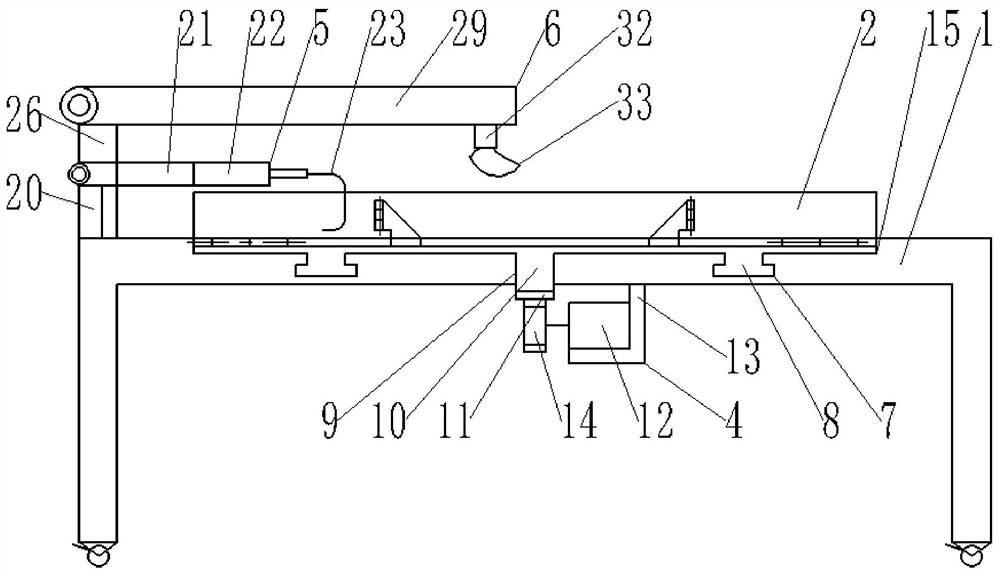
