuses. The disclosure also provides methodology for regulating tetrahydrocannabinolic acid synthase expression, for use in producing cigarettes, reconstituted cannabis, and other products having reduced Δ9-tetrahydrocannabinolic acid.
Owner:CABBACIS LLC
Methods for purification of non-psychoactive isoprenoid compounds from biological extracts
ActiveUS10323014B2Improve stabilityPrevent degradationOrganic chemistry methodsCentrifugal force sediment separationPrenylationDissolution
A method for the extraction and isolation of the terpene and isoprenoid compounds from plant material, followed by a centrifugal force induced selective crystallization of isoprenoids resulting in a separation of terpene and isoprenoid fractions. This this method is suitable for the extraction of cannabinoids from Cannabis and the enrichment tetrahydrocannabinolic acid and reduction of tetrahydrocannabinol in an extract. The purity of tetrahydrocannabinolic acid resulting from centrifugal crystallization is such that dissolution and selective recrystallization of tetrahydrocannabinolic acid is possible resulting in >99.9% pure tetrahydrocannabinolic acid, w / w.
Owner:CONCENTRATED CONSULTING GRP LLC
Parenteral formulations
ActiveUS11229612B2Hydroxy compound active ingredientsPharmaceutical delivery mechanismActive agentSurface-active agents
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
