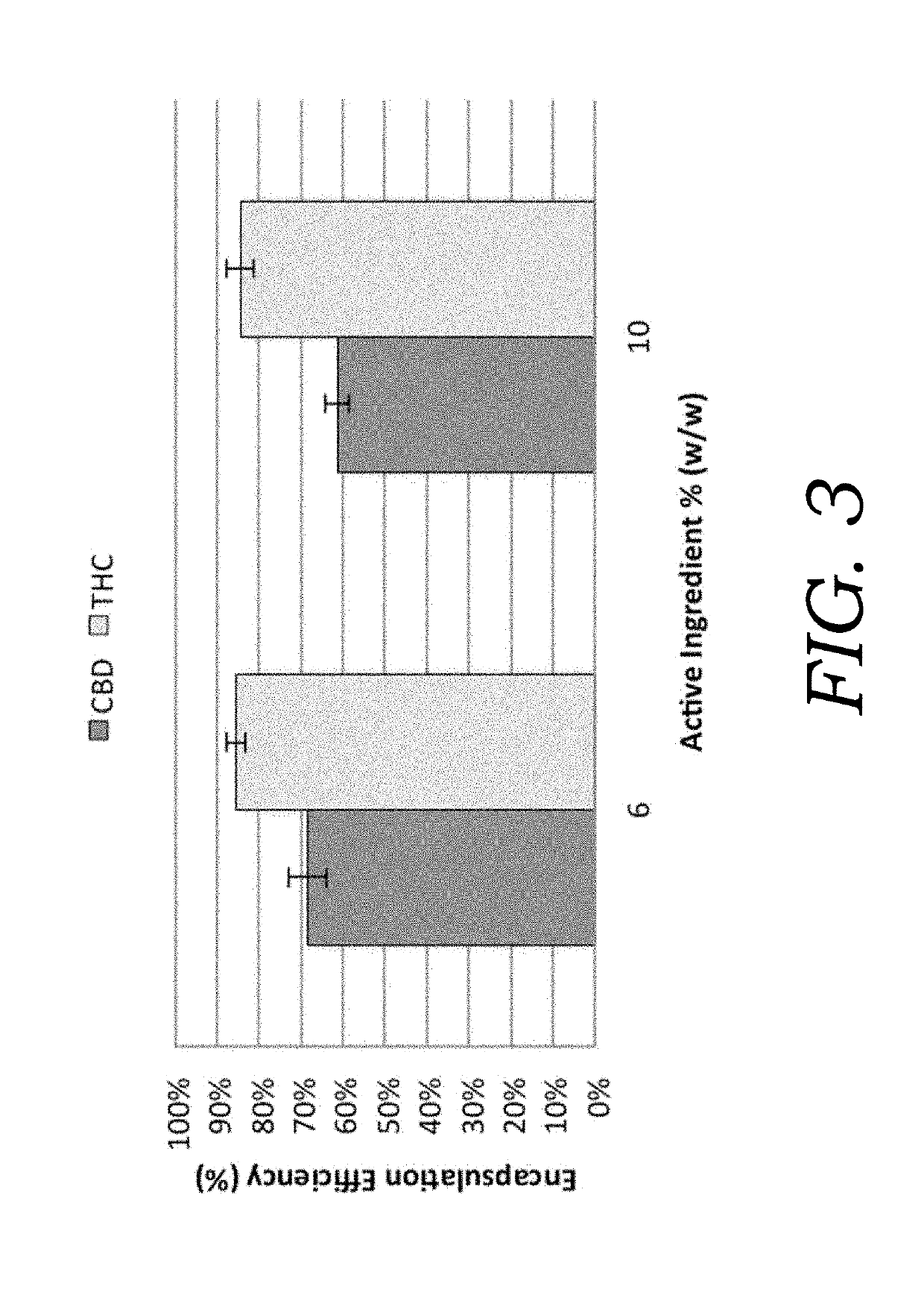
Patents
Literature
64 results about "Cannabigerol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
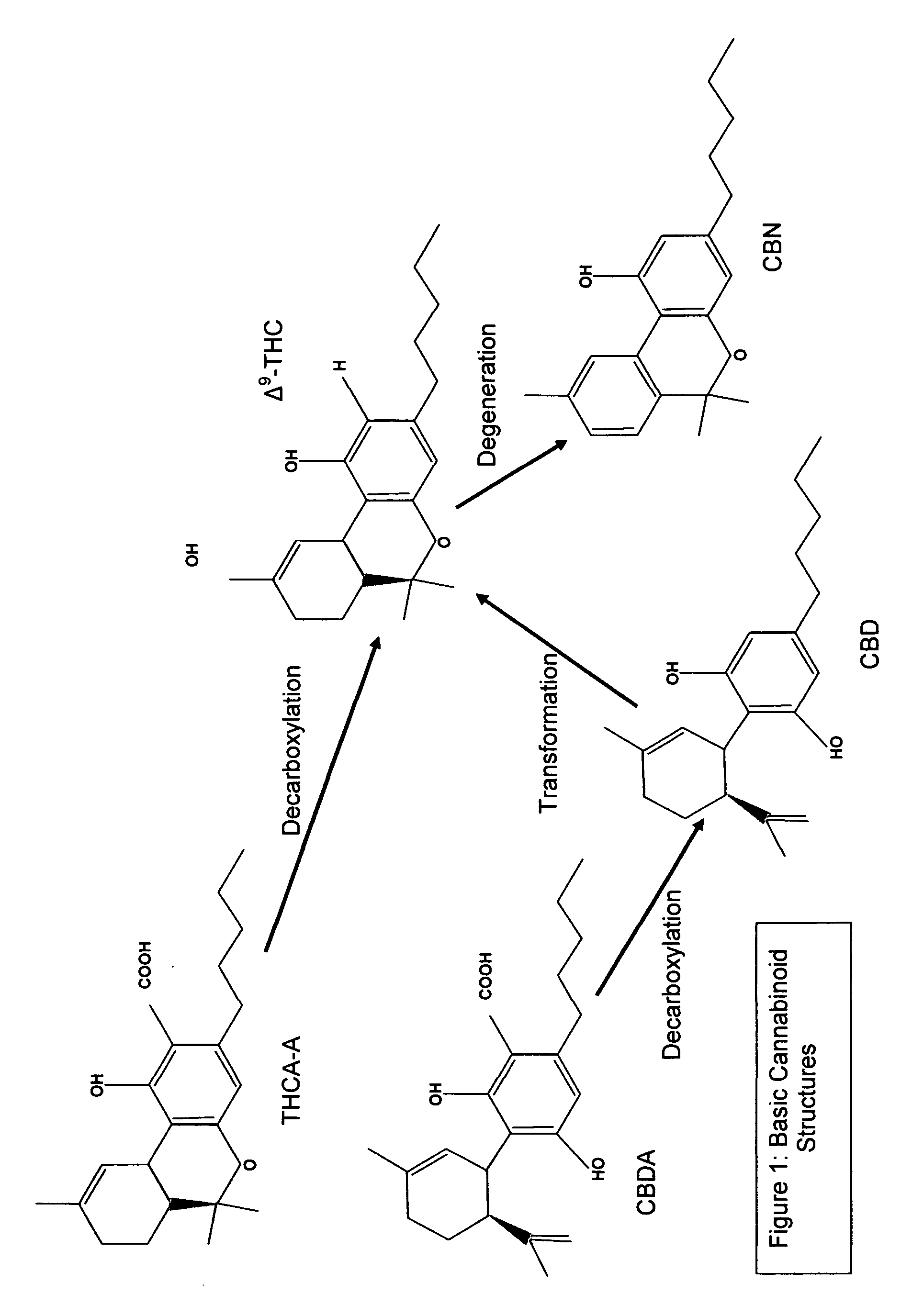
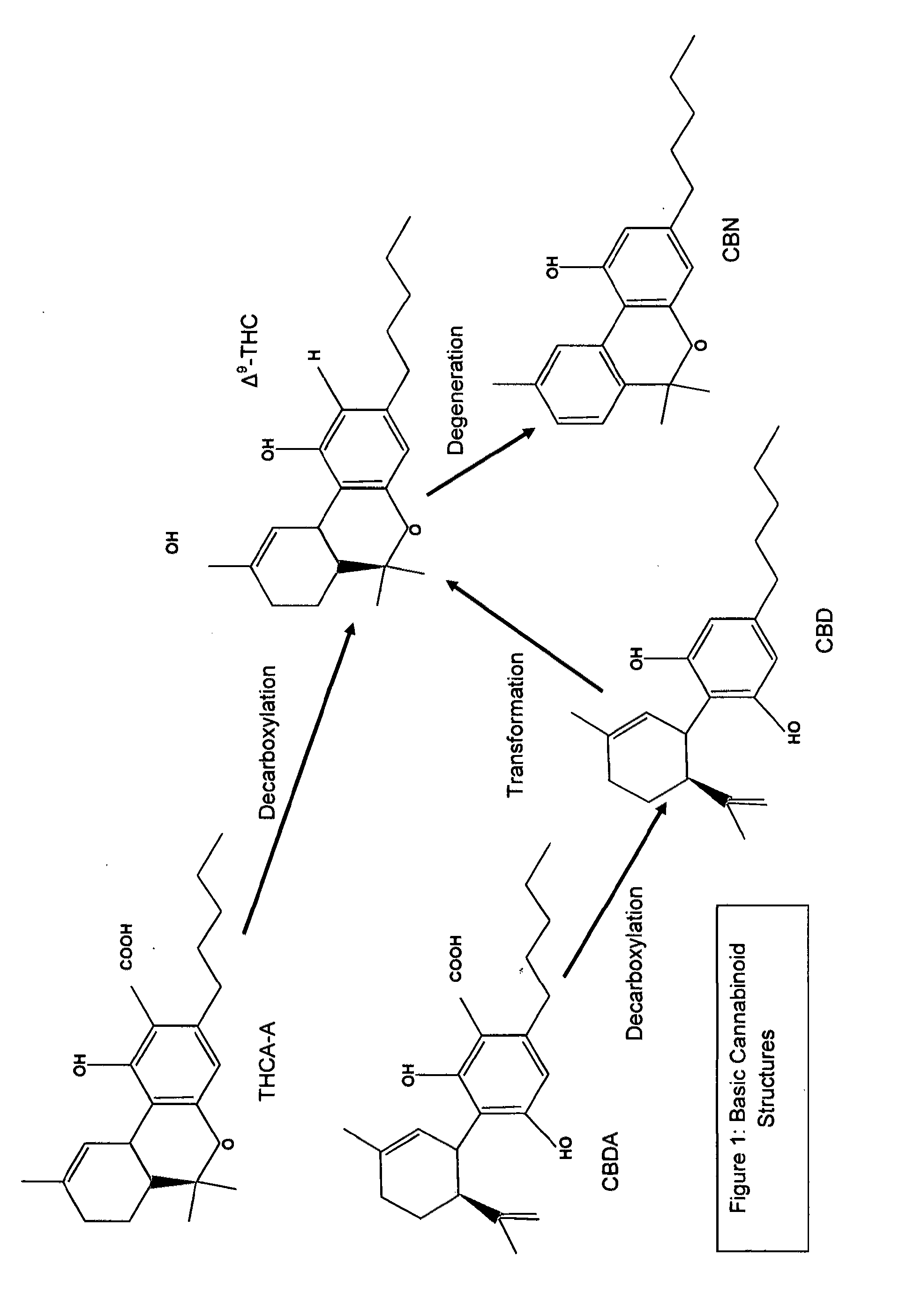
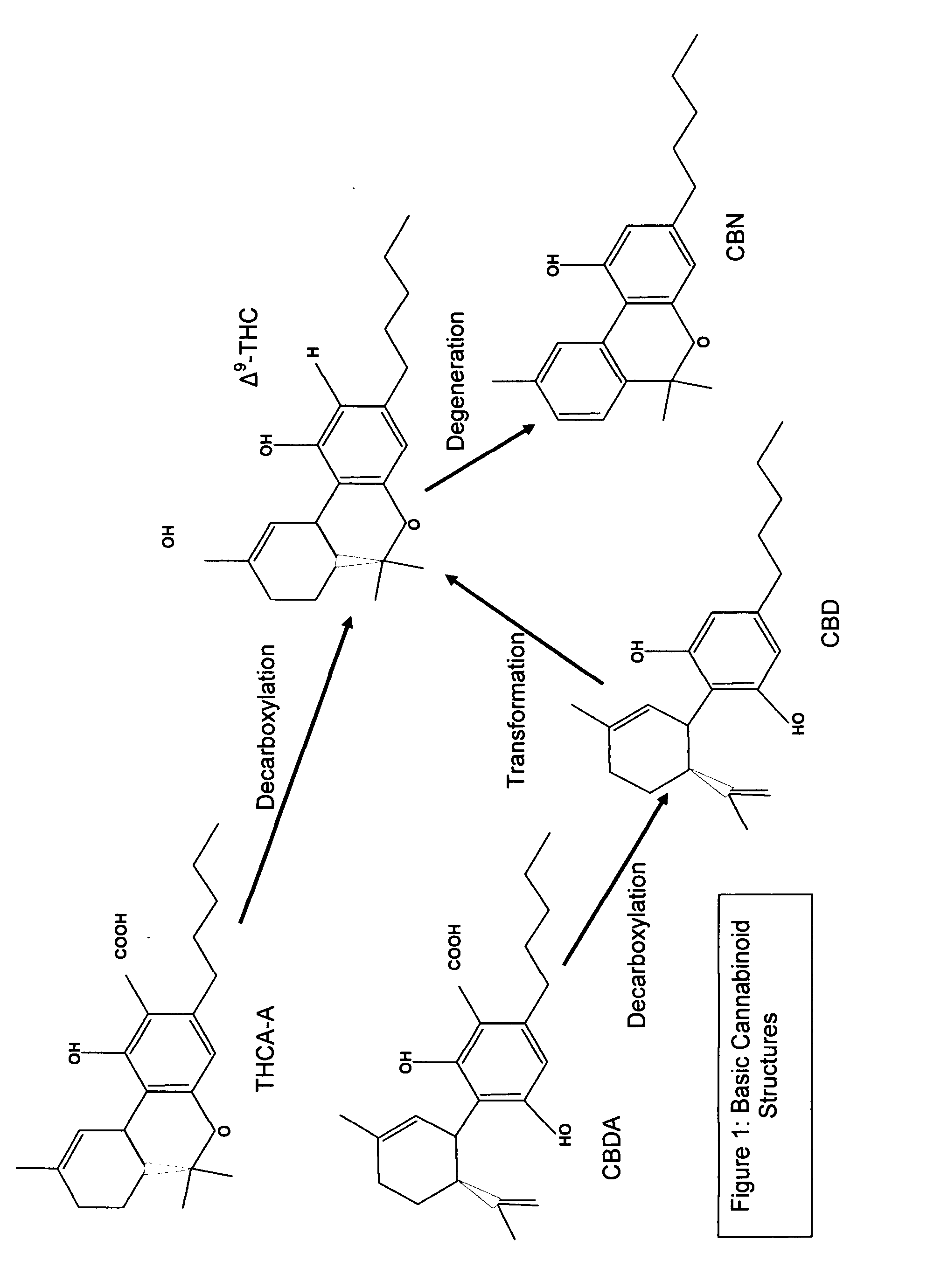
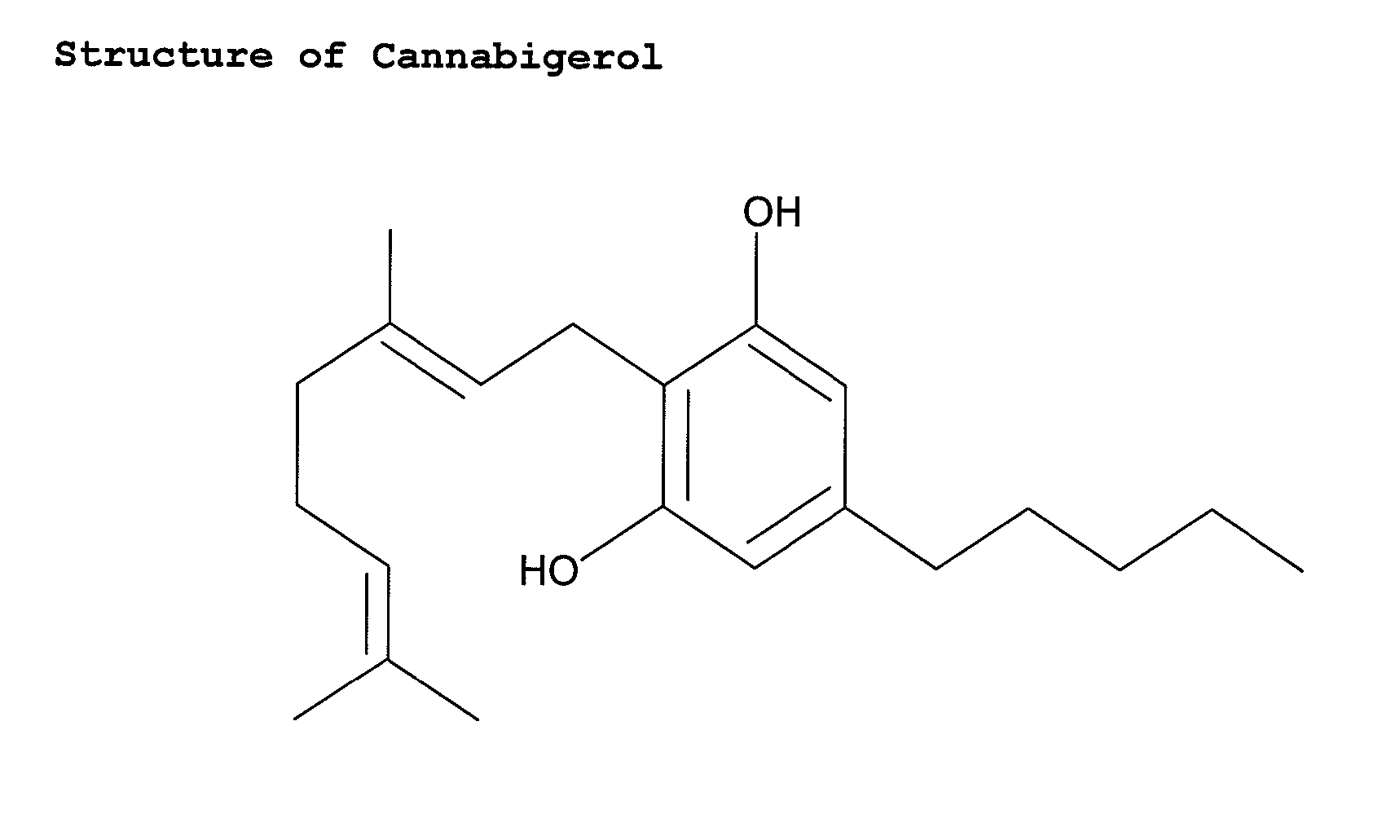
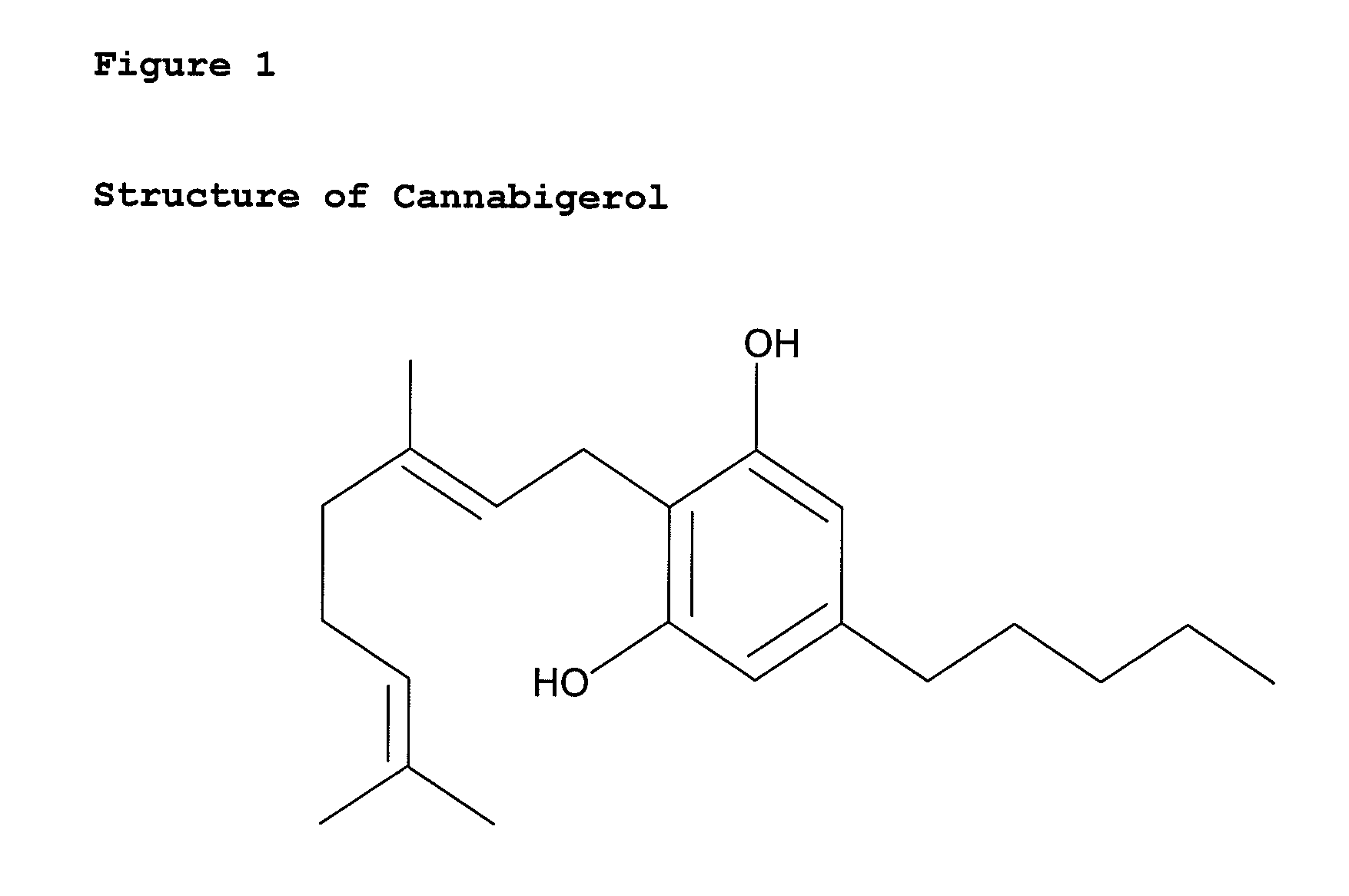
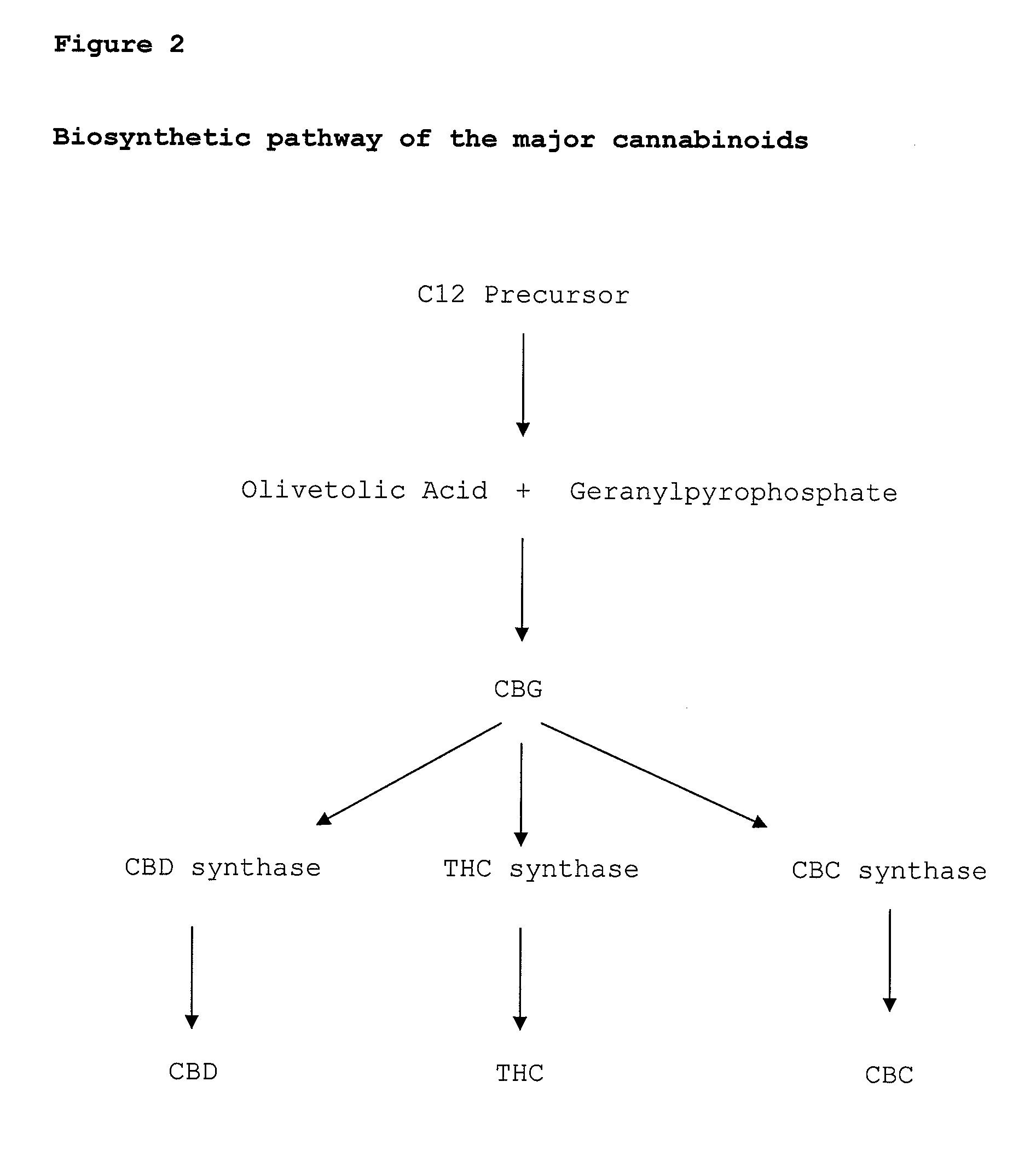
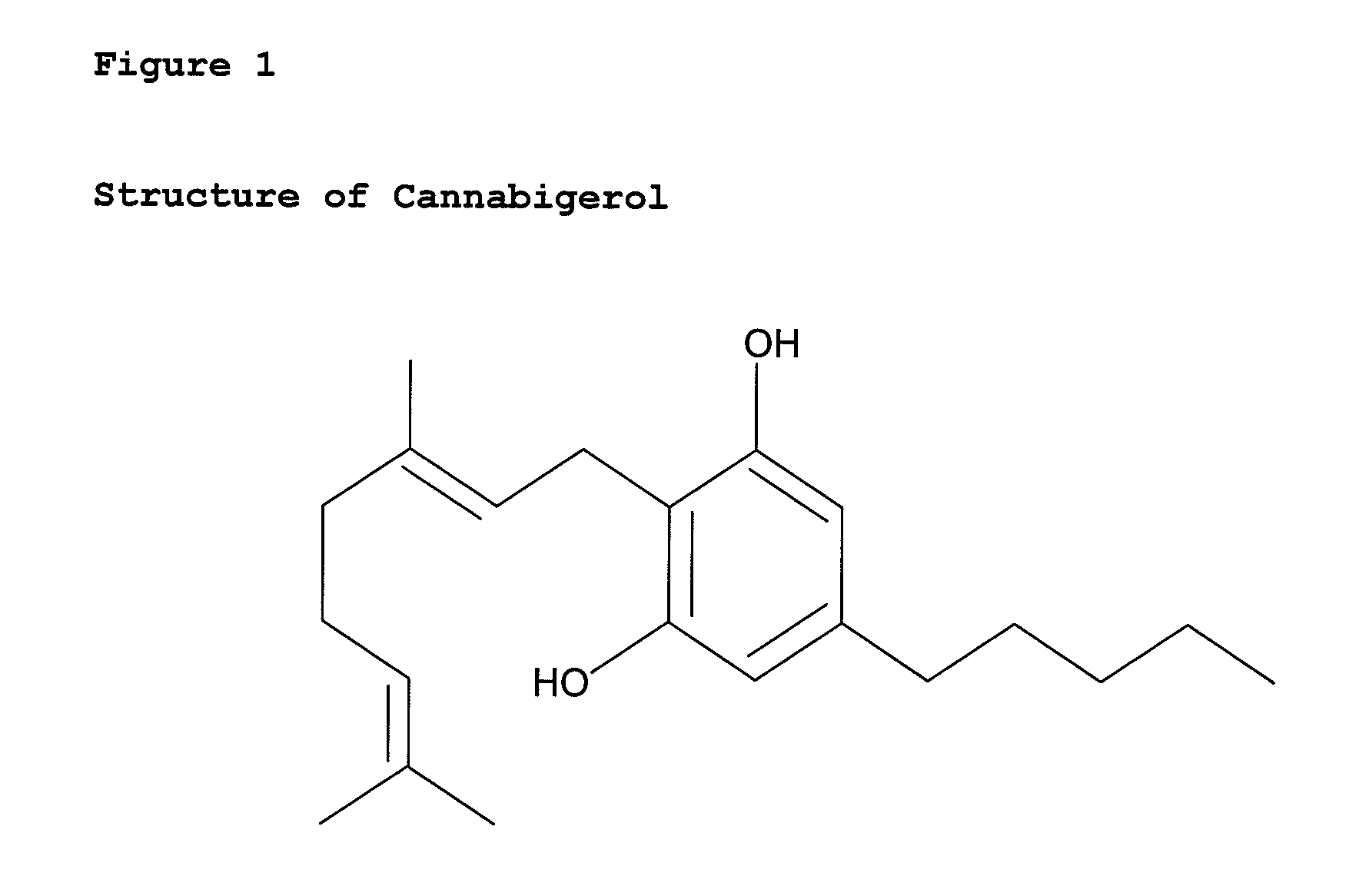
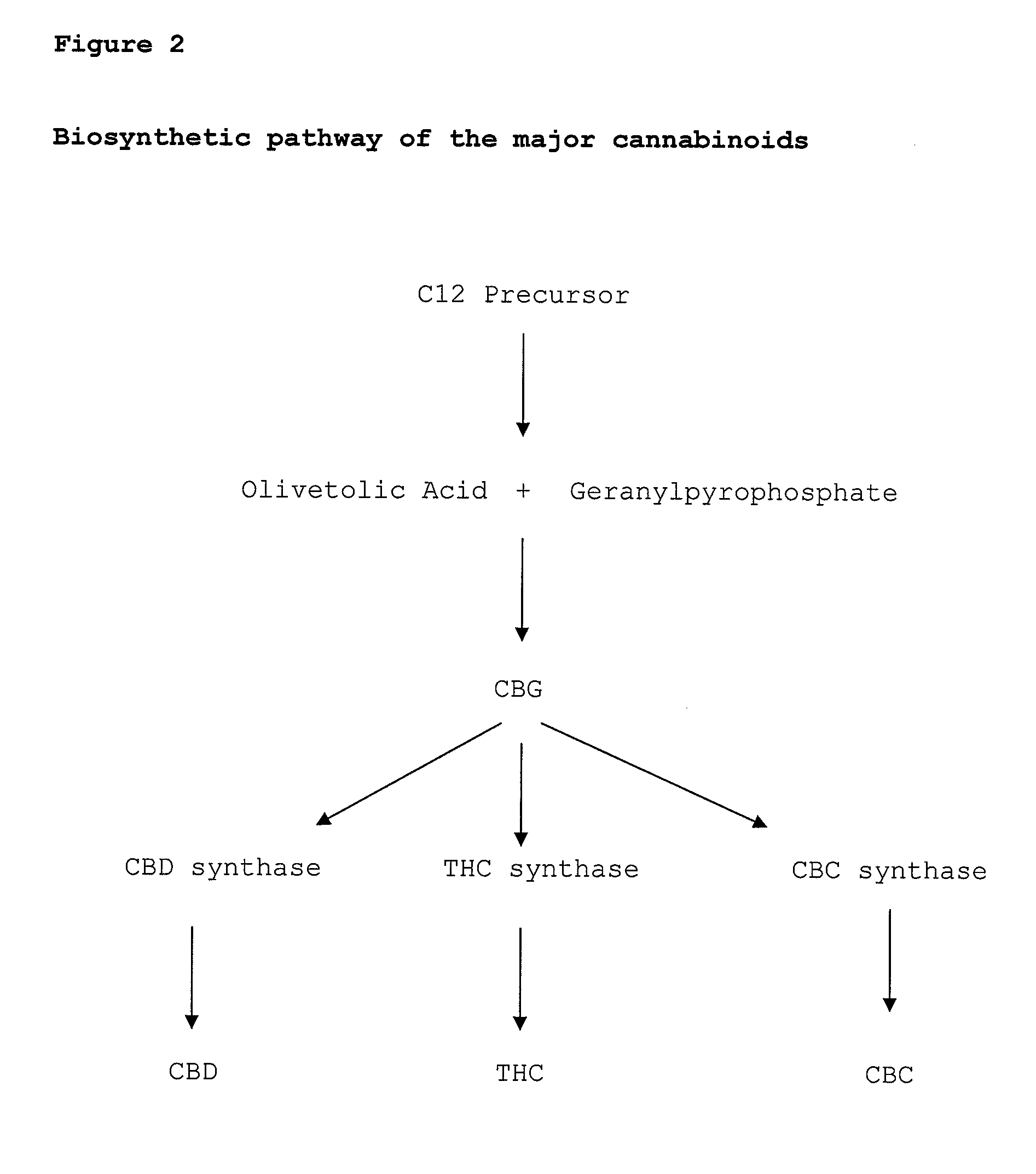
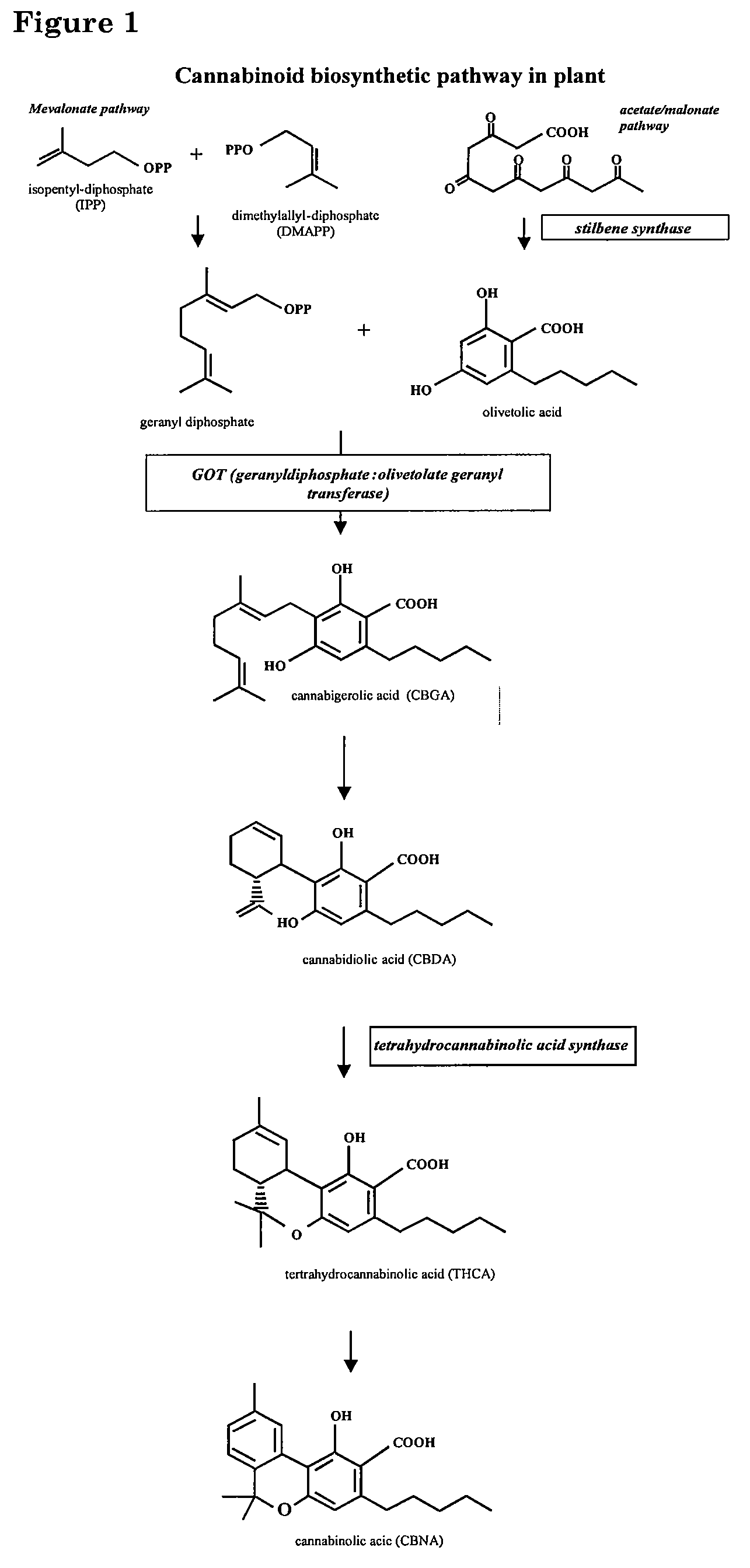
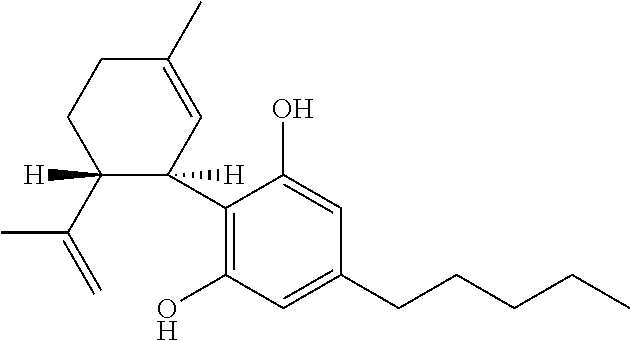
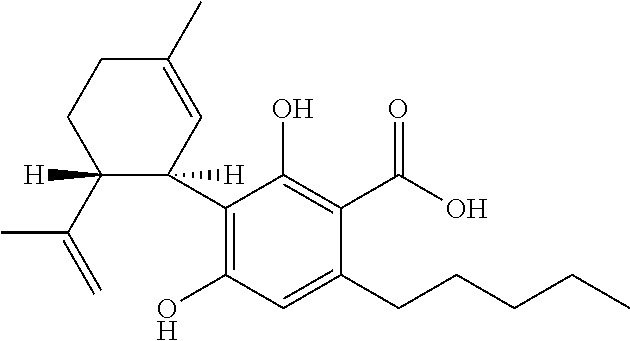
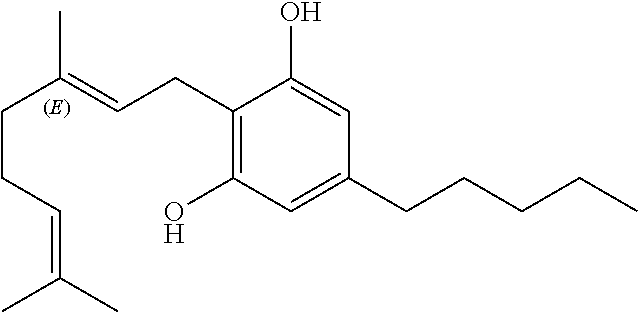
Cannabigerol (/ˌkænəbəˈdʒærɔːl/) (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis. Cannabigerol is the non-acidic form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized. Cannabigerol is a minor constituent of cannabis. During growth, most of the cannabigerol is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), leaving about 1% cannabigerol in the plant.