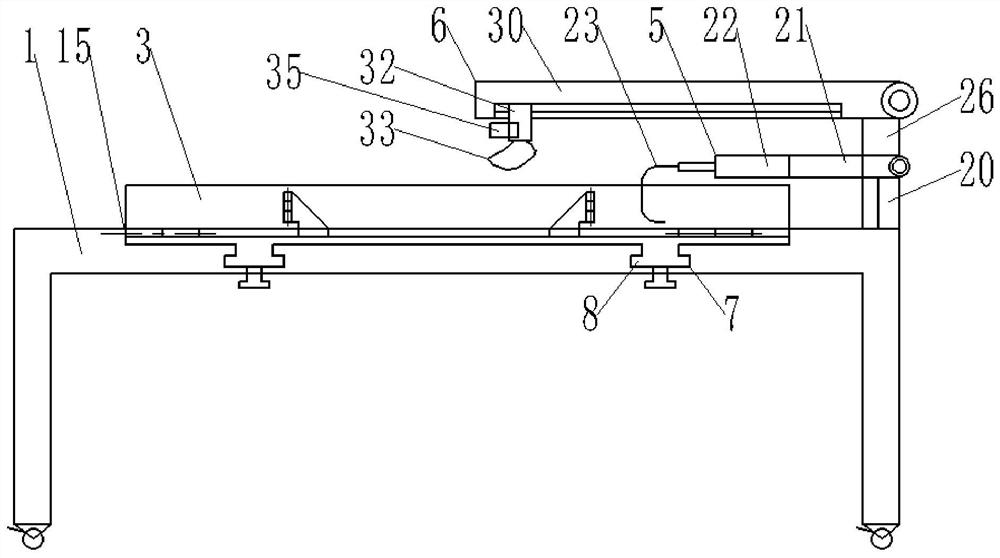
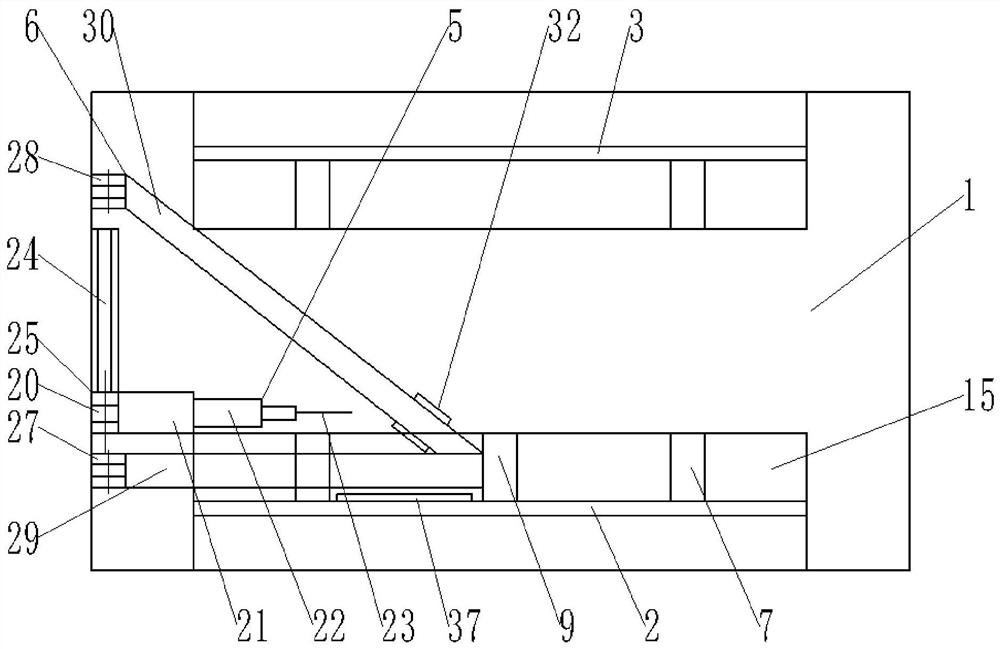