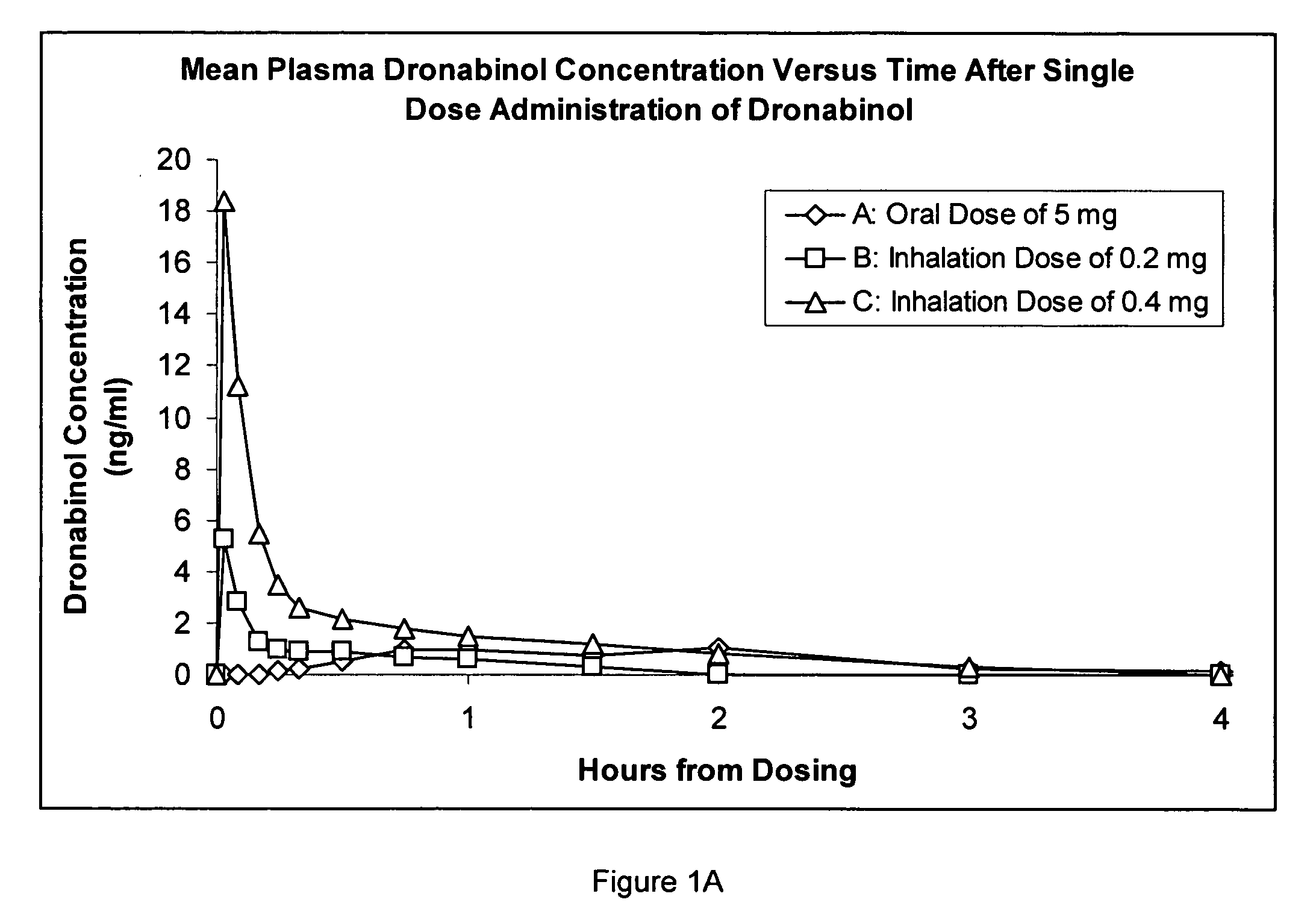
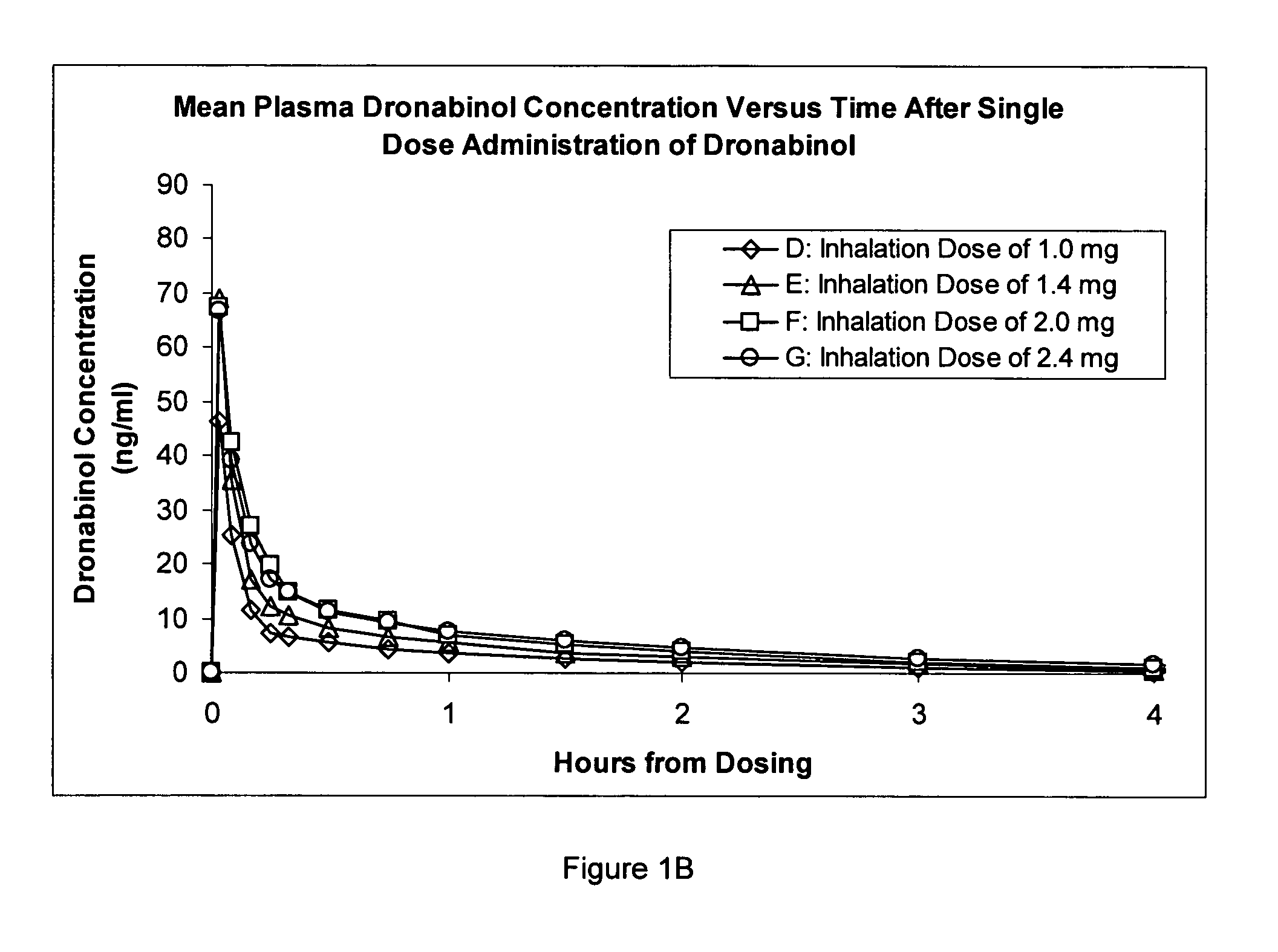
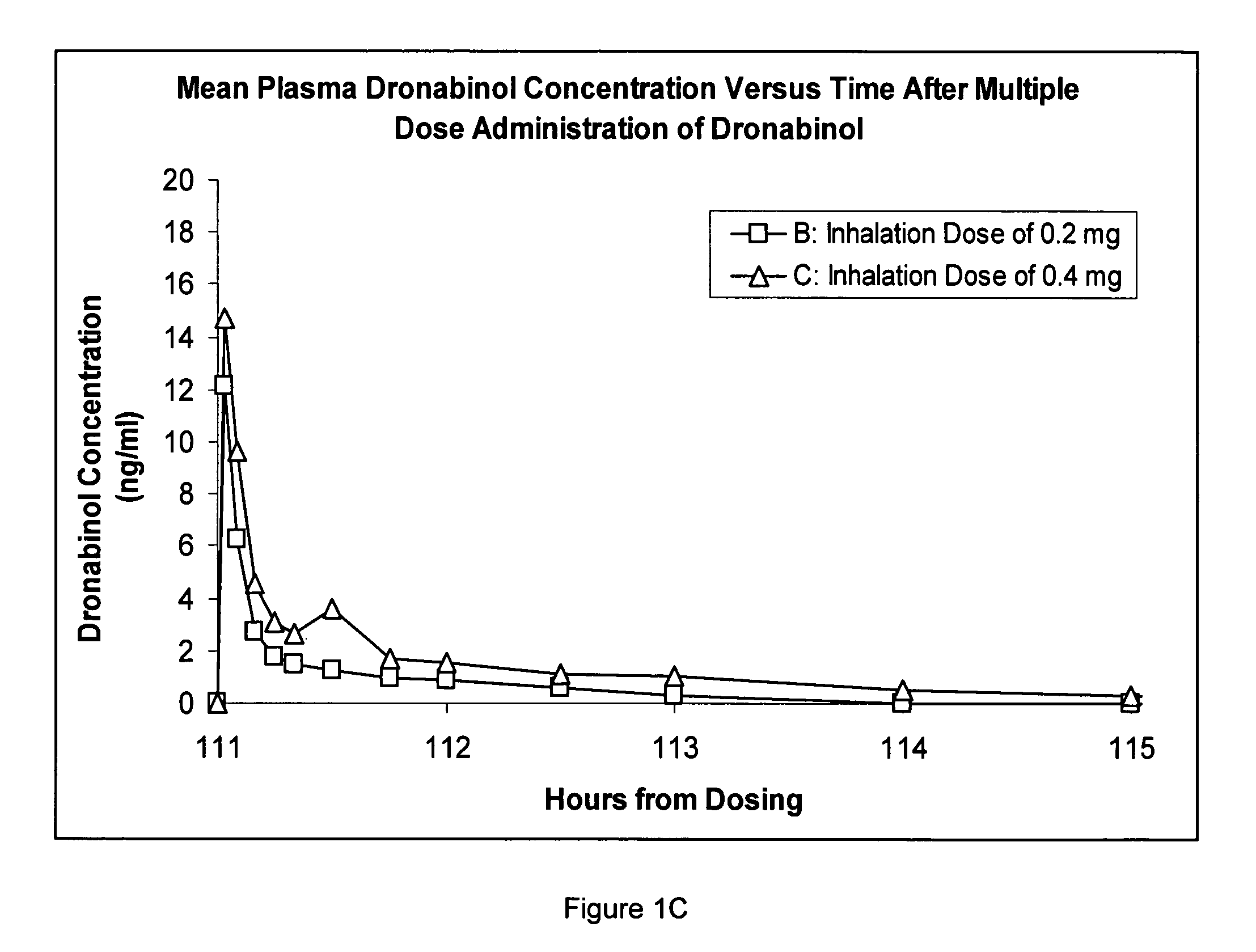
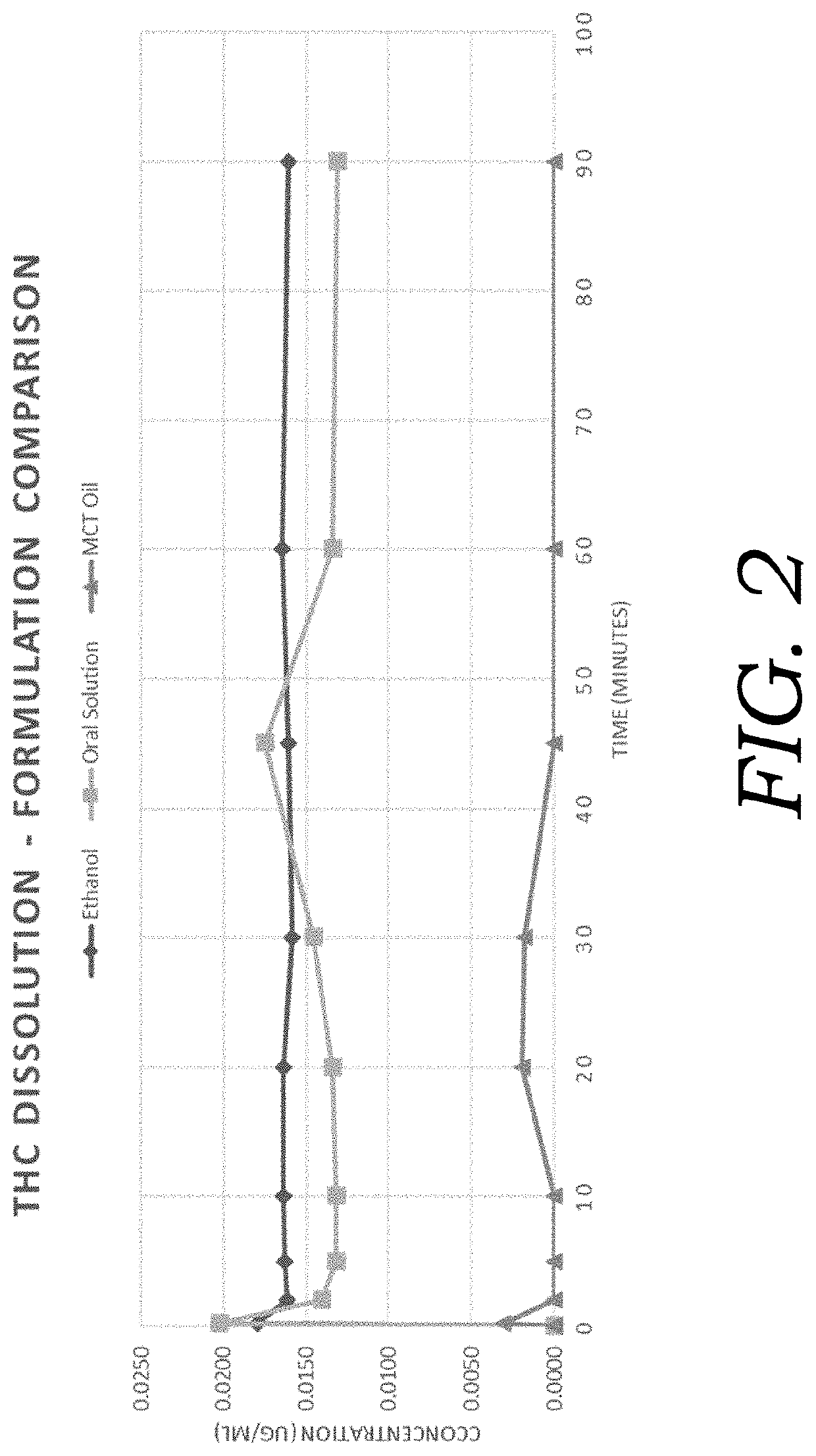
Composition for inhalation comprising delta-9-tetrahydrocannabinol in a semiaqueous solvent
A stable composition for rapid delivery by inhalation to the lungs, and subsequently to the bloodstream, is provided. The composition comprises a therapeutically effective amount of delta-9-tetrahydrocannabinol in a pharmaceutically-acceptable semiaqueous solvent comprising an alcohol, water and a glycol. A composition comprising volumetric ratios of ethanol:water:propylene glycol selected from those in the range of from 10-70:10-30:20-80, respectively, having