Method of relieving analgesia and reducing inflamation using a cannabinoid delivery topical liniment
InactiveUS6949582B1Good effectSafe and effectiveBiocideHydroxy compound active ingredientsSide effectCannabinoid receptor
A method of relieving analgesia and reducing inflammation using a cannabinoid delivery topical liniment composition containing from about 97.5% to about 99.5% by weight a 70% monohydric alcohol solution, and from about 0.5% to about 2.5% by weight of a synergistic cannabinoid mixture extracted from the female plant Cannabis sativa L, including in combination: 9-Tetrahydrocannabinol (delta-9-THC), 9-THC Propyl Analogue (THC-V), Cannabidiol (CBD), Cannabidiol Propyl Analogue (CBD-V), Cannabinol (CBN), Cannabichromene (CBC), Cannabichromene Propyl Analogue (CBC-V), Cannabigerol (CBG), terpenoids, and flavonoids. The liniment is applied topically, preferably by spraying, and the constituents of the mixture are absorbed through the skin and interact with cannabinoid receptors in the body and tissues of a human patient to produce therapeutic analgesic and anti-inflammatory effects without undesirable psychotropic side effects.
Owner:WALLACE WALTER H
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Use of cannabinoids in combination with an anti-psychotic medicament
ActiveUS9017737B2High activityLess degree of activityBiocideSenses disorderTypical antipsychoticPsychosis drug
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Medicinal cannabis added in food
The invention is a product and a process wherein cannabinoids such as Medicinal Δ9-THC and / or other substances associated with medicinal cannabis, including yet not necessarily limited to cannbidiols, cannabigerol are added to a foodstuff where the medicinal cannabis is not evenly distributed throughout the foodstuff where the food stuff contains a known weight of medicinal cannabis. Another provision of the invention is providing controlled amounts or ratios of Δ9-THC as compared to CBD in or on a foodstuff.