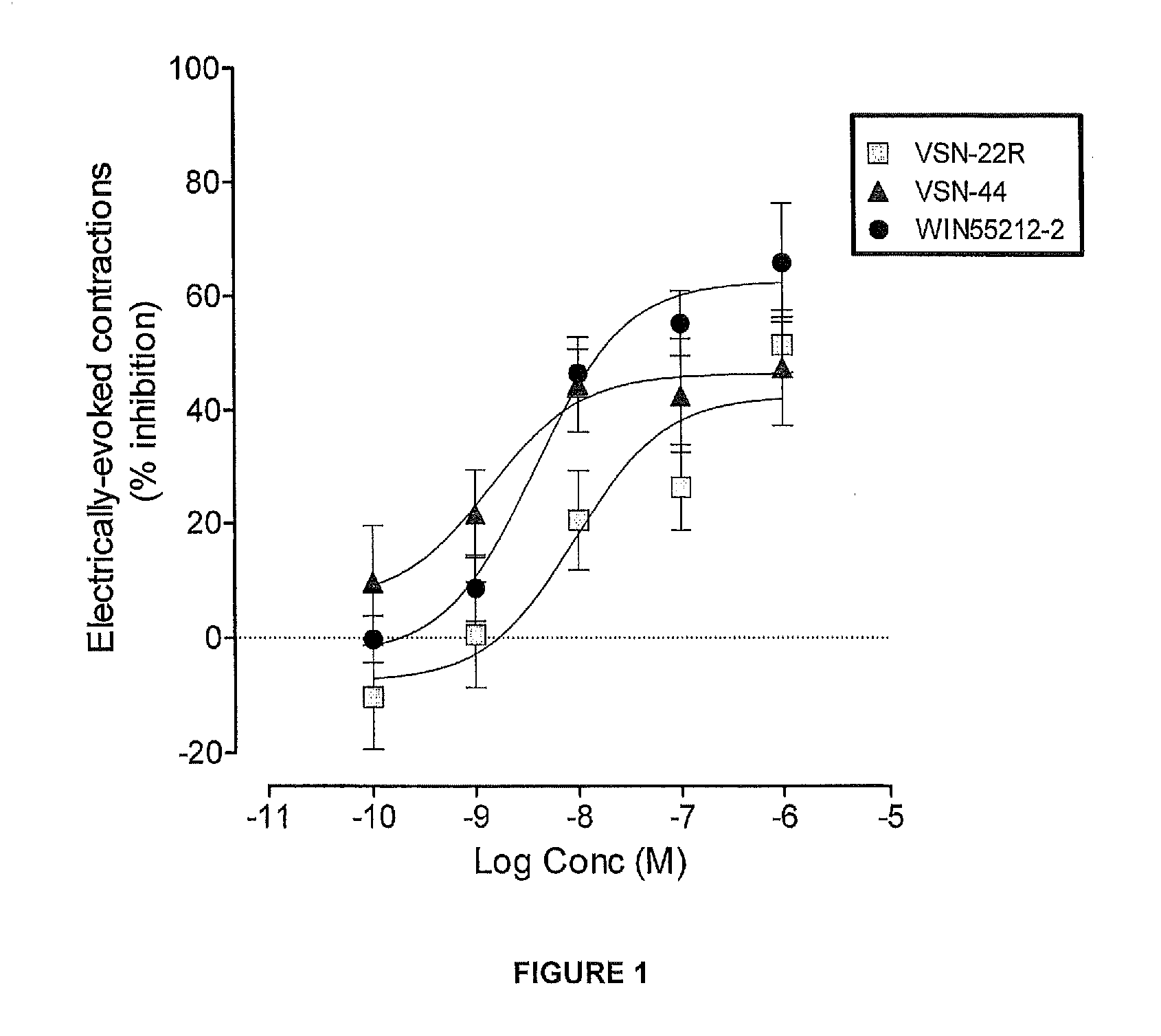
Patents
Literature