a combined total of 100 is also provided. A sterile and / or preserved sealed unit-or multi-unit dosage form of delta-9-tetrahydrocannabinol is further provided.
Owner:ALKEM LAB LTD
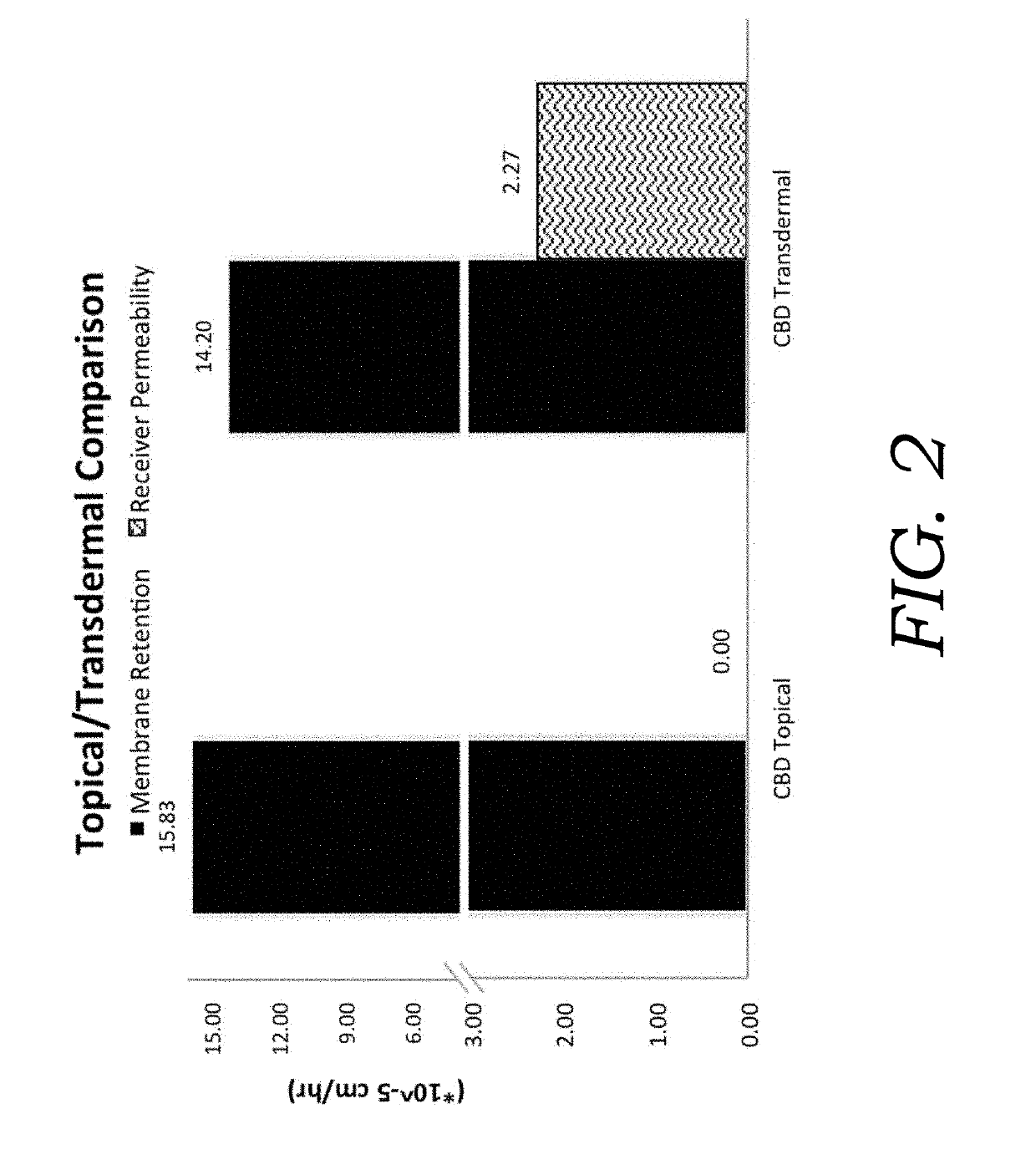
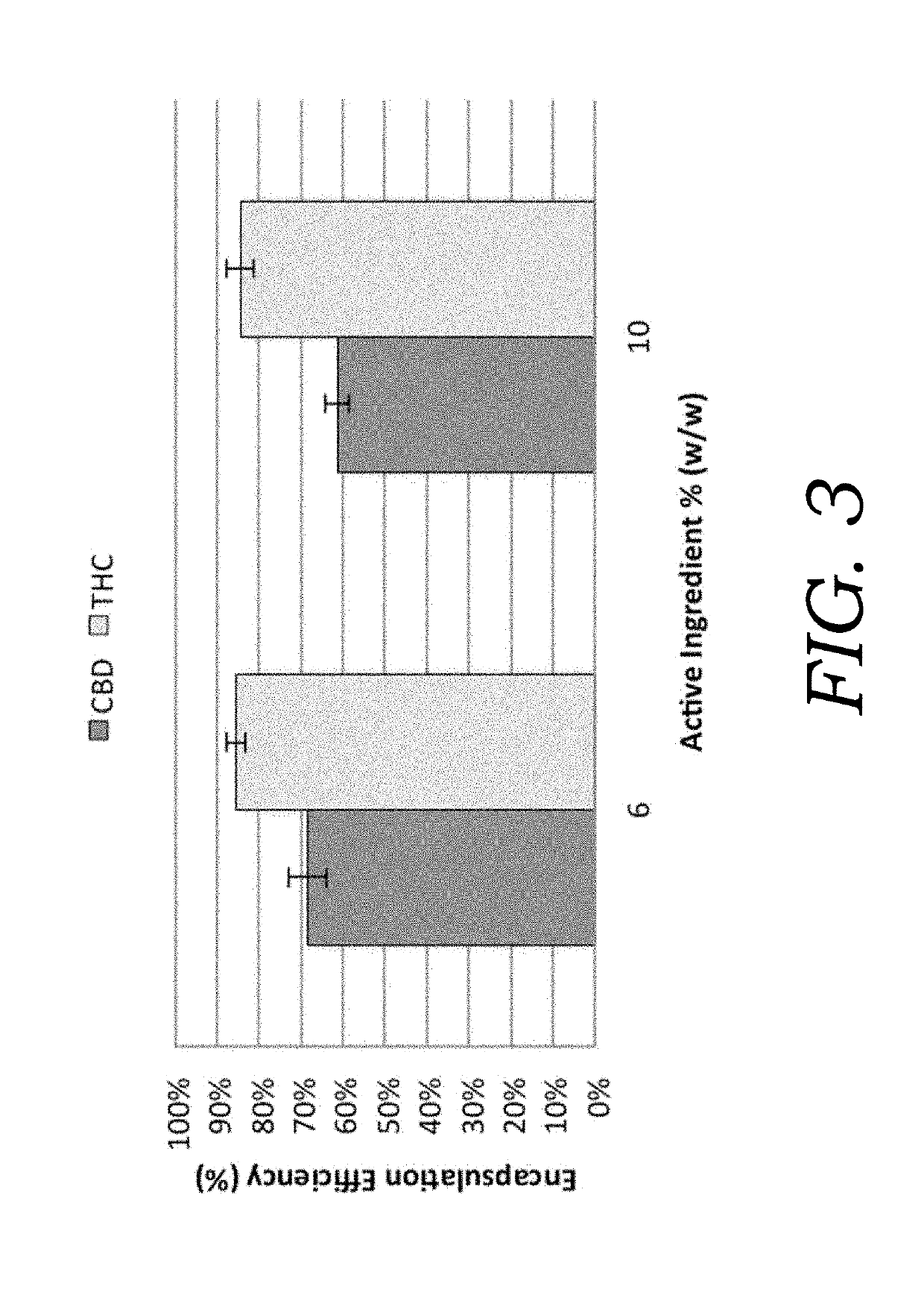
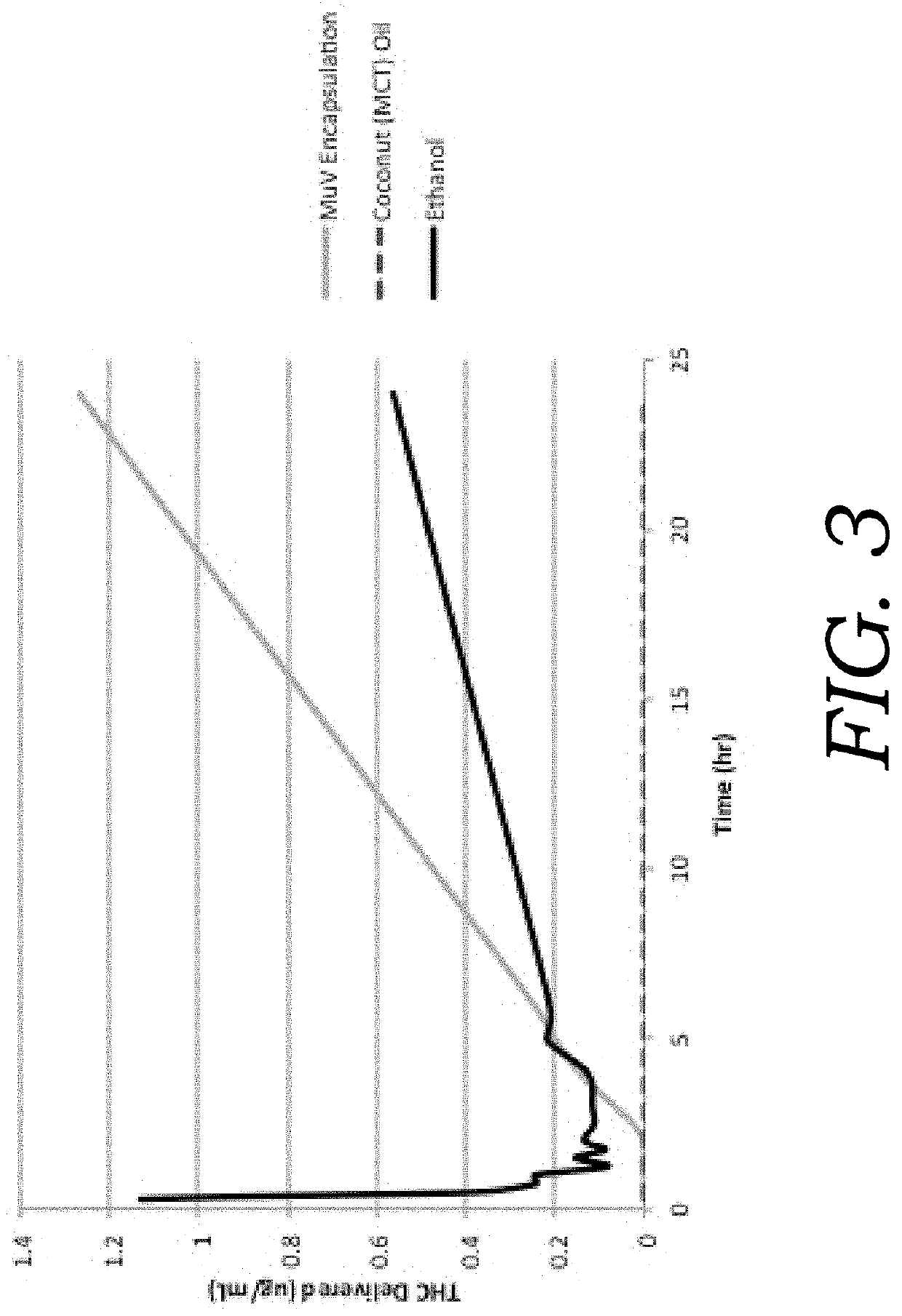
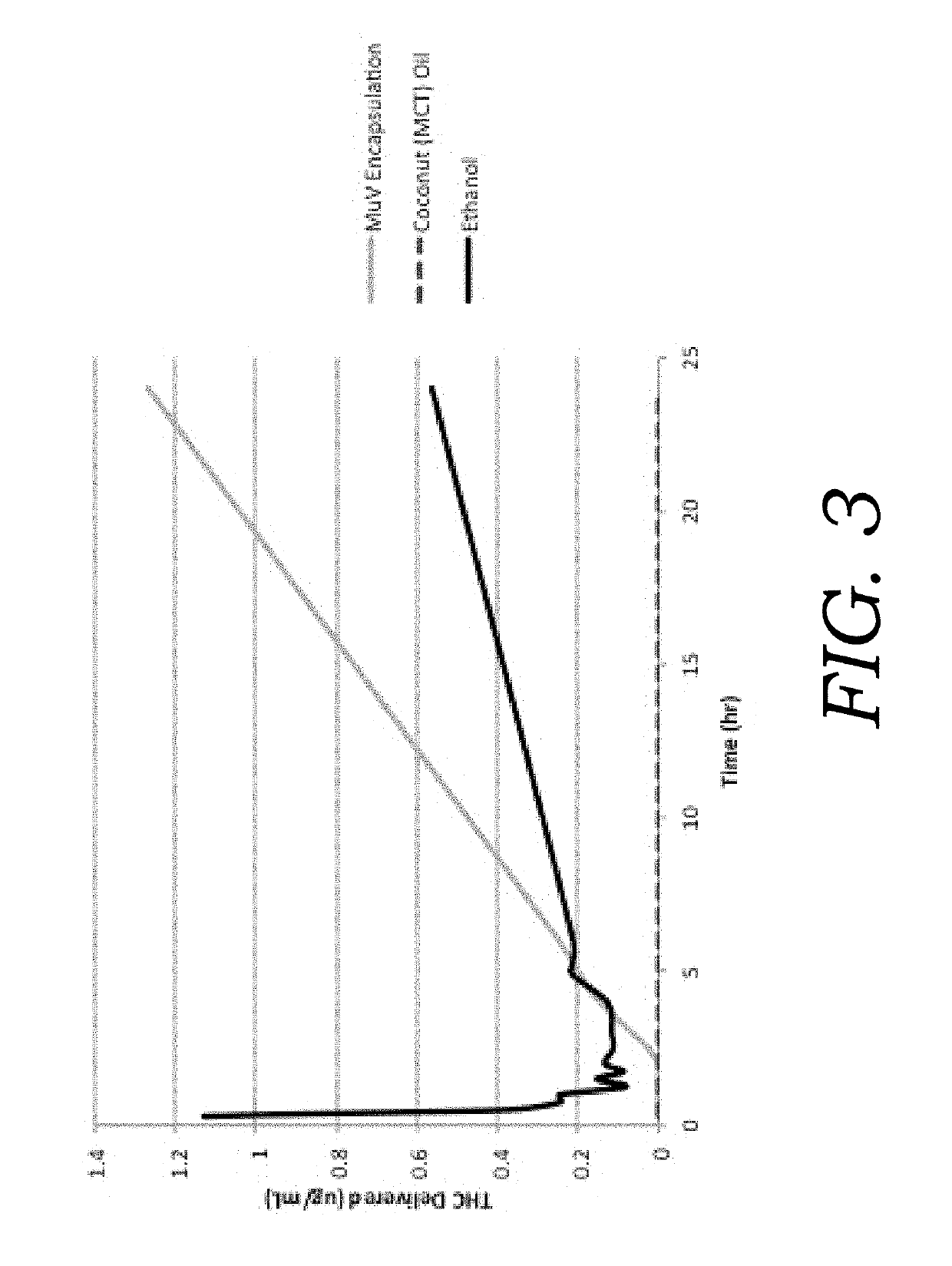
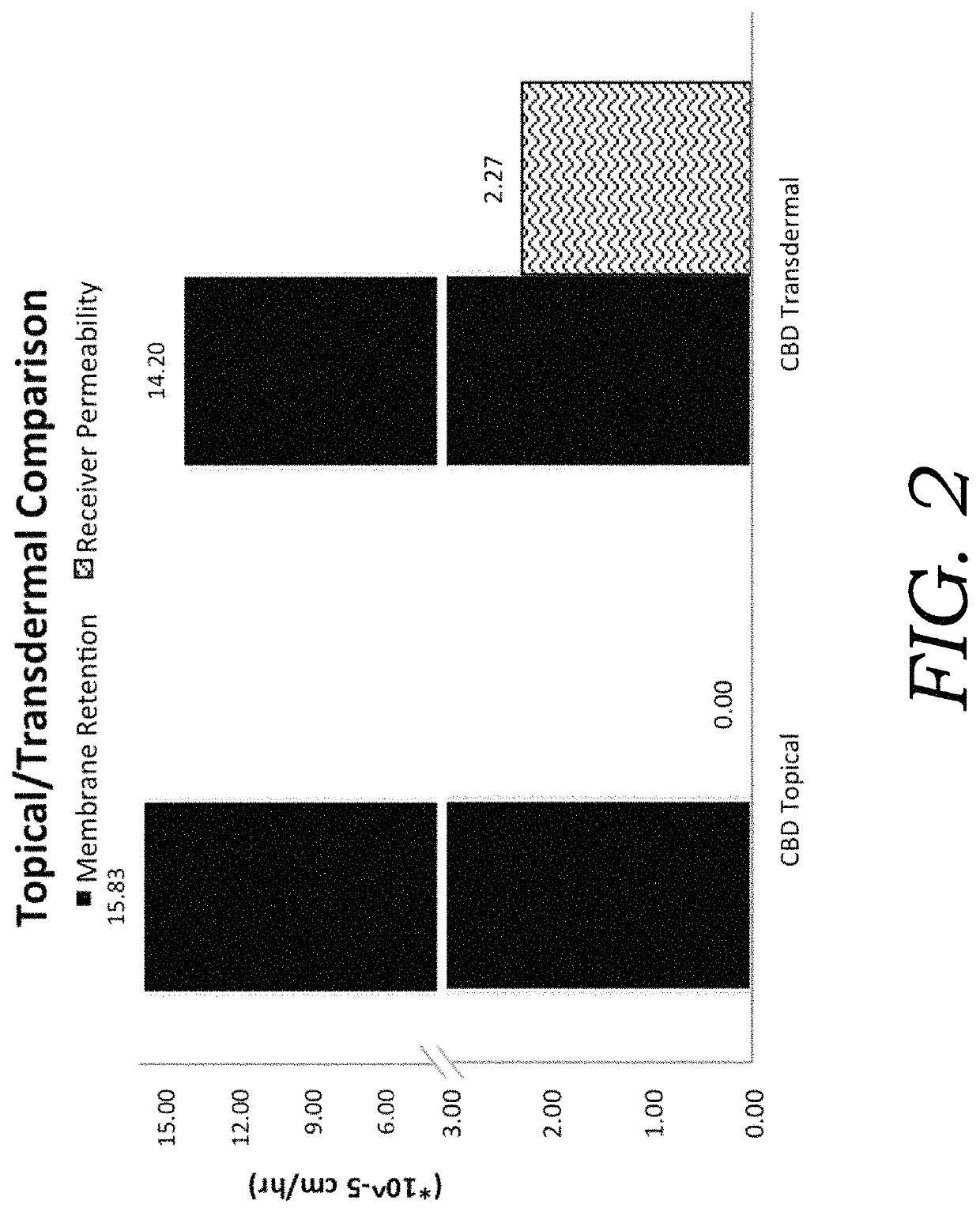
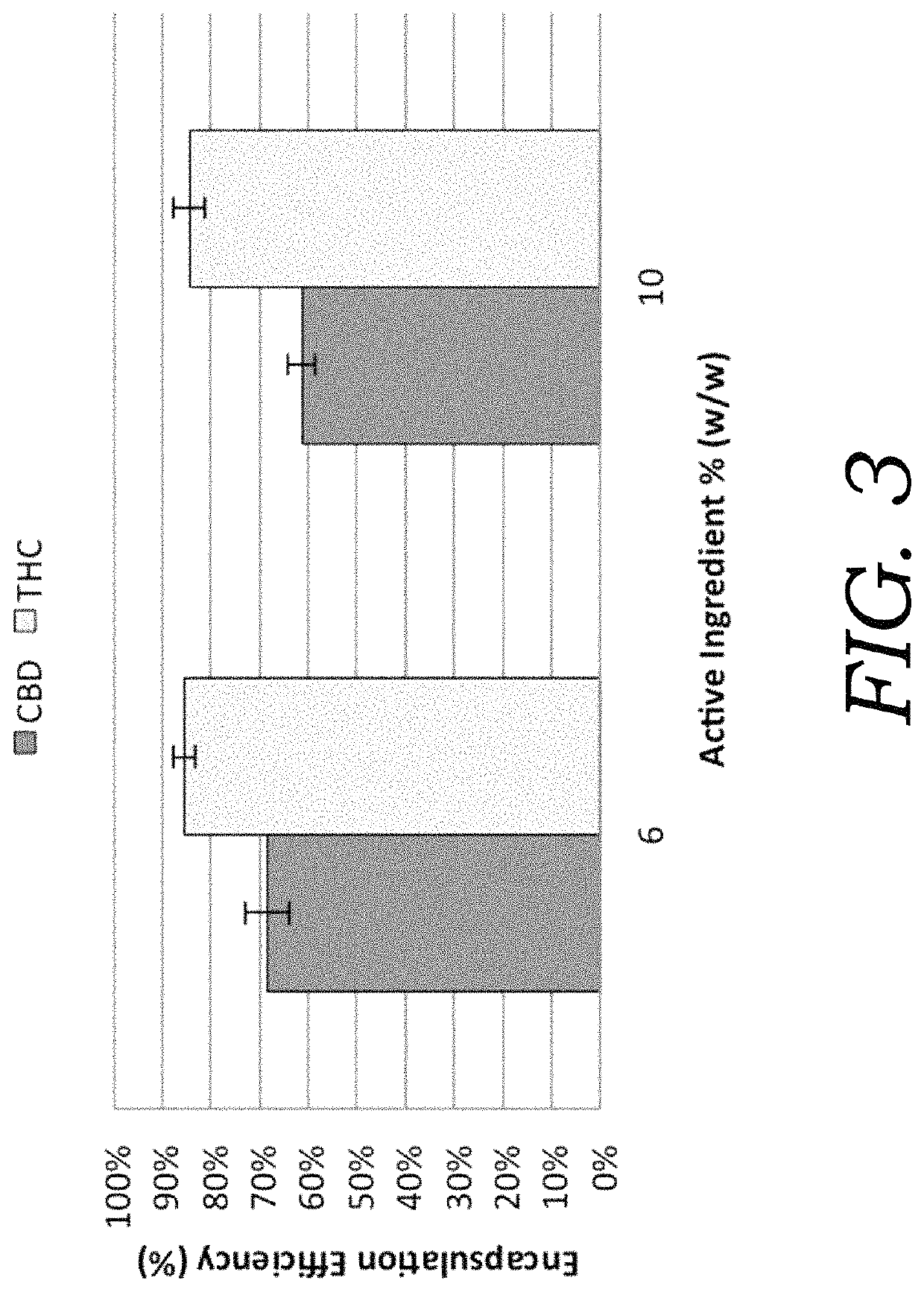
Encapsulated cannabinoid formulations for transdermal delivery
ActiveUS20190216870A1Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. The present invention demonstrates the viability of transdermal delivery with gels and patches for consistent and sustained cannabinoid dosing.