Owner:HOSPODOR ANDREW DAVID
Medicinal cannabis fatty foodstuff
InactiveUS20120043242A1Organic active ingredientsNervous disorderDelta-9-tetrahydrocannabinolMedicine
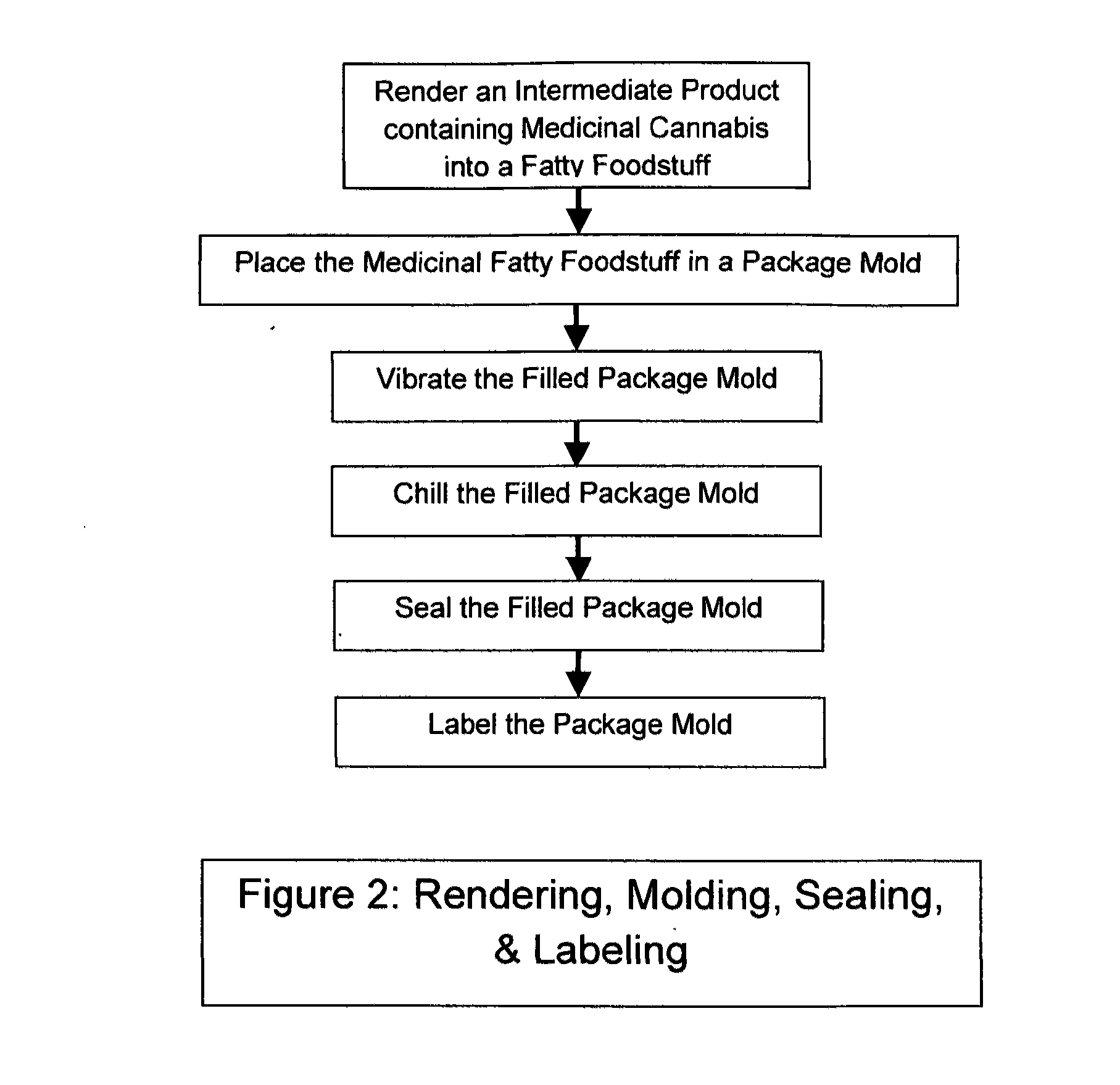
The invention is a product and a process wherein Medicinal Delta-9 tetrahydrocannabinol (Δ9-THC) and potentially other cannabinoids (medicinal cannabis substances) associated with decarboxylated cannabis, including yet not necessarily limited to cannbidiols, and cannabigerol are rendered into a fatty foodstuff and then molded into a mold that also acts as a package. The best mode of the invention is a blister pack containing a plurality of voids or receptacles of desired sizes. A product that is characterized by a controlled amount of medicinal cannabis per unit volume of a fatty foodstuff base material is inserted into the mold, then cooled, and finally sealed. Each void or receptacle contains a known amount of medicinal cannabis that are independently dispensable.
Owner:HOSPODOR ANDREW DAVID
Parenteral formulations
ActiveUS20190314296A1Improve solubilityLow toxicityHydroxy compound active ingredientsPharmaceutical delivery mechanismSURFACTANT BLENDPharmacology
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
Medicinal cannabis uniform in food
InactiveUS20120095088A1Low baking temperatureExtend the baking timeBiocideNervous disorderCannabinoidMedicine
The invention is a product and a process wherein cannabinoids such as Medicinal Δ9-THC and / or other substances associated with medicinal cannabis, including yet not necessarily limited to cannbidiols, cannabigerol are contained or processed into foodstuffs or medicinal compounds in controlled ways and with specific characteristics. First a medicinal substance with a known about of medicinal cannabis is mixed into a foodstuff such that the medicinal cannabis is distributed uniformly in the foodstuff. Foodstuffs consistent with this invention include baked goods, hard candies, ice cream, bases, ice cream, and yogurt. The product is characterized by a controlled amount of cannabinoids per unit volume of the foodstuff. Another provision of the invention is providing controlled amounts or ratios of Δ9-THC as compared to CBD in a foodstuff.
Owner:HOSPODOR ANDREW DAVID
Oral cannabinoid formulations
ActiveUS20190365667A1Ensure palatabilityEnsure stabilityNervous disorderDispersion deliveryNon ionicSURFACTANT BLEND
The present invention relates to an oral formulation containing one or more cannabinoids. Preferably one or more cannabinoids dissolved in a solvent system consisting essentially of: a non-ionic surfactant and water together with other components which ensure the cannabinoids stability and the formulations palatability. Furthermore, the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerol propyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
Pharmaceutical compositions comprising cannabigerol
ActiveUS20080031977A1High activityLess degree of activityBiocideNervous disorderCannabinoidCompound (substance)
The present invention relates to the use of cannabigerol (CBG) type compounds and derivatives thereof in the treatment of mood disorders.
Owner:GW PHARMA LTD
Psilocybin and/or psilocin in combination with cannabinoids and/or terpenes
One or more cannabinoids and / or terpenes in combination with psilocybin and / or psilocin may be used in the prevention or treatment of psychological or brain disorders. The one or more cannabinoids may be taken from the group of cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA).
Owner:PROCARE BEHEER BV
Pharmaceutical compositions comprising cannabigerol
ActiveUS8481085B2High activityLess degree of activityBiocideNervous disorderCannabigerolCannabidivarin
Owner:GW PHARMA LTD
Psilocybin and/or psilocin in combination with cannabinoids and/or terpenes
Owner:PROCARE BEHEER BV
Medicinal acidic cannabinoids
ActiveUS7807711B2Promote resultsReduce excretionBiocideNervous disorderOrganic chemistryCannabigerolic acid
The invention relates to an acidic cannabinoid for medical use and to a cannabis extract comprising an acidic cannabinoid. The extract may comprise one or more compounds selected from the group consisting of cannabidiolic acid (CBD-A), cannabidiol (CBD), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabinolic acid (CBN-A) and cannabinol. The invention further relates to a method for preparing a preparation comprising extracting an acidic cannabinoid from cannabis.
Owner:FYTA VERMOGENSVERWALTUNG GMBH
The topical composition with active compounds from cannabis sativa and calendula officinalis for reduction of skin lesions
ActiveUS20190111093A1Deep hydrationReduces skin poreCosmetic preparationsOrganic active ingredientsCalendula officinalis extractUltraviolet
Disclosed is a topical composition including essential combination of synergistically acting phyto-active materials, non-psychotropic phytocannabinoids from the plant of Cannabis sativa: Cannabidiol, Cannabidiolic acid, Cannabivarin Cannabigerol in combination with extract of Calendula flower and the formulation of the base to ensure the features of anti-inflammation, anti-oxidation, emollient, and bactericidal components. The topical composition is an emollient dedicated for reduction of skin lesions caused by atopic dermatitis, urticaria, radiotherapy and UV induced skin damage and acne. In addition the topical composition could reduce secretion of fats, facilitate deep skin hydration, reduce pores and exert soothing effect.
Owner:UAB SATIMED
Medicinal Acidic Cannabinoids
InactiveUS20110021617A1Promote resultsReduce excretionBiocideNervous disorderOrganic chemistryMedical treatment
The invention relates to an acidic cannabinoid for medical use and to a cannabis extract comprising an acidic cannabinoid. The extract may comprise one or more compounds selected from the group consisting of cannabidiolic acid (CBD-A), cannabidiol (CBD), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabinolic acid (CBN-A) and cannabinol. The invention further relates to a method for preparing a preparation comprising extracting an acidic cannabinoid from cannabis.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST NATUURWETENSCHAPPELIJK ONDERZOEK TNO
Phytocannabinoids for use in the treatment of intestinal inflammatory diseases
The present invention relates to one or more of the phytocannabinoids tetrahydrocannabivarin (THCV); cannabigerol (CBG); cannabichromene (CBC); and cannabidivarin (CBDV) for use in the treatment of intestinal inflammatory diseases. Preferably the intestinal inflammatory disease is either ulcerative colitis or Crohn's disease.