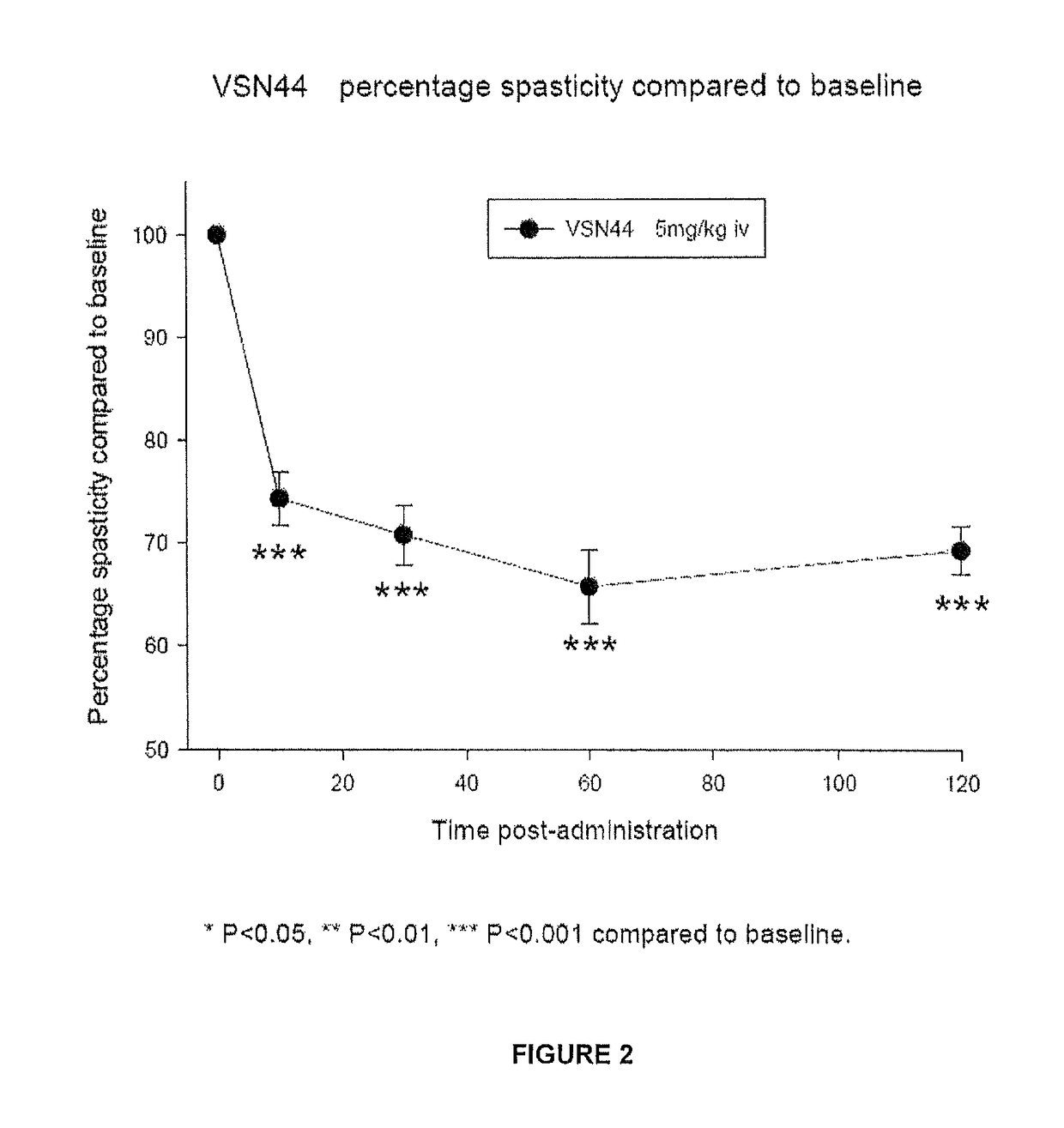
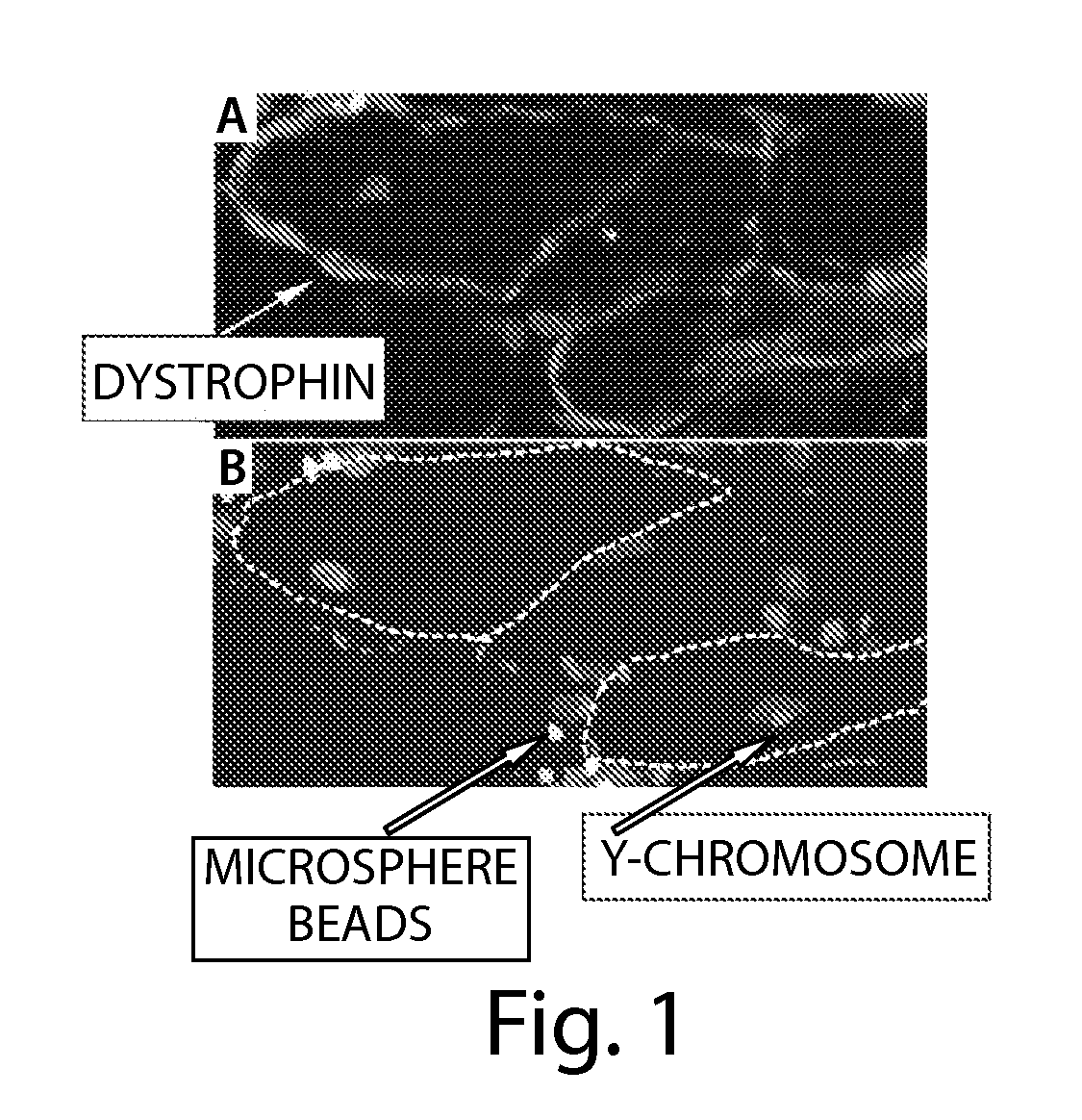