Owner:NUTRAE LLC
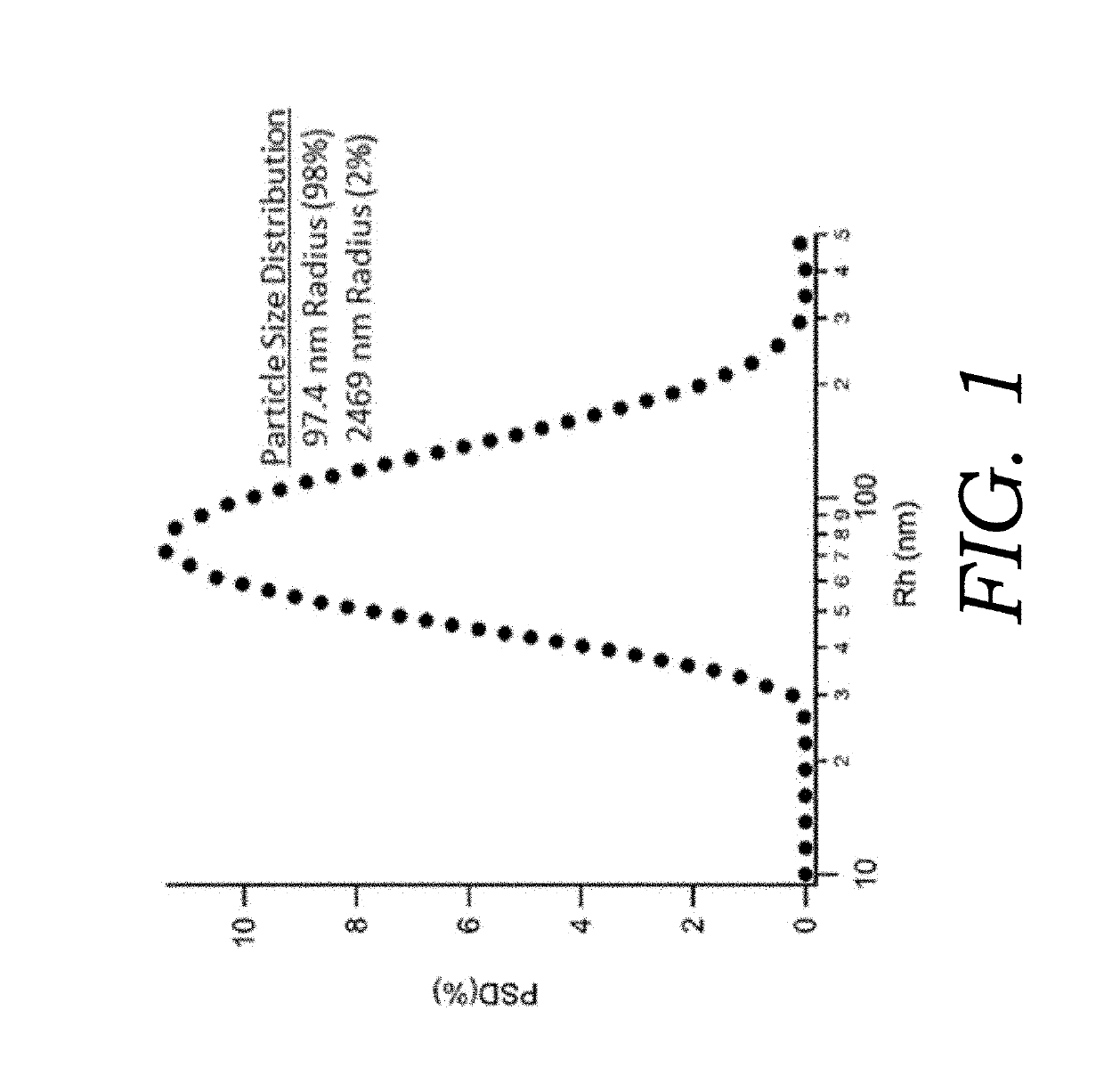
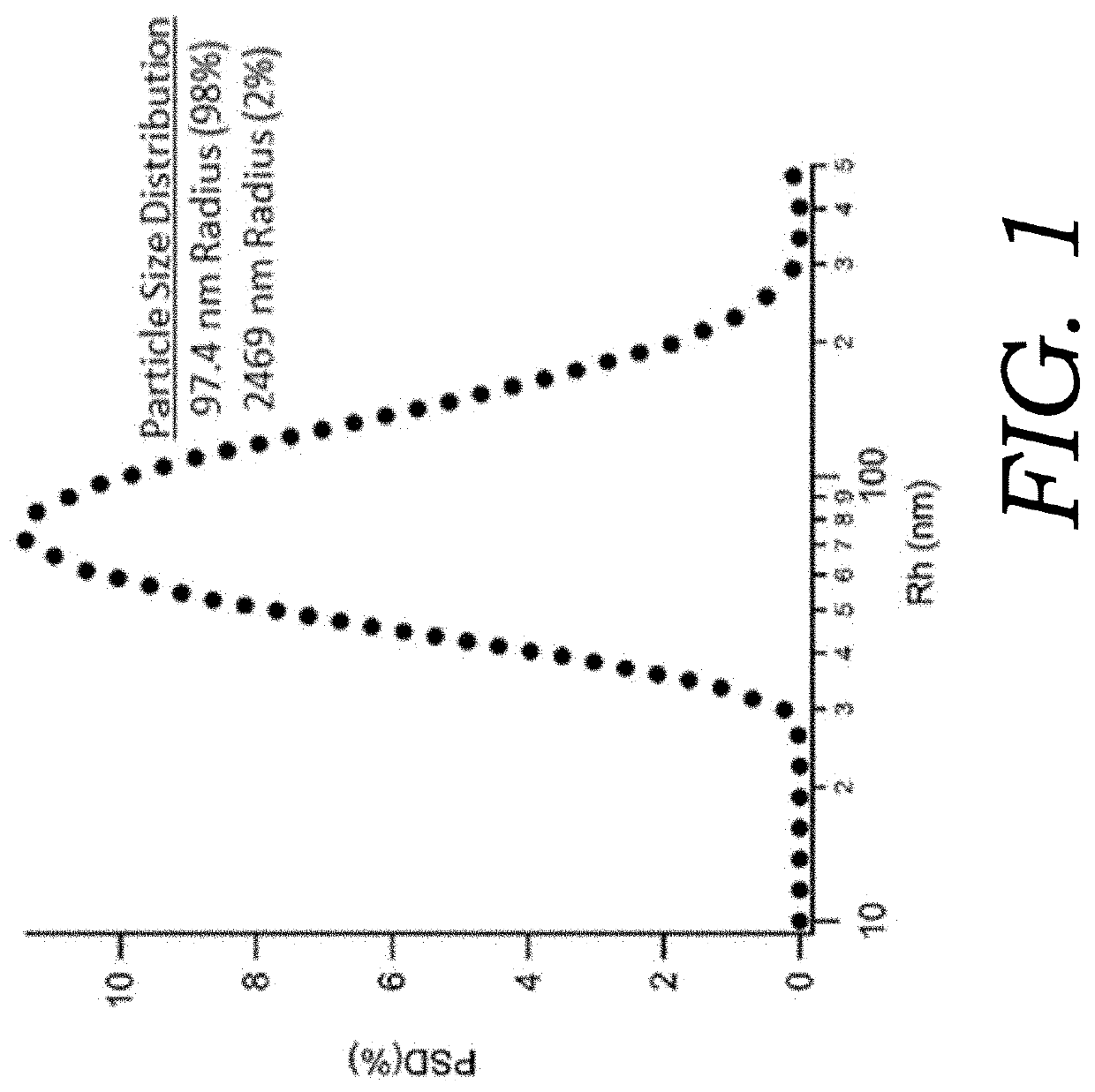
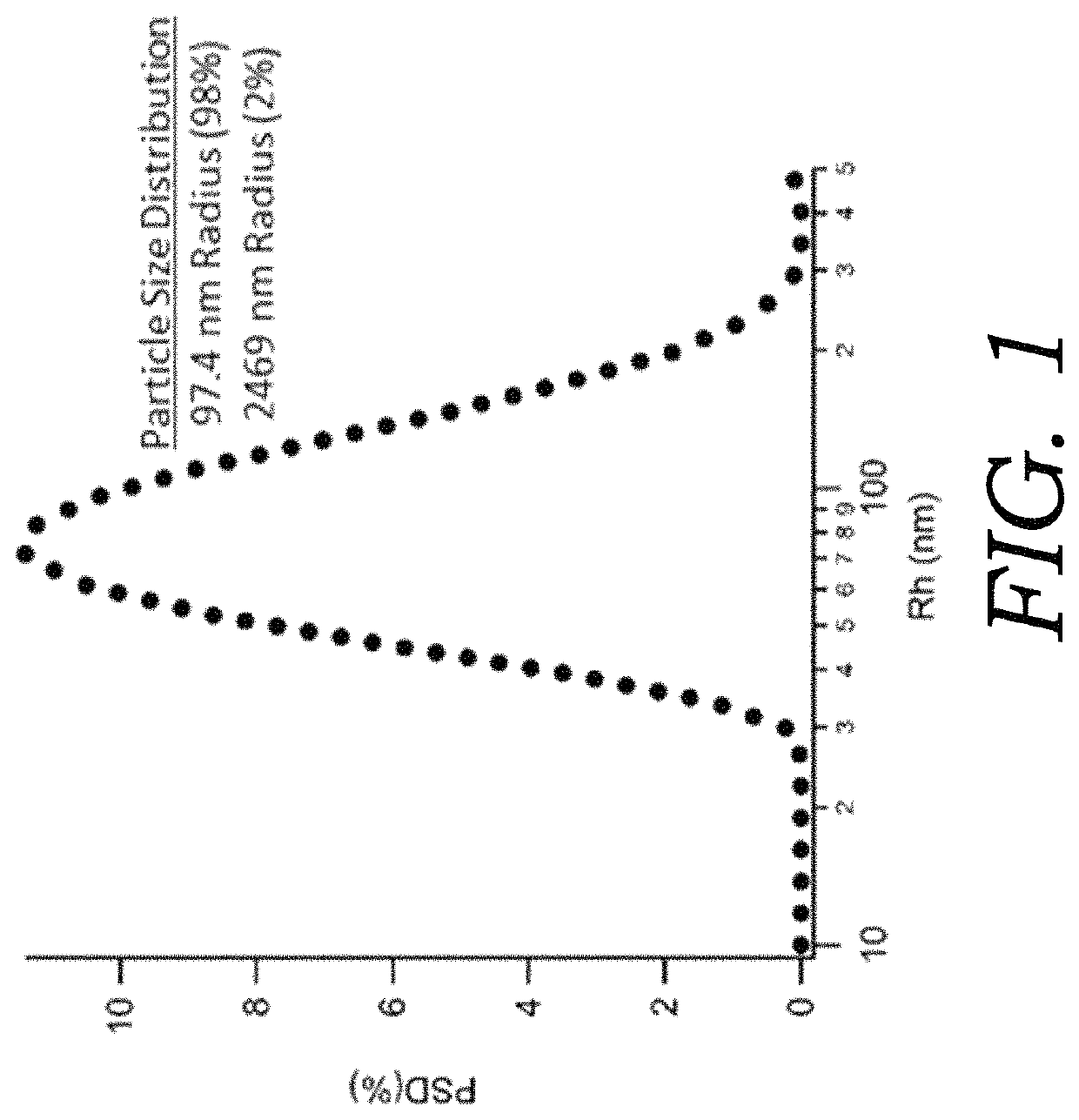
Nanoencapsulated delta-9-tetrahydrocannabinol
Embodiments of the present invention are directed to articles of manufacture and methods of making such articles having utility for the delivery of cannabinoids as a therapeutic. One embodiment of the present invention directed to the article of manufacture comprises a lyophilized particle or sphere having a diameter of about 100 to 500 nanometers having a shell and comprising a biodegradable polymer containing a cannabinoid. A featured cannabinoid is delta-9-tetrahydrocannabinol (delta-9-THC).