Owner:GW PHARMA LTD
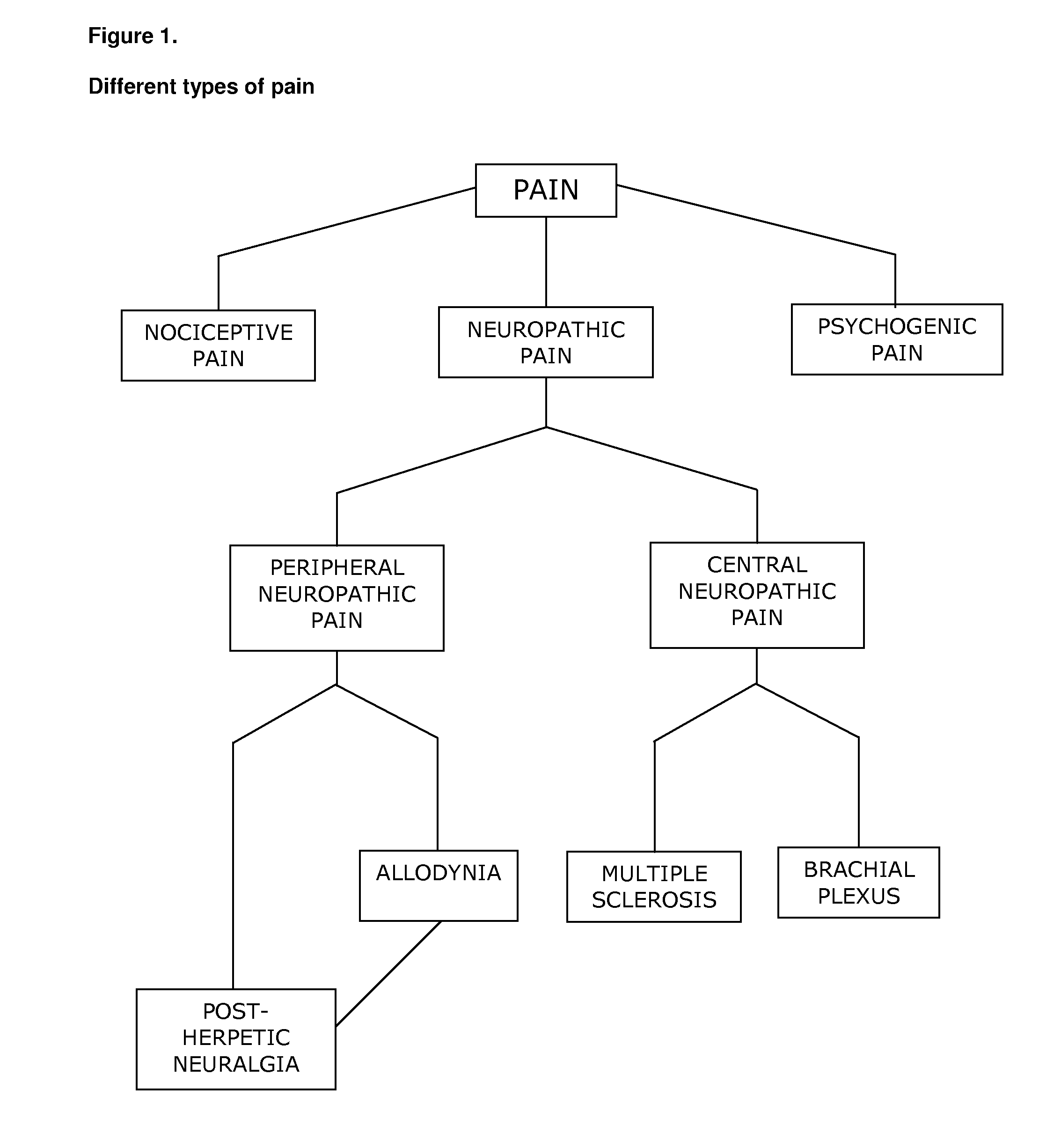
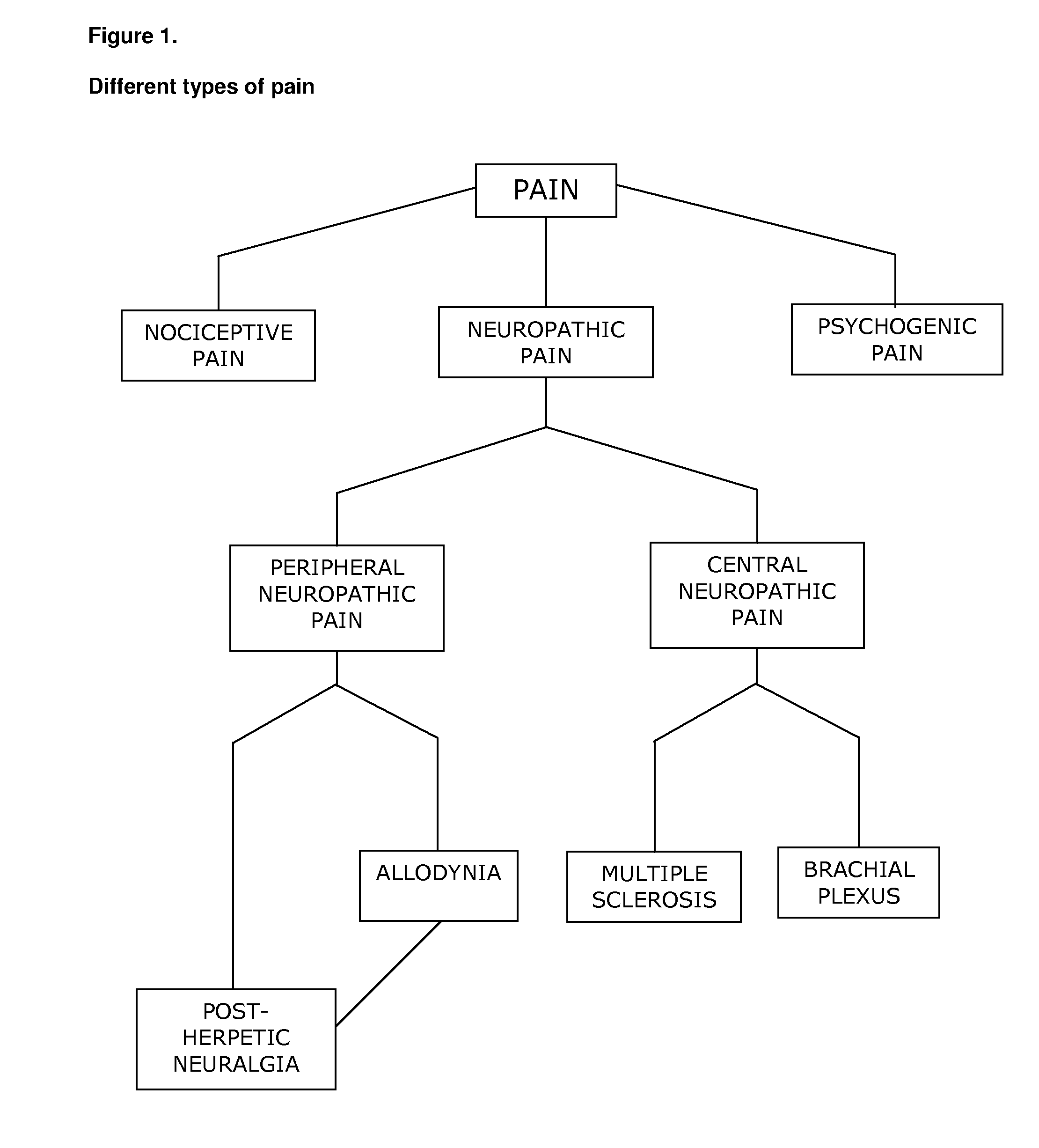
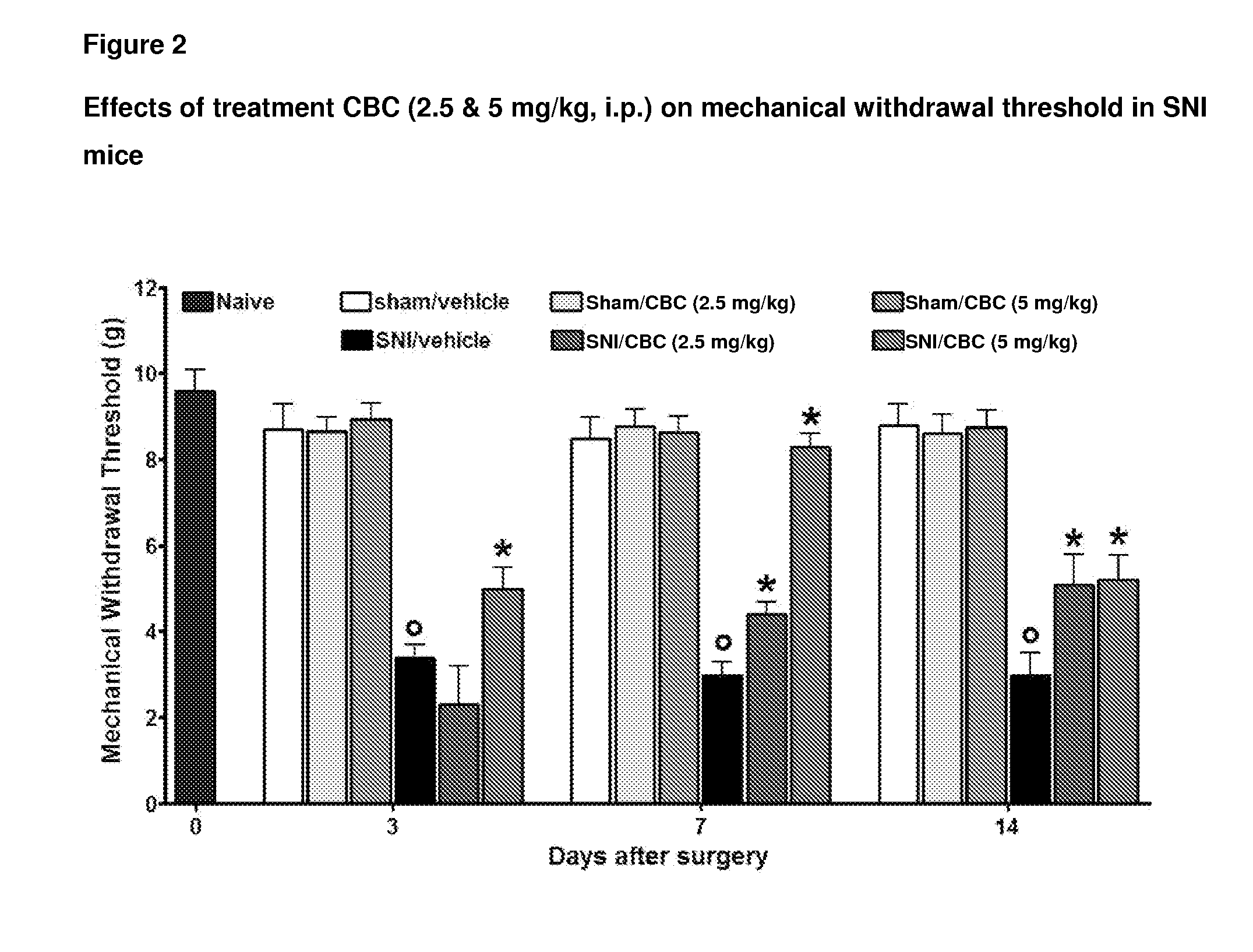
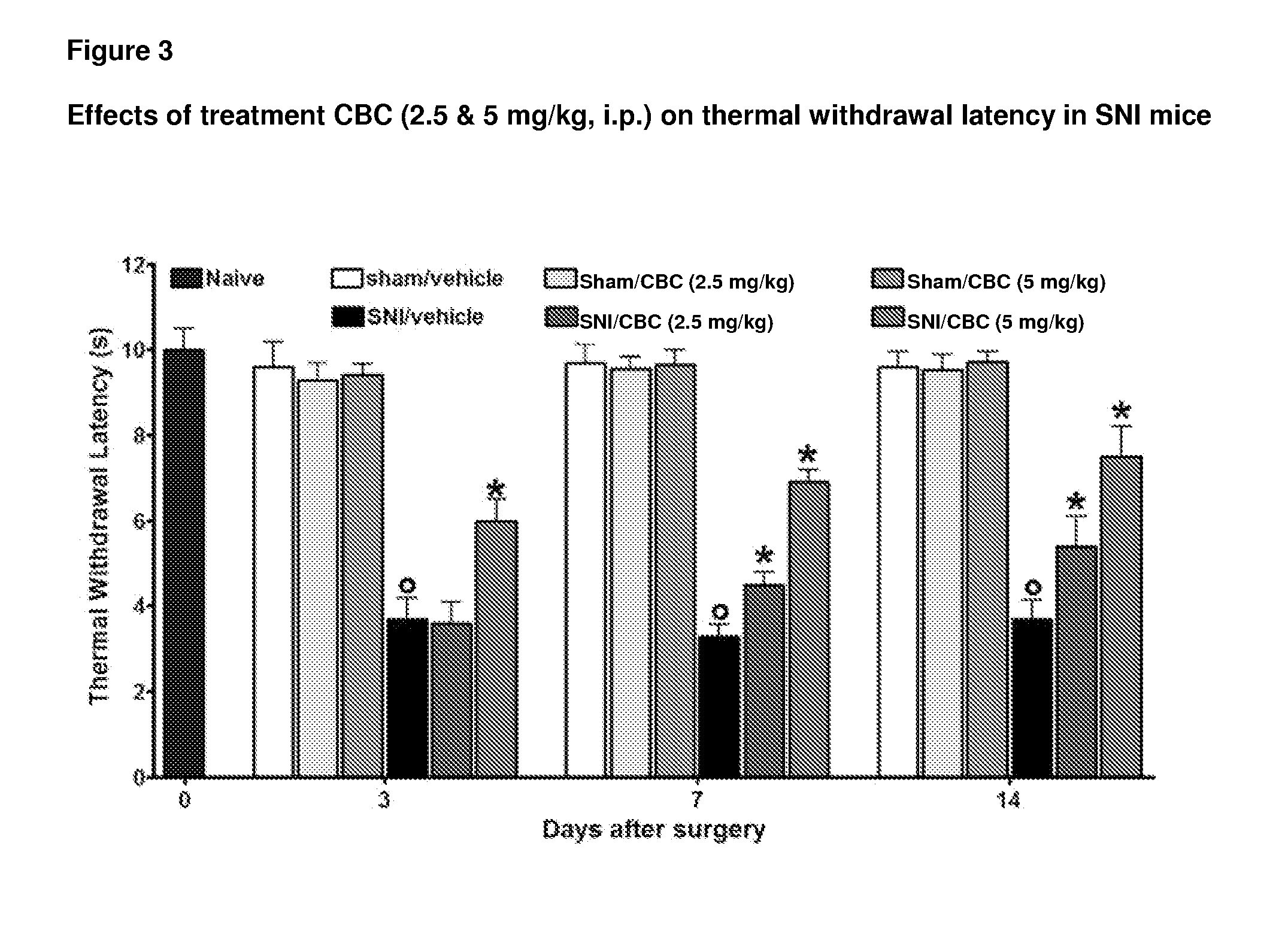
Cannabinoids for use in the treatment of neuropathic pain
ActiveUS20140107192A1Relieve neuropathic painExtended maintenance periodBiocideNervous disorderNeuropathic painCannabichromene
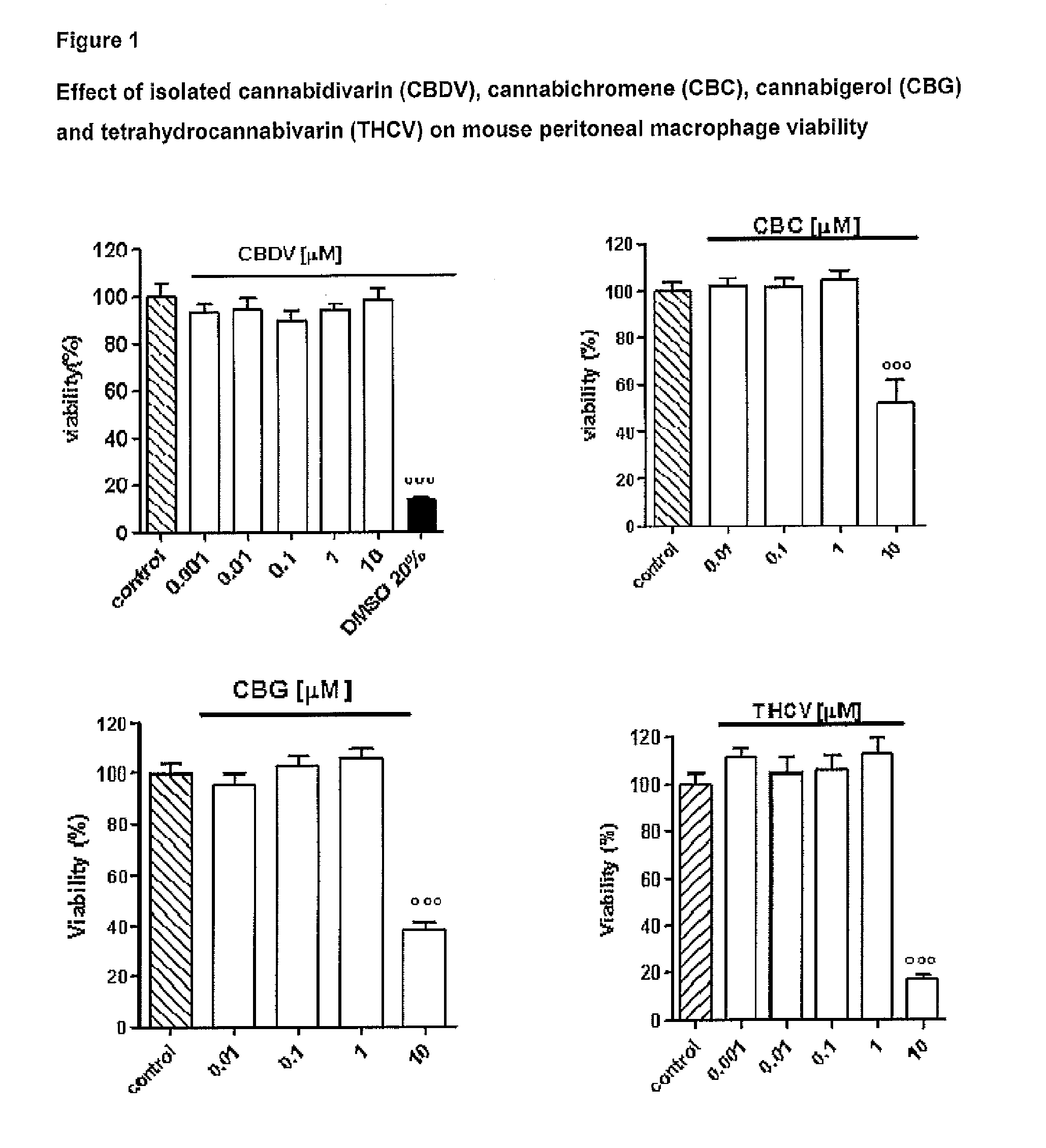
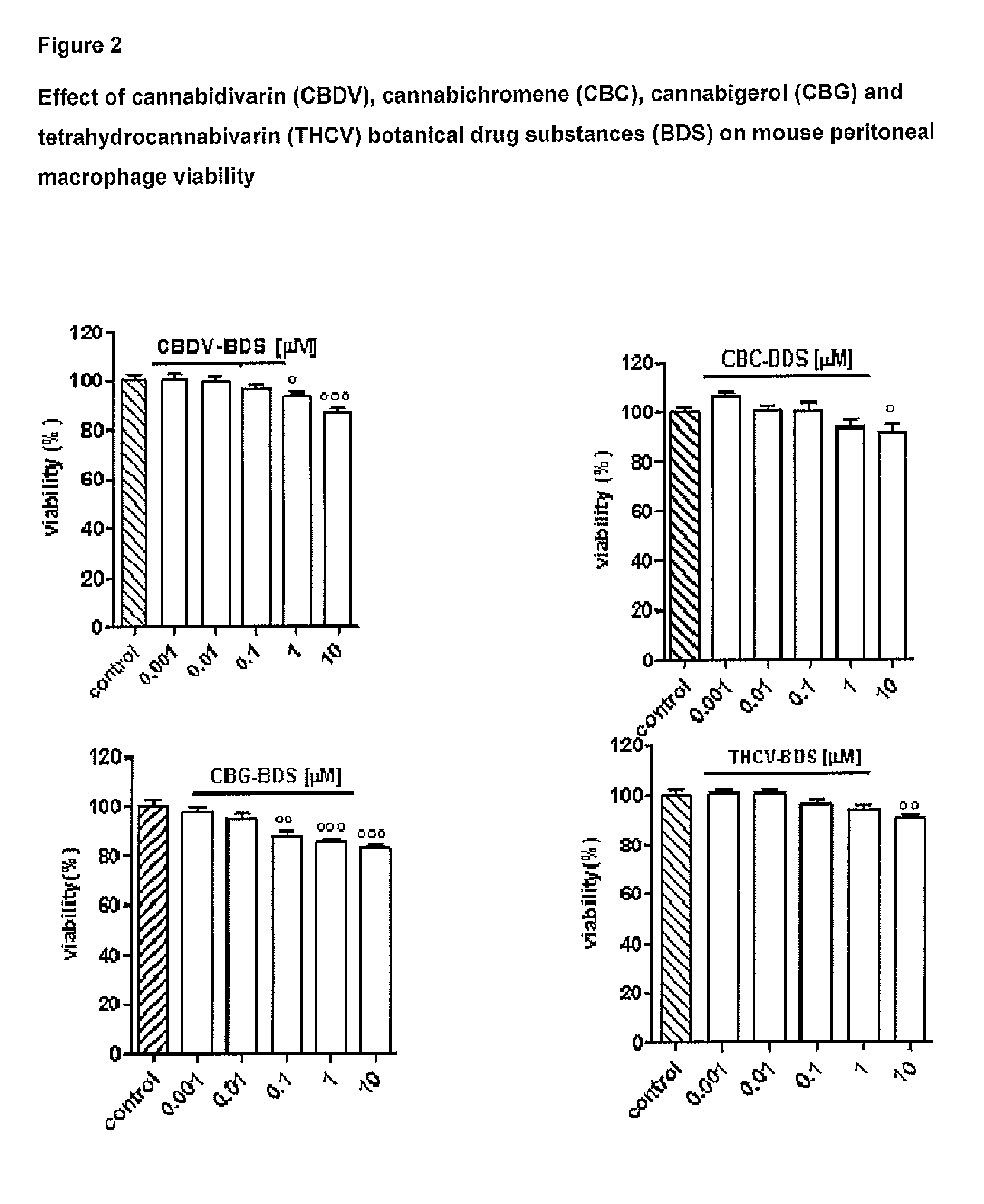
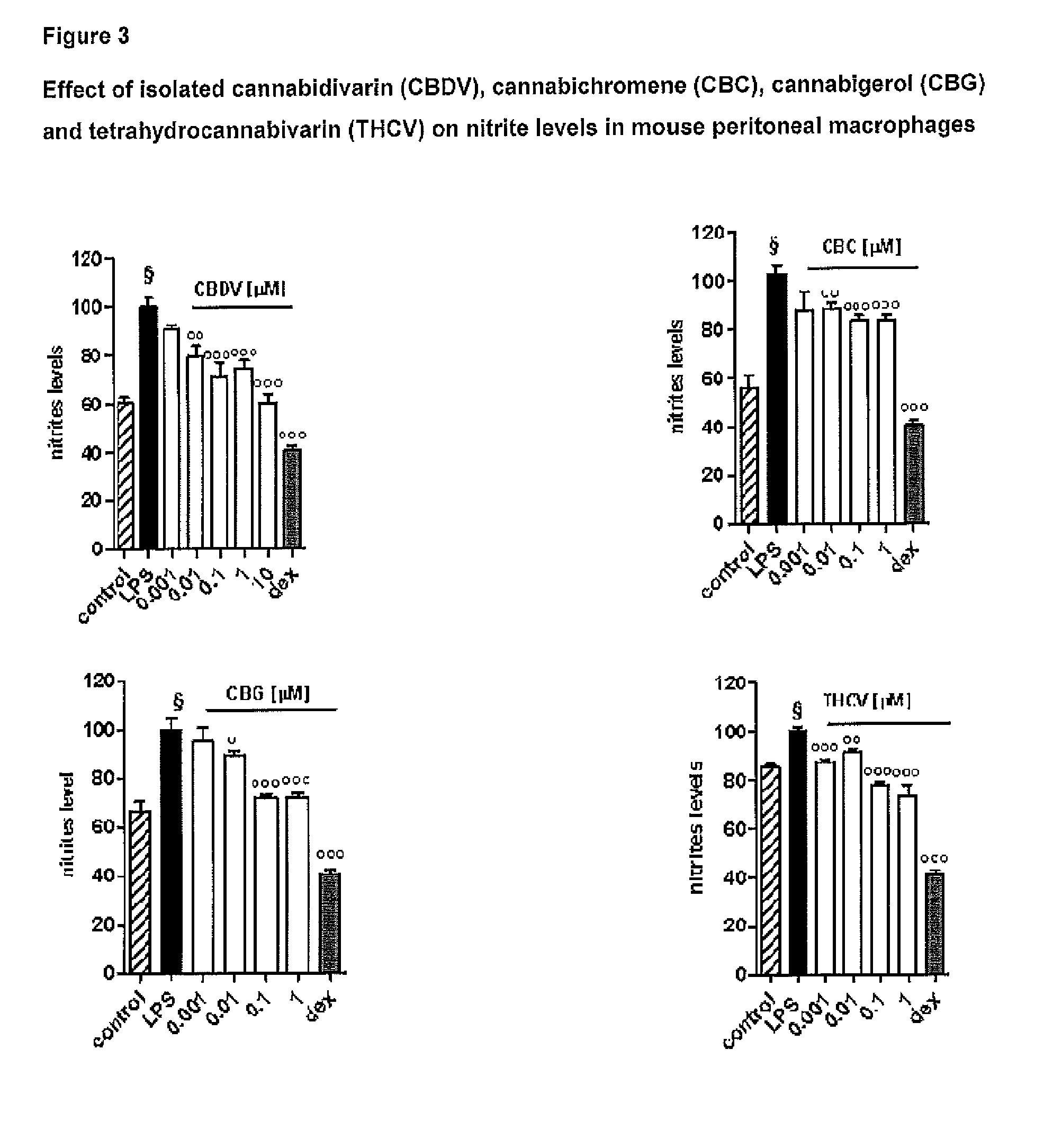
The present invention relates to cannabinoids for use in the treatment of neuropathic pain. Preferably the cannabinoids are one or more phytocannabinoids of: cannabigerol (CBG), cannabichromene (CBC), cannabidivarin (CBDV) or tetrahydrocannabivarin (THCV). More preferably the phytocannabinoids are isolated and / or purified from cannabis plant extracts.
Owner:GW PHARMA LTD
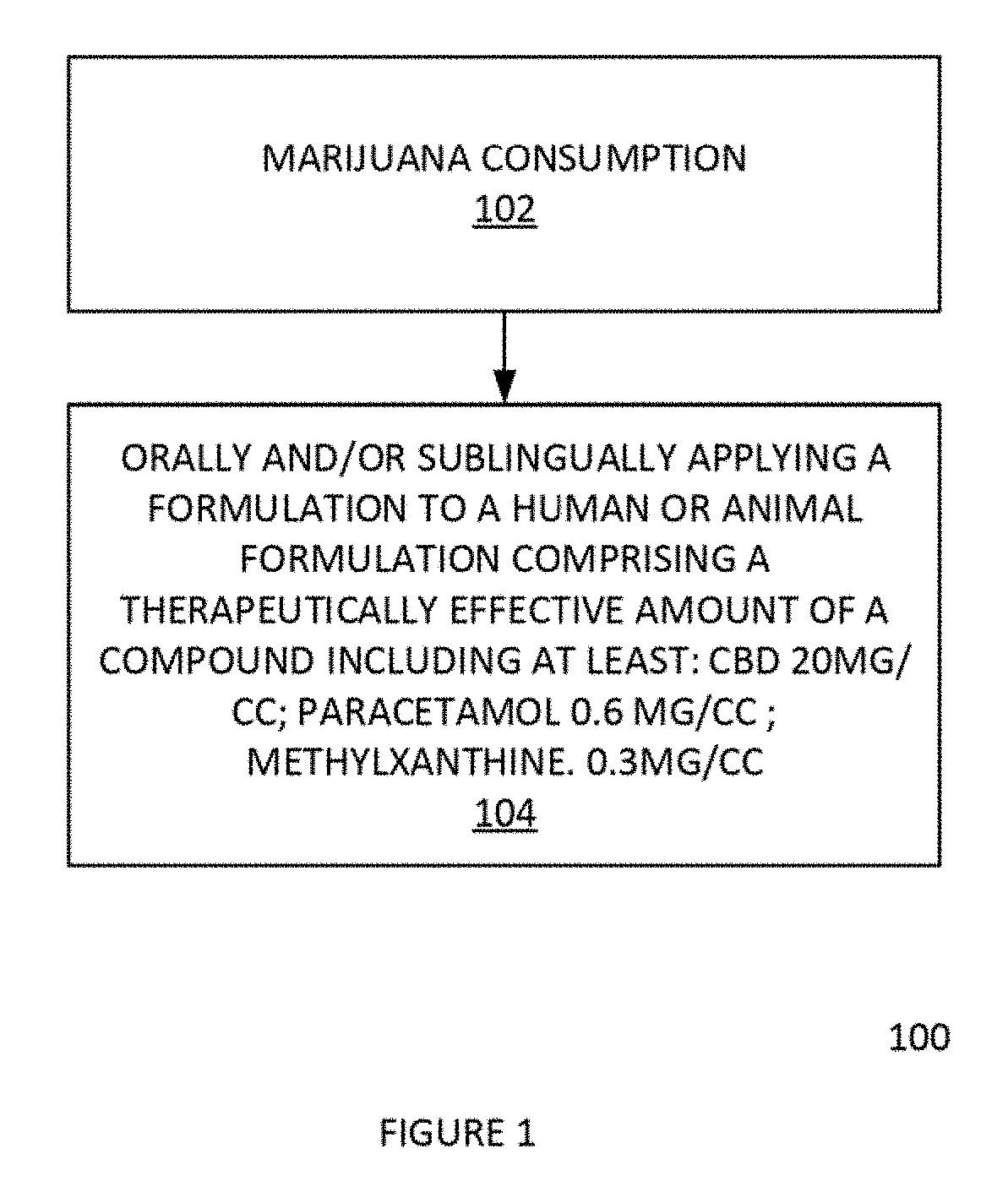
Method for reduction, suppression, or elimination of anxiety or marijuana/cannabis effects and related marijuana/cannabis product by process
InactiveUS20190134121A1Hydroxy compound active ingredientsSuppositories deliveryMonomethyl etherMedicine
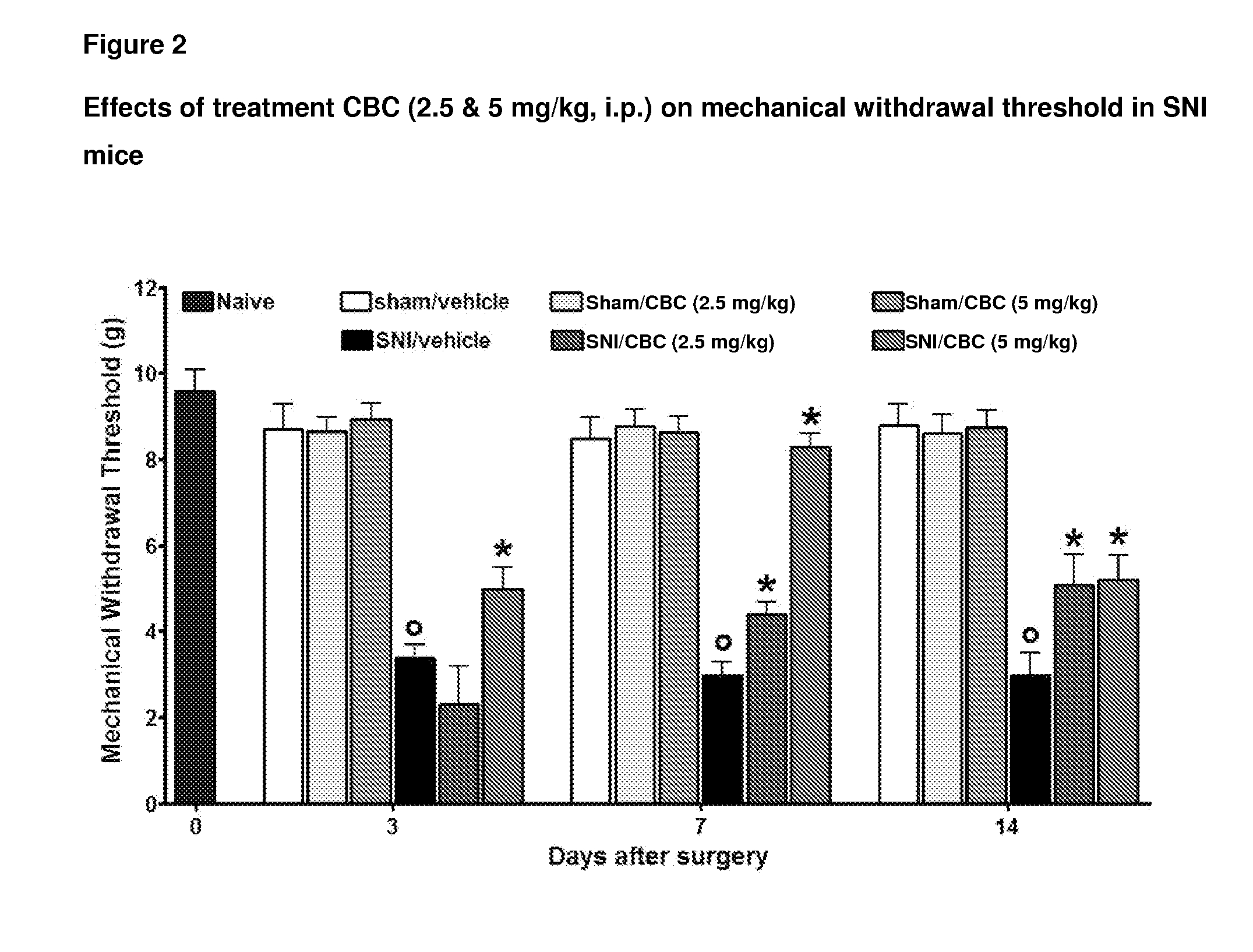
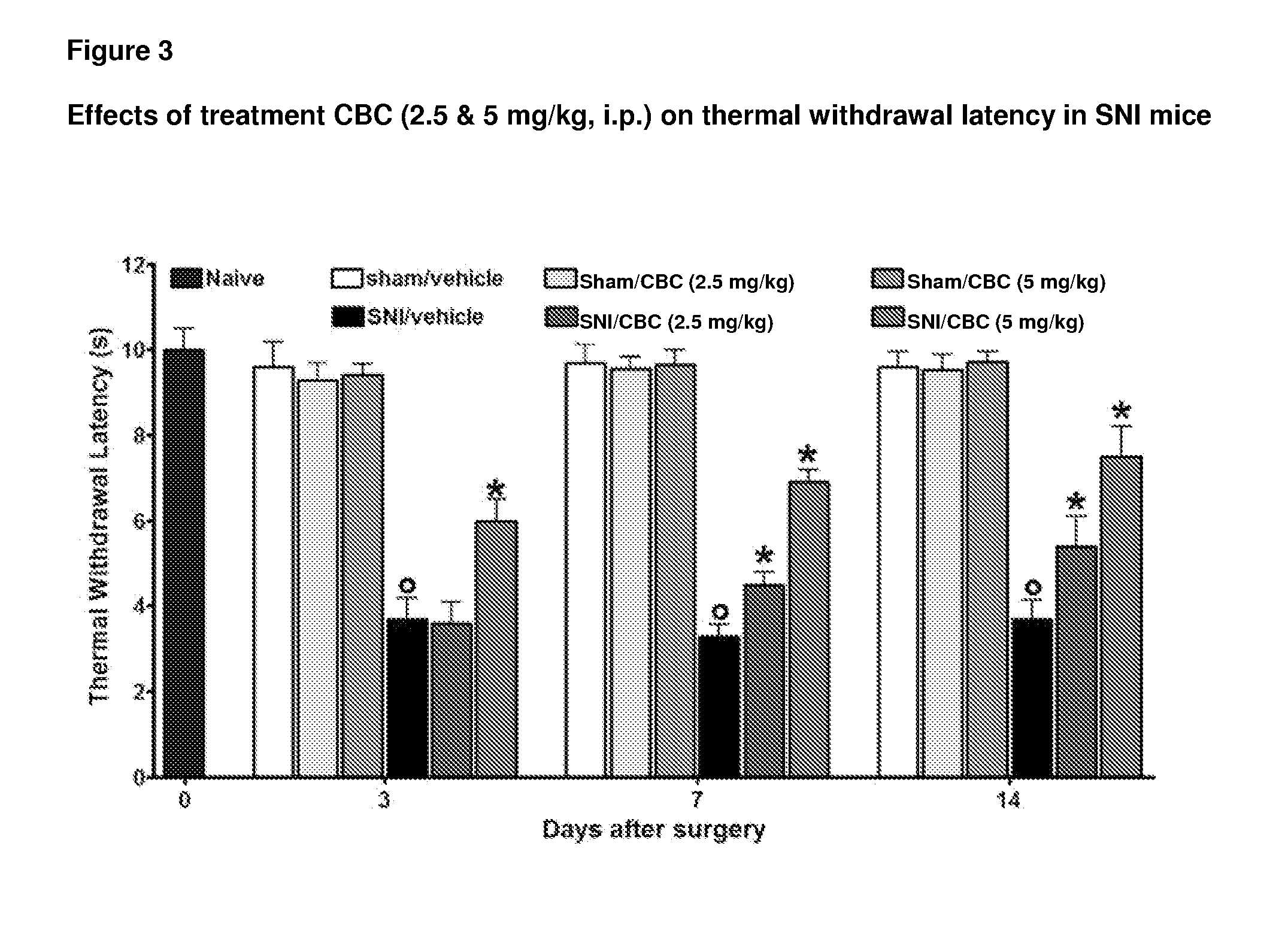
In one aspect, a formulation comprising cannabinoids, paracetamol, methylxanthines, salicylates, terpenes, humulus oil, or amino acids individually or any combination or omission thereof for the reduction, alleviation, elimination, and / or suspension of effects of THC exposure and anxiety. The formulation can be the effective amount of cannabinoid is between 5 mg and 5000 mg. The formulation can be the cannabinoid comprises a Cannabidiol (CBD), a cannabidolic acid (CBDA), a Cannabinol (CBN), a Cannabigerol (CBG), a Cannabichromene (CBC), a Cannabicyclol (CBL), a Cannabivarin (CBV), a Tetrahydrocannabivarin (THCV), a Cannabidivarin (CBDV), a Cannabichromevarin (CBCV), a Cannabigerovarin (CBGV), a Cannabigerol monomethyl ether (CBGM), a Cannabielsoin (CBE), or a cannabicitran (CBT). The formulation can be the effective amount of paracetamol is between 0 mg-1000 mg.
Owner:BERMUDEZ STEVEN +1
Clathrate containing non-psychoactive cannabinoid and preparation method of clathrate
InactiveCN110123876AConducive to subsequent preparation moldingEasy to operateCosmetic preparationsHydroxy compound active ingredientsOrganic solventCannabielsoin