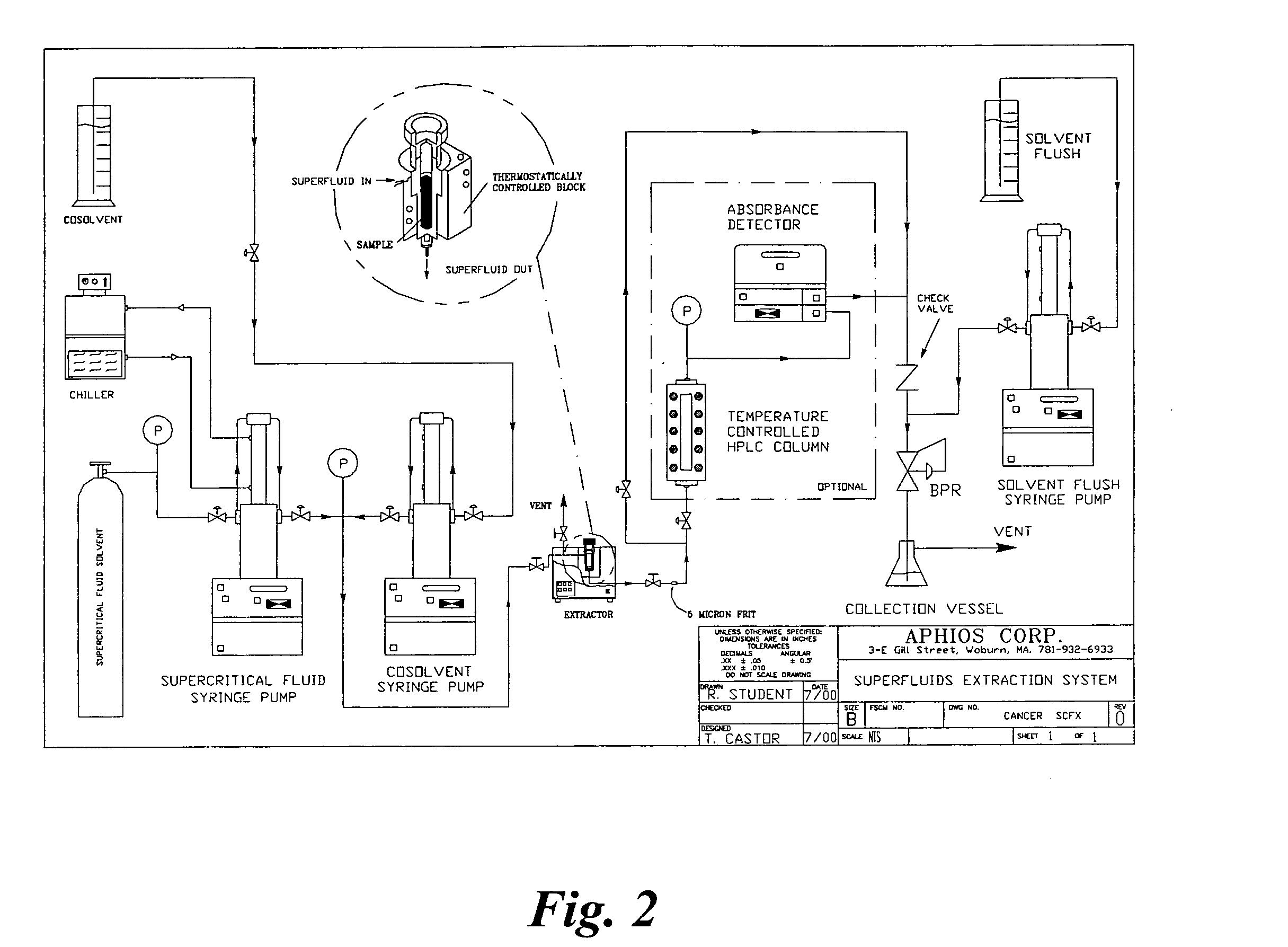
Owner:APHIOS
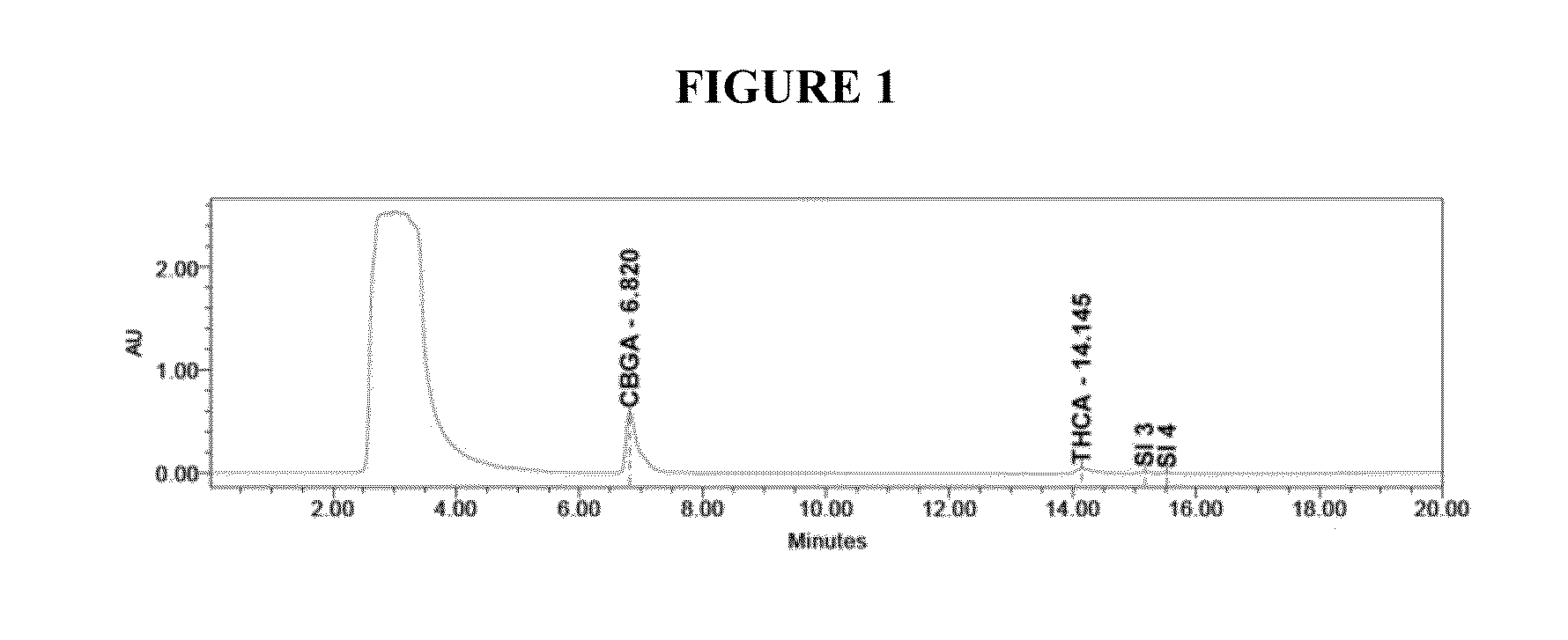
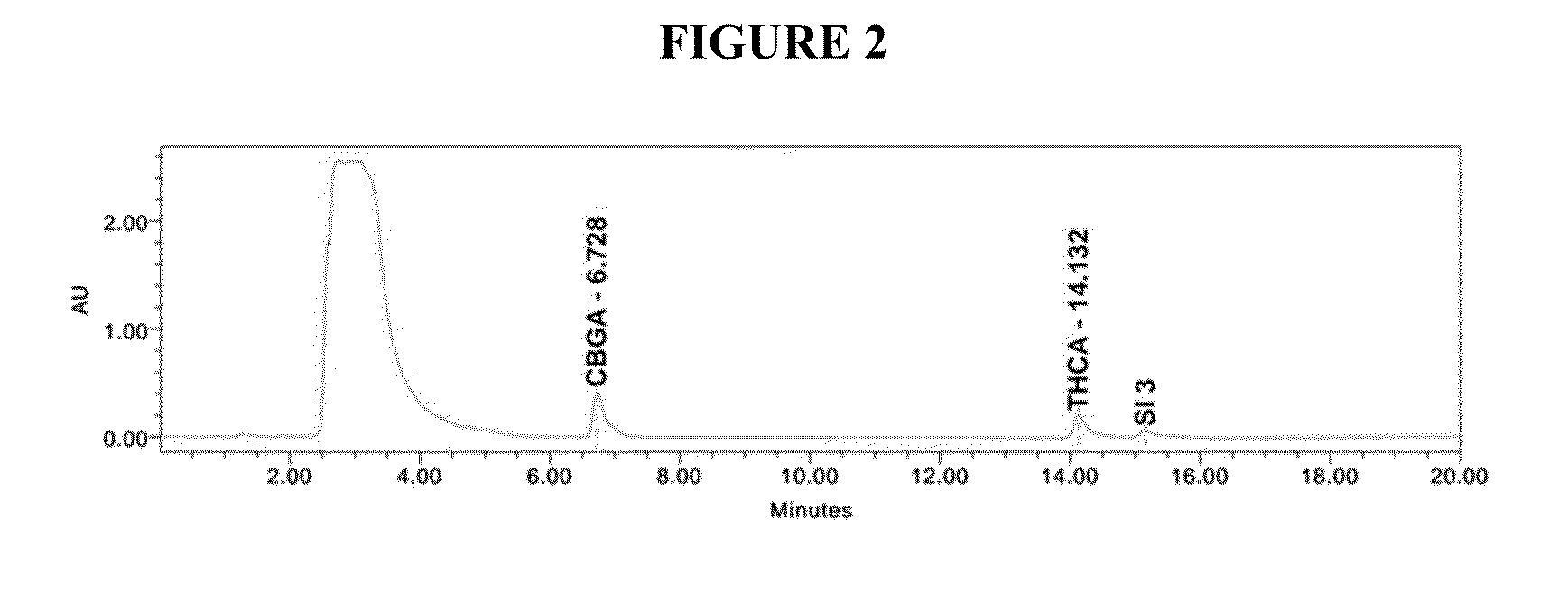
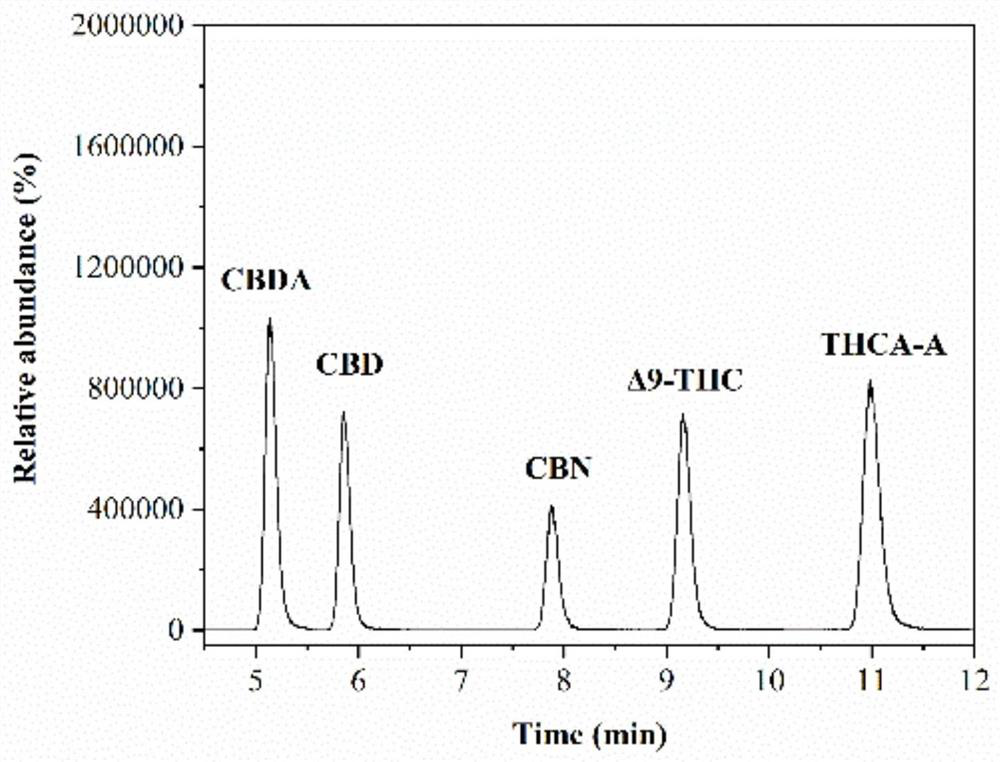
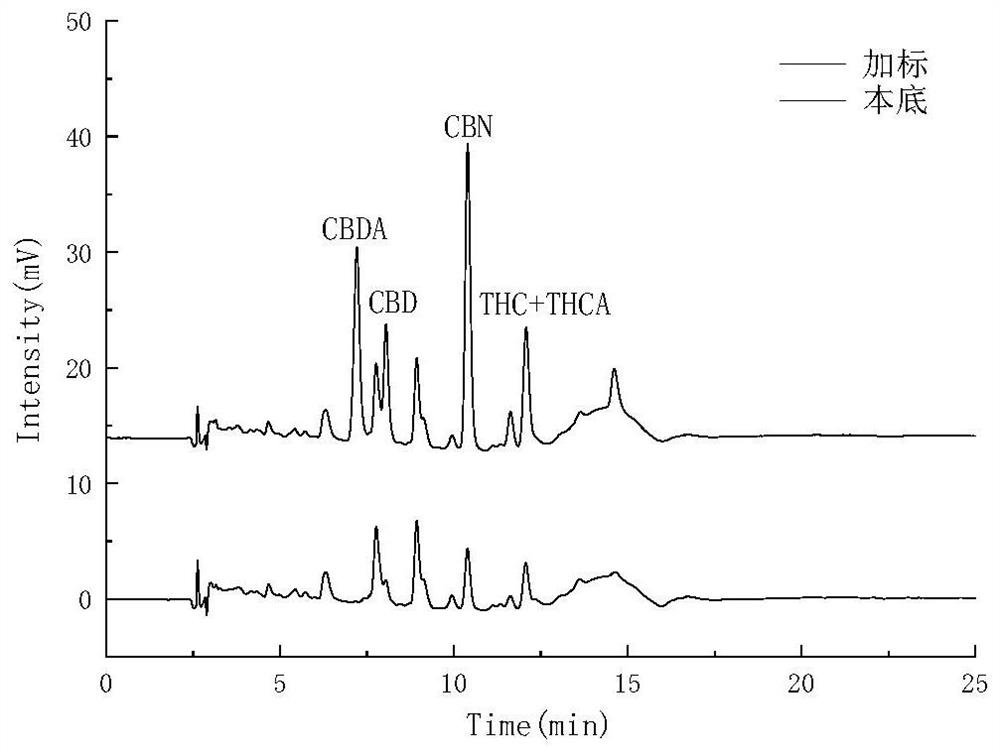
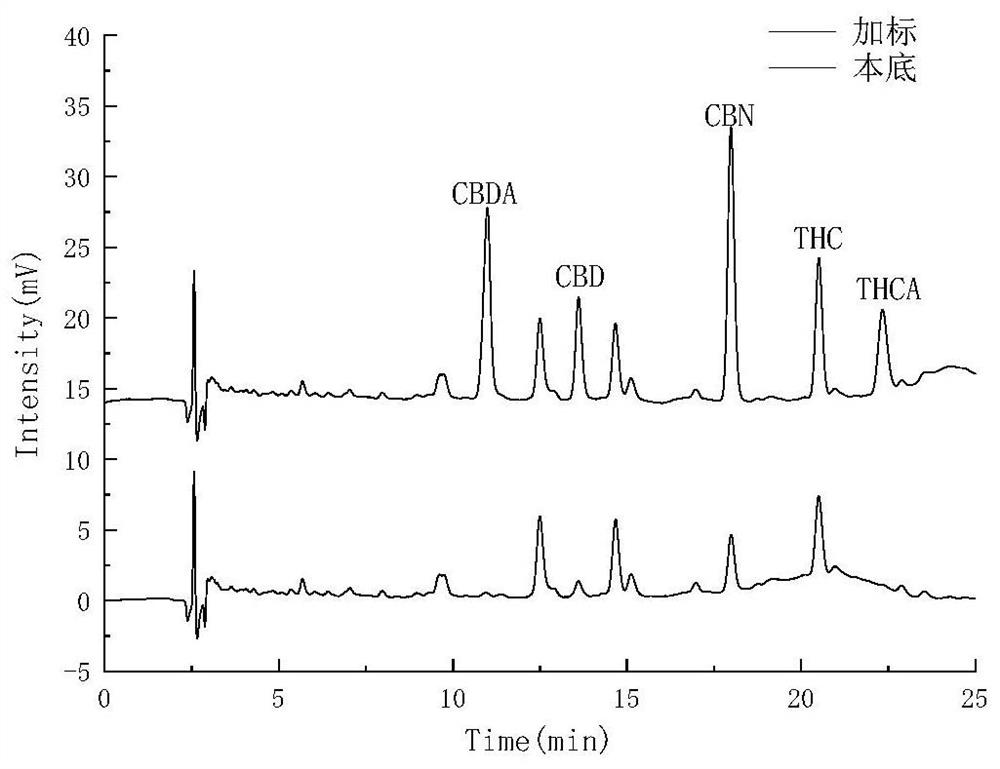
Method for detecting five cannabinol compounds in cannabis oil by utilizing HPLC method
PendingCN112730696ASimple and fast operationEfficient separationComponent separationHplc methodGradient elution
The invention belongs to the field of analytical chemistry, and relates to a method for detecting five cannabinol compounds in cannabis oil by high performance liquid chromatography (HPLC). According to the method, a C18 chromatographic column is adopted, ultrapure water is used as a mobile phase A, acetonitrile is used as a mobile phase B for gradient elution, and a sample enters a detector for detection. The cannabinol compounds are delta 9-tetrahydrocannabinol, cannabidiol, cannabinol, tetrahydrocannabinolic acid and cannabidiolic acid. The technical scheme provided by the invention can lay a methodological foundation for the establishment of related detection method standards, and has important meanings for preventing cannabis oil with overproof cannabinol content from flowing into the market and promoting the effective supervision of industrial cannabis food at home and abroad.
Owner:CHINA NAT INST OF STANDARDIZATION
Nanoencapsulated delta-9-tetrahydrocannabinol
ActiveUS20120052119A1Stable and bioavailableBiocideNervous disorderDelta-9-tetrahydrocannabinolMedicine
Embodiments of the present invention are directed to articles of manufacture and methods of making such articles having utility for the delivery of cannabinoids as a therapeutic. One embodiment of the present invention directed to the article of manufacture comprises a lyophilized particle or sphere having a diameter of about 100 to 500 nanometers having a shell and comprising a biodegradeable polymer containing a cannabinoid. A featured cannabinoid is delta-9-tetrahydrocannabinol (delta-9-THC).
Owner:APHIOS
Methods and products to facilitate smokers switching to a tobacco heating product or e-cigarettes
The present disclosure relates to tobacco products, including methodology, devices and compositions, for assisting a smoker to transition from conventional tobacco cigarettes to either e-cigarettes or a tobacco heating device. Provided herein are methodology for switching, along with kits, products and apparatuses. The disclosure also provides methodology for regulating tetrahydrocannabinolic acid synthase expression, for use in producing cigarettes, reconstituted cannabis, and other products having reduced Δ9-tetrahydrocannabinolic acid.
Owner:CABBACIS LLC
Method for simultaneously detecting five cannabinol compounds by using HPLC-MS/MS
PendingCN112730697AReduce difficultyHigh detection sensitivityComponent separationCannabielsoinGradient elution
The invention belongs to the field of analytical chemistry, and particularly relates to a method for simultaneously detecting five cannabinol compounds by using a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). According to the method, a special chromatographic column for cannabinol is adopted, a formic acid aqueous solution is used as a mobile phase A, acetonitrile is used as a mobile phase B for gradient elution, and a sample enters a detector for detection. The cannabinol compound is tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabinol (CBN), cannabidiol (CBD) and delta9-tetrahydrocannabinol (THC). The method is high in detection sensitivity, good in recovery rate and easy to operate.