The invention discloses a clathrate containing non-psychoactive cannabinoid and a preparation method of the clathrate. The preparation method includes sequentially subjecting hemp extract to dissolution by organic solvents, clathration by clathration agents, decomposition and impurity removal by water-organic solvents, and vacuum drying and precipitation. The clathrate can completely remove psychoactive components such as tetrahydrocannabinol (THC) and delta 9-tetrahydrocannabivarin (THCV), and effectively enrich non-psychoactive components with the total content of more than 50%, such as cannabidiol (CBD), cannabigerol (CBG) and cannabichromene (CBC). The preparation method has the advantages of simple process and easiness in industrialization.
Owner:HANYI BIO TECH CO LTD
Use of phytocannabinoids in the treatment of ovarian carcinoma
ActiveUS10098867B2Hydroxy compound active ingredientsAntineoplastic agentsOvarian cancerCannabigerolic acid
The present invention relates to the use of phytocannabinoids in the treatment of ovarian cancer. Preferably the phytocannabinoid is selected from the group consisting of: cannabidiol (CBD); cannabidiol acid (CBDA); cannabigerol (CBG); cannabigerolic acid (CBGA); cannabigerol propyl variant (CBGV); and tetrahydrocannabivarin (THCV). In a further embodiment the one or more phytocannabinoids are used in combination with each other. Preferably the combination of cannabinoids consists of CBD and CBG.
Owner:GW PHARMA LTD +1
Cannabinoid Composition and Method For Treating Pain
ActiveUS20170027978A1Nervous disorderHydroxy compound active ingredientsTreatment painButyrospermum parkii
A combination of THC, CBD and Cobalamin (in a ratio of about 63%, 27% and 10%, respectively) is used with a topical carrier such as Shea butter cream to relieve pain. The THC and CBD mixture is extracted from a Cannabis Indica dominant strain using high pressure and carbon dioxide (CO2) as a solvent and comprises: Tetrahydrocannabinol “THC” (9-Tetrahydrocannabinol (delta-9 THC), 8-Tetrahydrocannabinol (Delta-8 THC) and 9-THC Acid), Cannabidiol “CBD”, Cannabinol “CBN”, Cannabichromene (“CBC”), Cannabigerol (“CBG”), terpenoids and flavonoids. The Shea butter is an extract from the Shea nut from the Shea tree (Vitellaria paradoxa).
Owner:INDIA GLOBALIZATION CAPITAL
Parenteral formulations
ActiveUS11229612B2Hydroxy compound active ingredientsPharmaceutical delivery mechanismActive agentSurface-active agents
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
Encapsulated cannabinoid formulations for transdermal delivery
ActiveUS20190216870A1Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. The present invention demonstrates the viability of transdermal delivery with gels and patches for consistent and sustained cannabinoid dosing.
Owner:NUTRAE LLC
Medical water-soluble cannabidiol CBD preparation formula