Owner:CHINA NAT INST OF STANDARDIZATION
Encapsulated cannabinoid formulations for oral delivery
ActiveUS10709747B2Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentCannabichromenePharmaceutical Substances
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as Cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. These preparations demonstrates the viability of sublingual, buccal, or oral delivery using an aqueous-based encapsulation method, including as a beverage or drink.
Owner:NUTRAE LLC
Cannabis plants having modified expression of THCA synthase
The invention relates to novel genetically modified plants and methods or materials, such as polynucleotides, expression cassettes, or vectors for producing the same. Moreover, the invention relates to altering the content of cannabinoids in plants and to medical compositions derived from such plants. In particular embodiments, the present invention relates to cannabis plants having modified expression of tetrahydrocannabinolic acid (THCA) synthase and methods of modifying the amount of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in cannabis by modifying expression of THCA synthase.
Owner:CANOPY GROWTH CORP
Encapsulated cannabinoid formulations for oral delivery
ActiveUS20190216869A1Improve bioavailabilitySmall particle sizePowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as Cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. These preparations demonstrates the viability of sublingual, buccal, or oral delivery using an aqueous-based encapsulation method, including as a beverage or drink.
Owner:NUTRAE LLC
Encapsulated cannabinoid formulations for transdermal delivery
ActiveUS10709748B2Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. The present invention demonstrates the viability of transdermal delivery with gels and patches for consistent and sustained cannabinoid dosing.
Owner:NUTRAE LLC
Microorganisms and Methods for the Fermentation of Cannabinoids
PendingUS20210348137A1Improve efficiencyOrganic active ingredientsCarbon-sulfur lyasesCyclaseBeta oxidation
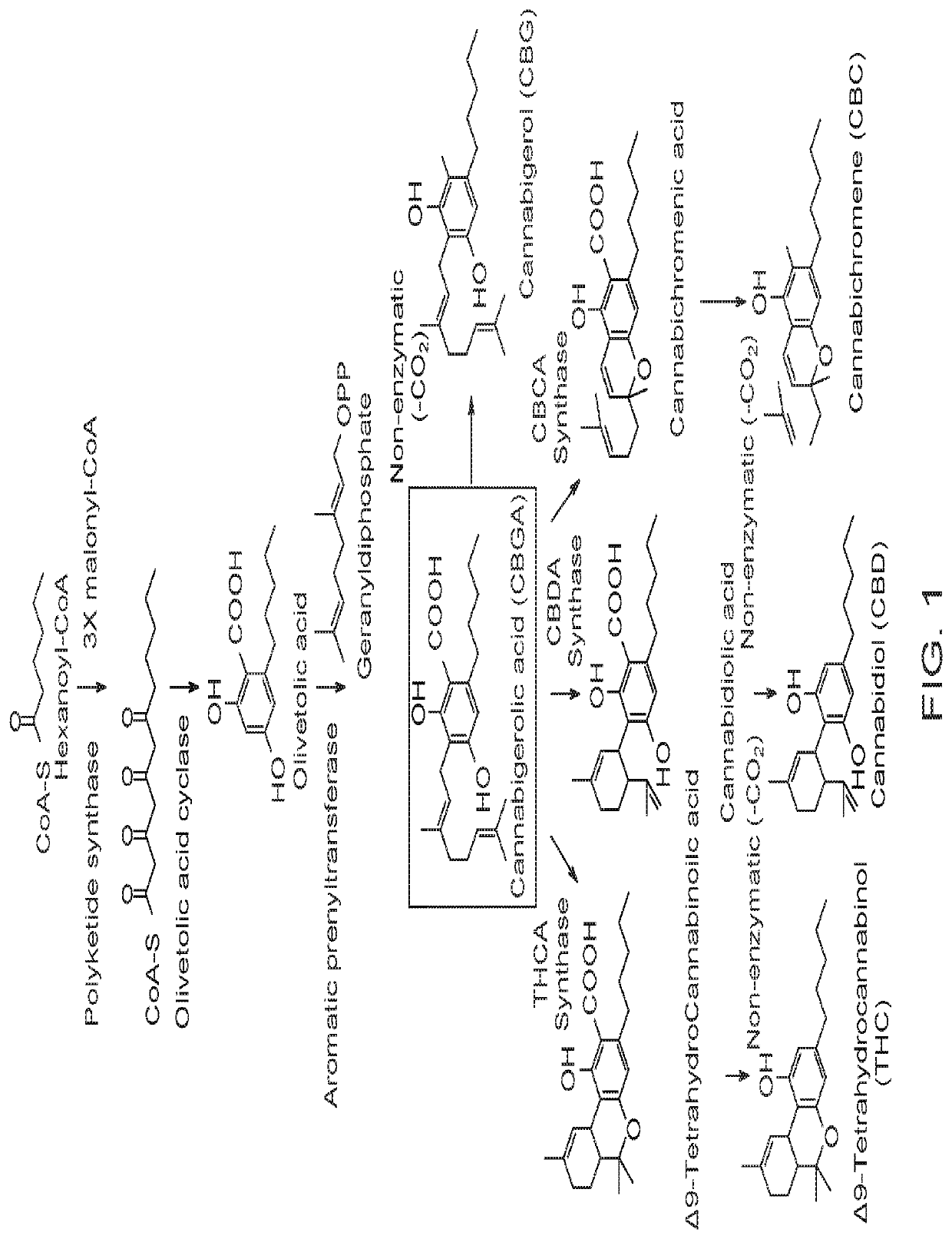
Disclosed herein are microorganism and methods that can be used for the synthesis of cannabigerolic acid (CBGA) and cannabinoids. The methods disclosed can be used to produce CBGA, Δ9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabichromenic acid (CBCA), Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabichromene (CBC). Enzymes useful for the synthesis of CBGA and cannabinoids, include but are not limited to acyl activating enzyme (AAE1), polyketide synthase (PKS), olivetolic acid cyclase (OAC), prenyltransferase (PT), THCA synthase (THCAS), CBDA synthase (CBDAS), CBCA synthase (CBCAS), HMG-Co reductase (HMG1), and / or farnesyl pyrophosphate synthetase (ERG20). The microorganisms can also have one or more genes disrupted, such as gene that that controls beta oxidation of long chain fatty acids.
Owner:ELESZTO GENETIKA INC
Compositions and methods for treating cancer
ActiveUS20200030282A1Hydrocarbon active ingredientsHydroxy compound active ingredientsDiseaseBULK ACTIVE INGREDIENT
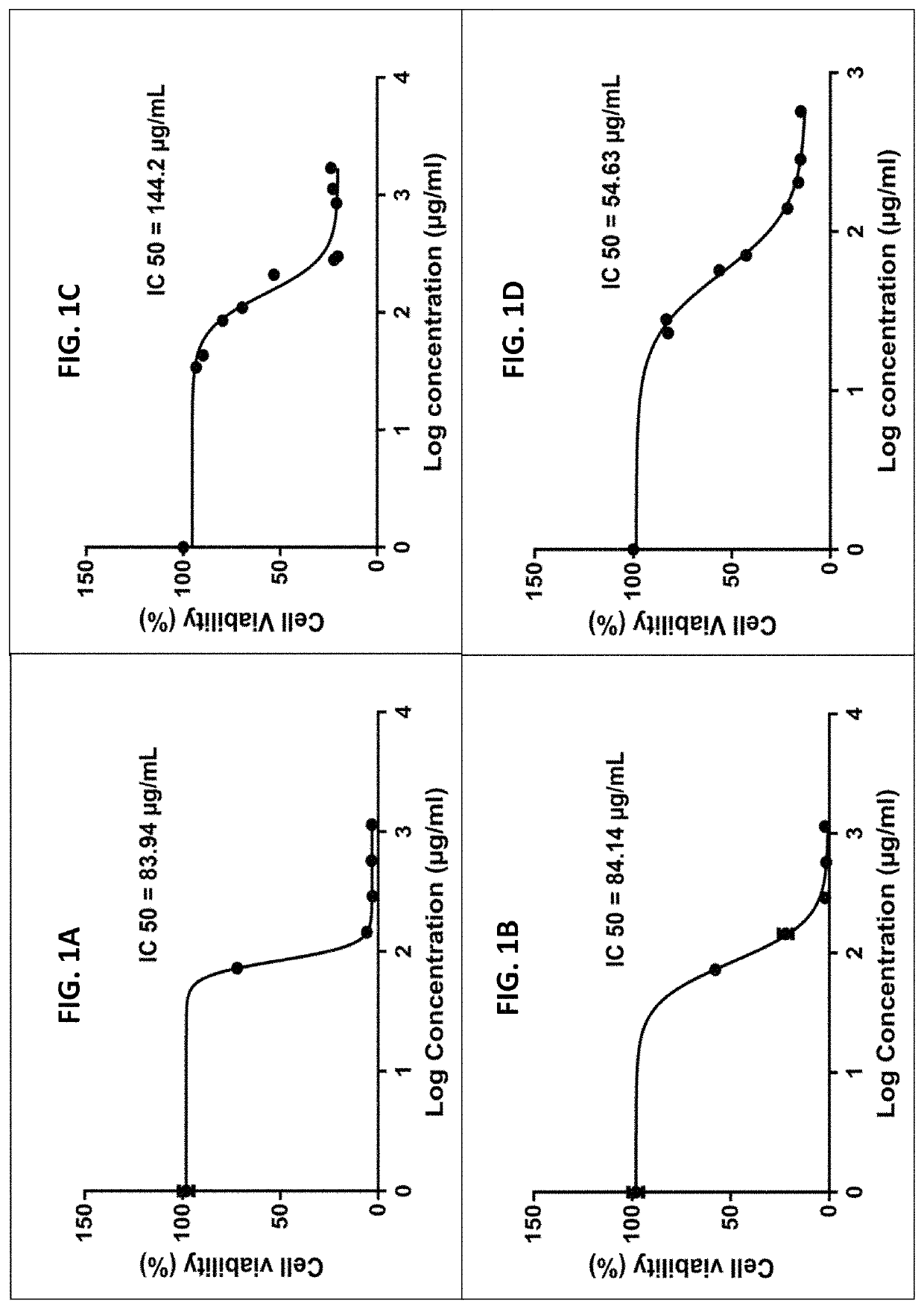
A method of treating a malignant disease in a subject in need thereof is provided. The method comprising administering to the subject a therapeutically effective amount of a first liquid chromatography fraction of a Cannabis extract comprising at least 75% tetrahydrocannabinolic acid (THCA), and a therapeutically effective amount of a second liquid chromatography fraction of a Cannabis extract comprising at least 75% cannabigerolic acid (CBGA), wherein the first liquid chromatography fraction comprises Cannabis derived active ingredients other than the THCA, and the second liquid chromatography fraction comprises Cannabis derived active ingredients other than the CBGA, thereby treating the malignant disease in the subject.
Owner:MOR RES APPL LTD +1
Compositions and methods for treating inflammatory diseases
PendingUS20200222359A1Lower Level RequirementsReduce expressionHydrocarbon active ingredientsHydroxy compound active ingredientsDiseaseFluid phase
A method of treating an inflammatory disease in a subject in need thereof is provided. The method comprising administering to the subject a therapeutically effective amount of a liquid chromatography fraction of a cannabis extract comprising at least 75% tetrahydrocannabinolic acid (THCA), wherein the fraction comprises cannabis derived active ingredients other than the THCA.
Owner:MOR RES APPL LTD +1
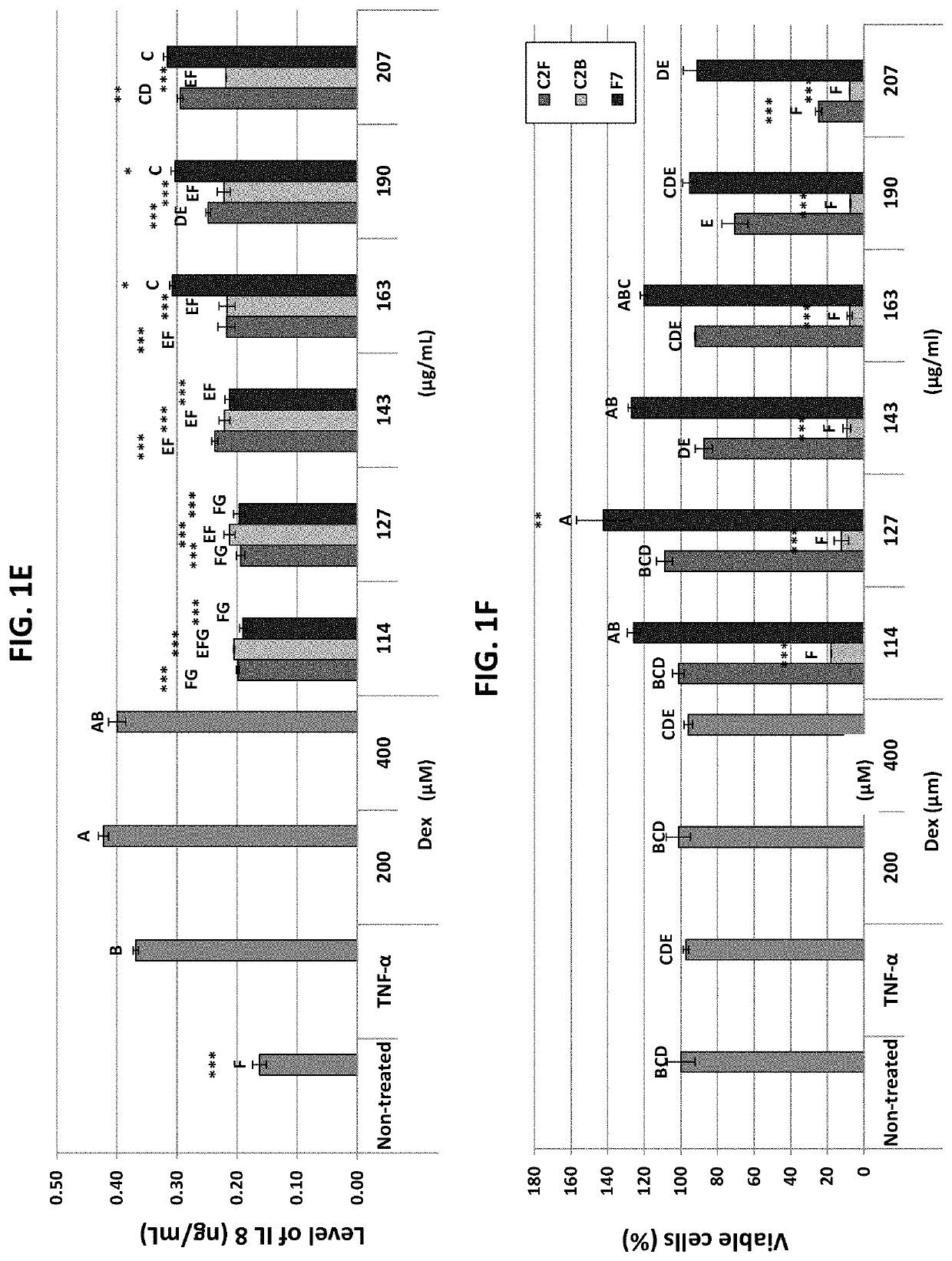
Complete animal food having cannabinoids in trace concentrations to avoid toxicity
ActiveUS20210106556A1Assure food safetyImprove stabilityHydroxy compound active ingredientsAnimal feeding stuffBiotechnologyAnimal food
A nutritionally complete animal food including, vitamins, and minerals to sustain the animal's health and wellness. The one or more ingredients containing cannabinoids added to the nutritionally complete animal food at the time of manufacturing. These cannabinoids are selected from the group consisting of Cannabichromenic acid (CBCA), Cannabidiolic acid (CBDA), Cannabidivarinic acid (CBDVA), Cannabigerolic acid (CBGA), Cannabinolic acid (CBNA), Δ9-Tetrahydrocannabinolic acid (THCA),Tetrahydrocannabinolic acid (THCVA) and combinations thereof. The concentrations of each of the cannabinoids are each less than 100 parts per million (ppm) in the at least one ingredient. In an alternate embodiment, the one or more ingredients have cannabinoids include in an aggregate concentration of less than 100 parts per million (ppm).
Owner:HIGH PLAINS NUTRITION LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com