InactiveCN110448598APlay a repairing roleNo dependencyNervous disorderAntimycoticsDiseaseTransdermal patch
The invention relates to a medical water-soluble cannabisdiol CBD preparation formula. The preparation is prepared from, by weight, 60-80 parts of water-soluble cannabisdiol CBD, 5-10 parts of water-soluble hypocannabidiol CBDa, 1-3 parts of and water-soluble cannabigerol CBG, 1-5 parts of water-soluble cannabis Elson CBE, 2-3 parts of water-soluble cannabis color CBC and 5-8 parts of water-soluble CBN. Compared with the prior art, the formula has the advantages that the water-soluble cannabisdiol CBD serves as the main component, does not contain addictive ingredients, has no psychoactive activity, does not produce dependence, and repairs cells and neuronal systems, and meanwhile the pharmacological action is achieved for resisting spasticity, anxiety, neurogenic diseases, pain and cancer; the preparation can be made to be effervescent tablets, dissolved medicines, capsules, soft capsules, tablets, sprays, emulsified oils, creams, transdermal patches, suppositories, expectorant tablets and sprays, the bioavailability is high, and the formula can play a good effect on cell repair of postoperative chemotherapy and radiotherapy for cancer and treatment of neuropathic diseases.
Owner:汉康生物科技(深圳)有限公司
Method for simultaneously separating secondary cannabidiol and cannabigerol
ActiveCN110590511ANo pollution in the processLarge amount of preparationOrganic chemistryOrganic compound preparationCountercurrent chromatographyCannabigerol
The invention relates to a method for simultaneously separating secondary cannabidiol and cannabigerol. The method comprises the following steps: sufficiently oscillating a solvent system, allowing the solvent system to stand, and separately collecting an upper phase and a lower phaser; dissolving an industrial hemp full-spectrum essential oil sold in the market into the upper phase, performing separation by using high-speed countercurrent chromatography by taking the upper phase as an immobile phase and the lower phase as a mobile phase so as to respectively obtain a mixed liquid of secondarycannabidiol and the mobile phase and a mixed liquid of cannabigerol and the mobile phase, and removing the mobile phase, so as to obtain the secondary cannabidiol and the cannabigerol. By adopting the method, a high-speed countercurrent chromatography technique is adopted for a first time to simultaneously separate and purify secondary cannabidiol (CBDV) of which the purity is greater than 98% and cannabigerol of which the purity is greater than 97% from the industrial hemp full-spectrum essential oil.
Owner:SHANGHAI TAUTO BIOTECH CO LTD
Cannabinoid Composition and Method For Treating Pain
A combination of THC, CBD and Cobalamin (in a ratio of about 63%, 27% and 10%, respectively) is used with a topical carrier such as Shea butter cream to relieve pain. The THC and CBD mixture is extracted from a Cannabis Indica dominant strain using high pressure and carbon dioxide (CO2) as a solvent and comprises: Tetrahydrocannabinol “THC” (9-Tetrahydrocannabinol (delta-9 THC), 8-Tetrahydrocannabinol (Delta-8 THC) and 9-THC Acid), Cannabidiol “CBD”, Cannabinol “CBN”, Cannabichromene (“CBC), Cannabigerol (“CBG”), terpenoids and flavonoids. The Shea butter is an extract from the Shea nut from the Shea tree (Vitellaria paradoxa). The weight of THC, CBD and Cobalamin, excluding other ingredients in the Indica extract, is in the range of about 1.0% to 5% of the total weight of the Shea butter. The cream is applied to joints for indications such as Psoriatic Arthritis and Scleroderma. The mixture is absorbed through the skin and interacts with the peripheral nervous and immune systems via the A Delta and C nerve fibers and CB 2 skin receptors to provide relief from pain without psychotropic or other side effects normally associated with THC.
Owner:INDIA GLOBALIZATION CAPITAL INC
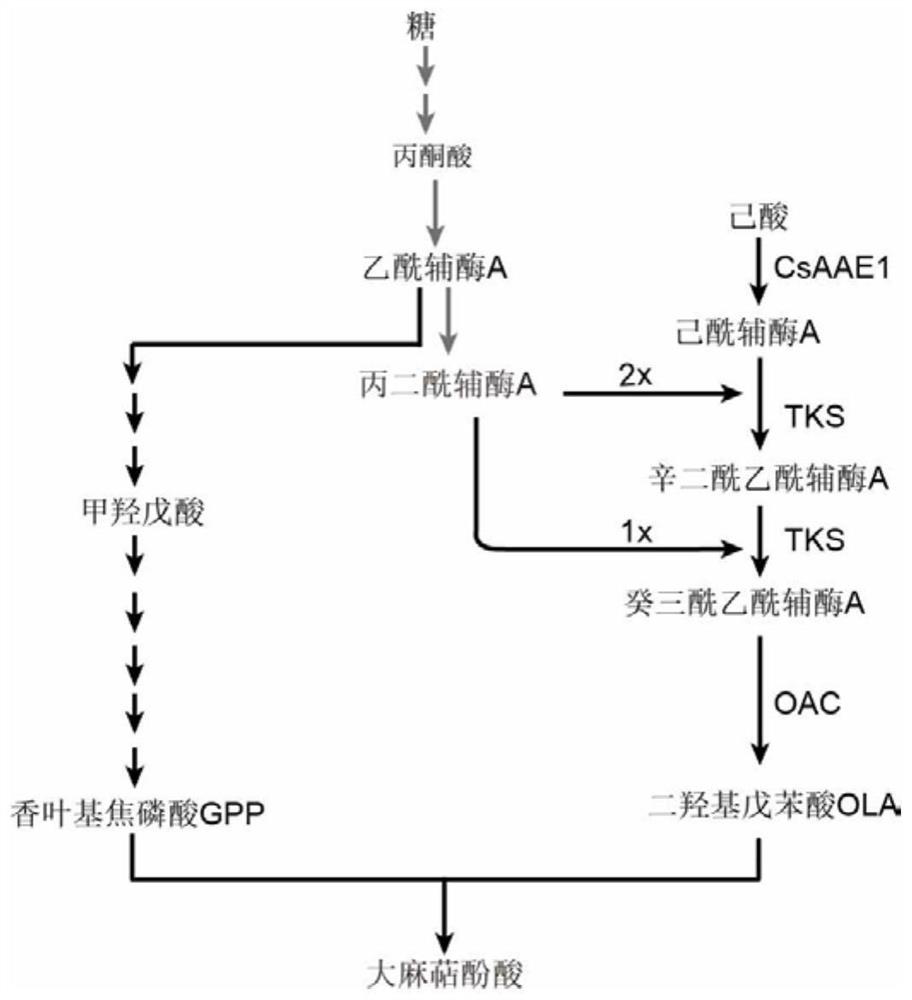
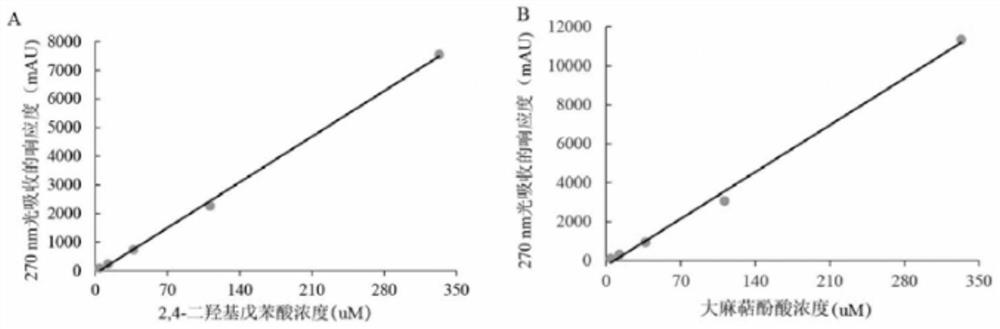
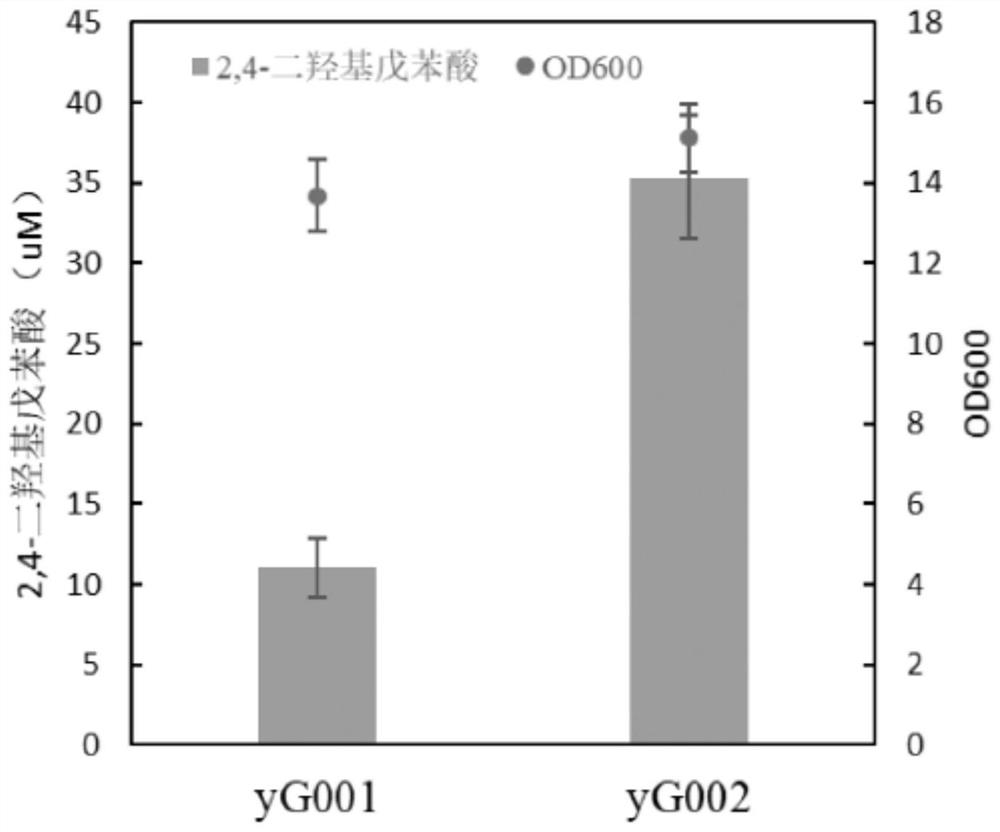
Saccharomyces cerevisiae strain with high yield of cannabigerol and construction method and application thereof
The invention discloses a saccharomyces cerevisiae strain with high yield of cannabigerol and a construction method and application thereof. The saccharomyces cerevisiae strain with high yield of cannabigerol is obtained by inserting TKS and OAC enzymes into a saccharomyces cerevisiae strain A capable of producing the cannabigerol through gene editing technology. According to the invention, the gene editing technology is used for regulating and controlling the gene copy numbers of the 2, 4-olivetol synthase TKS and the 2, 4-olivetolic acid synthase OAC, and the accumulation amount of OLA is increased. Finally, the metabolic flux of the cannabigerolic acid synthesis precursors (GPP and OLA) is balanced, and the purpose of high yield of cannabigerolic acid is achieved.
Owner:SENRIS BIOTECHNOLOGY (SHENZHEN) CO LTD
Phytoterpenoid facilitation of therapeutic onset and efficacy of sublingual cannabinoid administration
A cannabis plant extract based formulation to aid in stabilizing the therapeutic efficacy of cannabinoid containing treatments in patients affected with neurological diseases that comprises one or more of the following: Cannabidiol (CBD), tetrahydrocannabinol (THC), and terpenes; wherein the formulation comprises a high ratio of CBD to THC, with each of those cannabinoids in a relatively high concentration. The formulation also comprises Beta-caryophyllene that is used to further aid in neuroprotection by co-modulating CB1 and 2 receptors. Additionally, the formulation comprises the terpene humulene to assist in creating an “entourage effect” in conjunction with CBD, THC, and Beta-caryophyllene to stabilize and enhance treatment-related pharmacological actions. The formulation may also comprise Cannabichromene (CBC), Cannabigerol (CBG), and Cannabinol (CBN).
Owner:JC PHARMA INC
Topical formulations of resorcinols and cannibinoids and methods of use
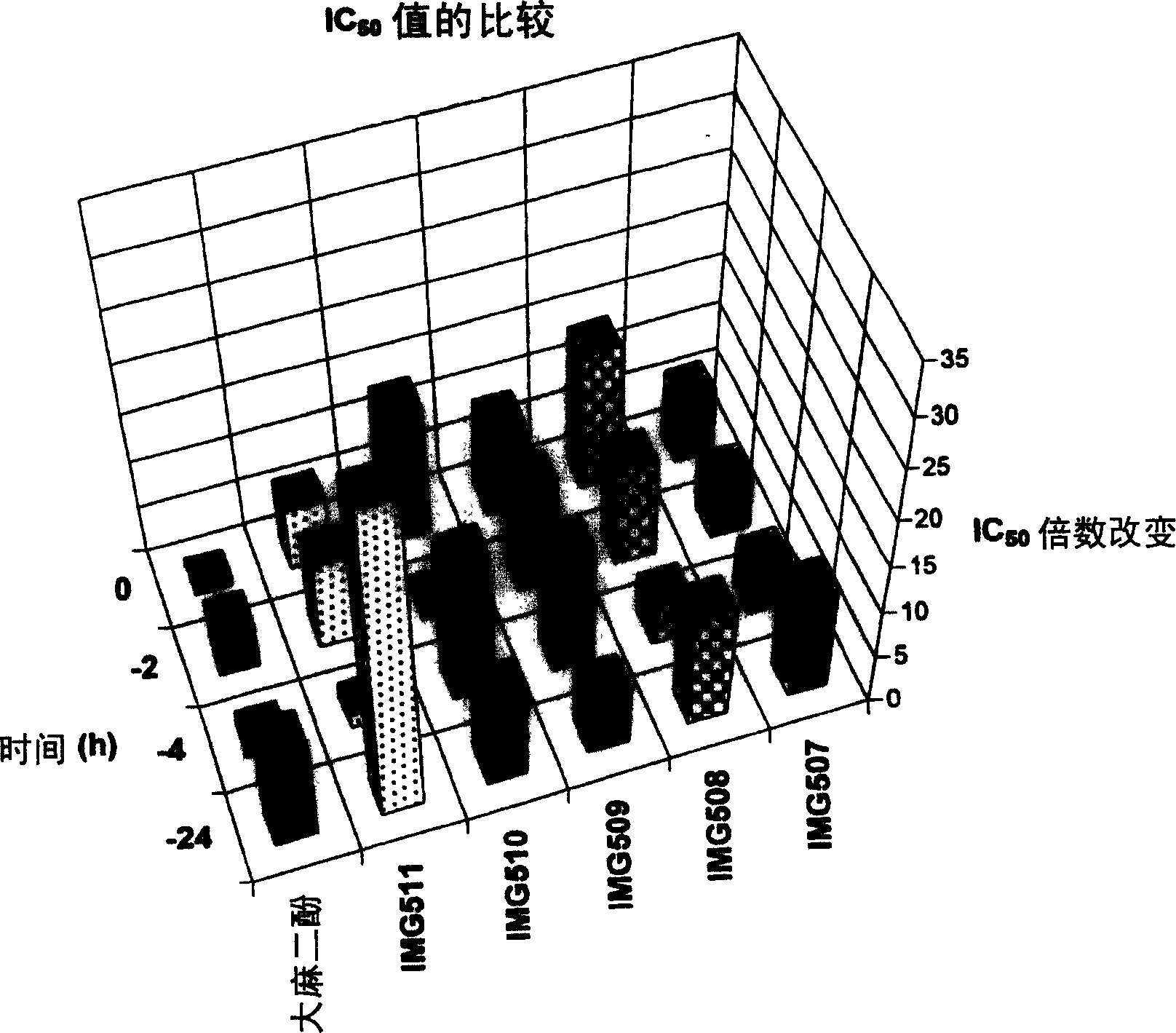
InactiveCN1652766AAvoid spreadingPharmaceutical delivery mechanismHeterocyclic compound active ingredientsWater insolubleMedicine
In one aspect, the invention provides a method for preventing the transmission of HIV from one individual to another. In accordance with the method, a pharmacologicallyacceptable composition including at least one resorcinol derivative compound and / or cannabinoid (e.g., cannabdol derivatives, Delta8-THC derivatives, cannabichromene derivatives, cannabidiol derivatives, cannabigerol derivatives) (including combinations thereof) is administered topically to a first individual harboring HIV, or to a second individual at risk of infection with HIV, proximate in time with contact between the first individual and the second individual. The invention also provides topical formulations of at least one resorcinol and / or cannabinoid and water insoluble polymers as hydrogels.
Owner:IMMUGEN PHARMA INC
Cannabinoids for use in the treatment of neuropathic pain
Owner:GW PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com