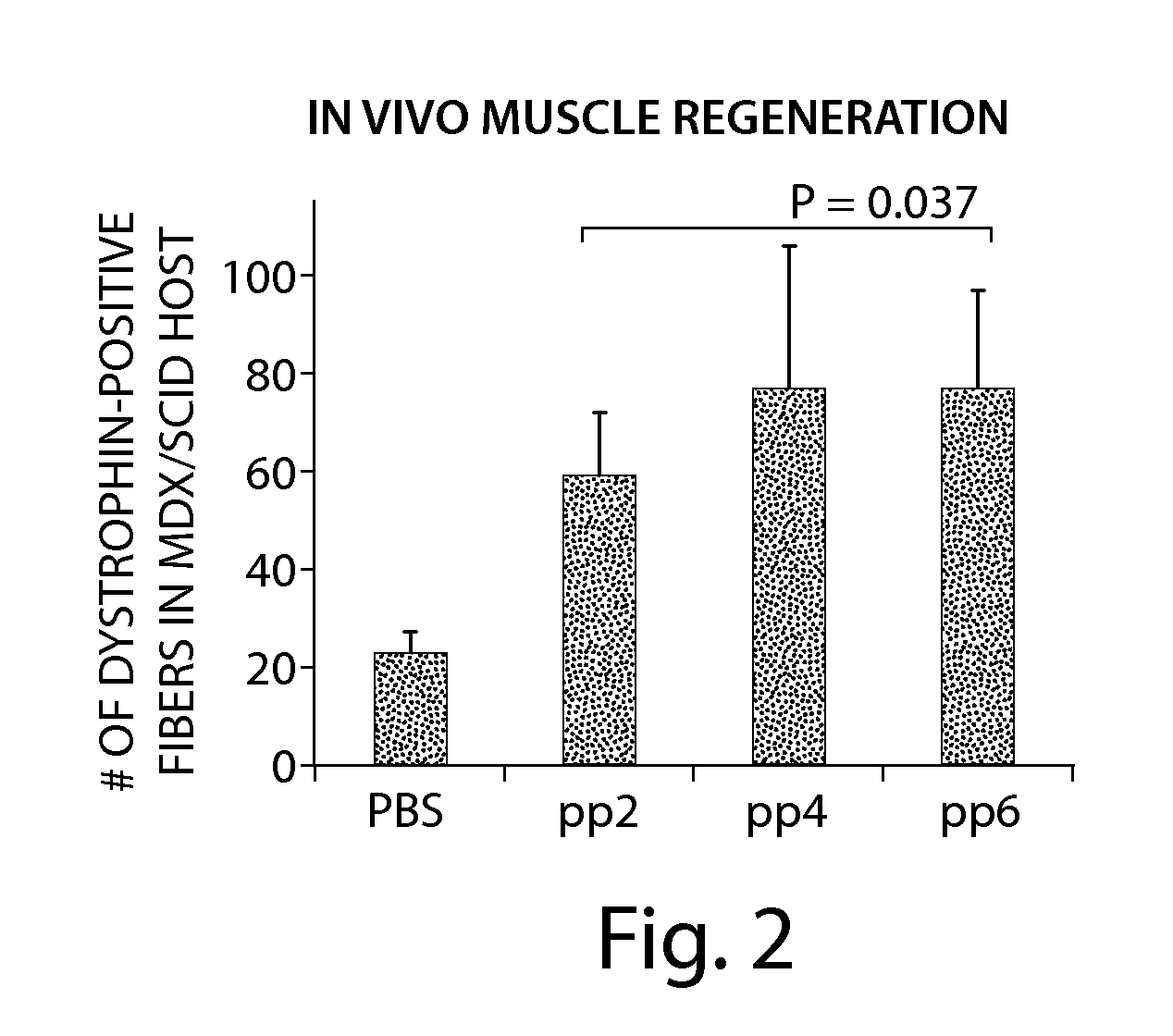
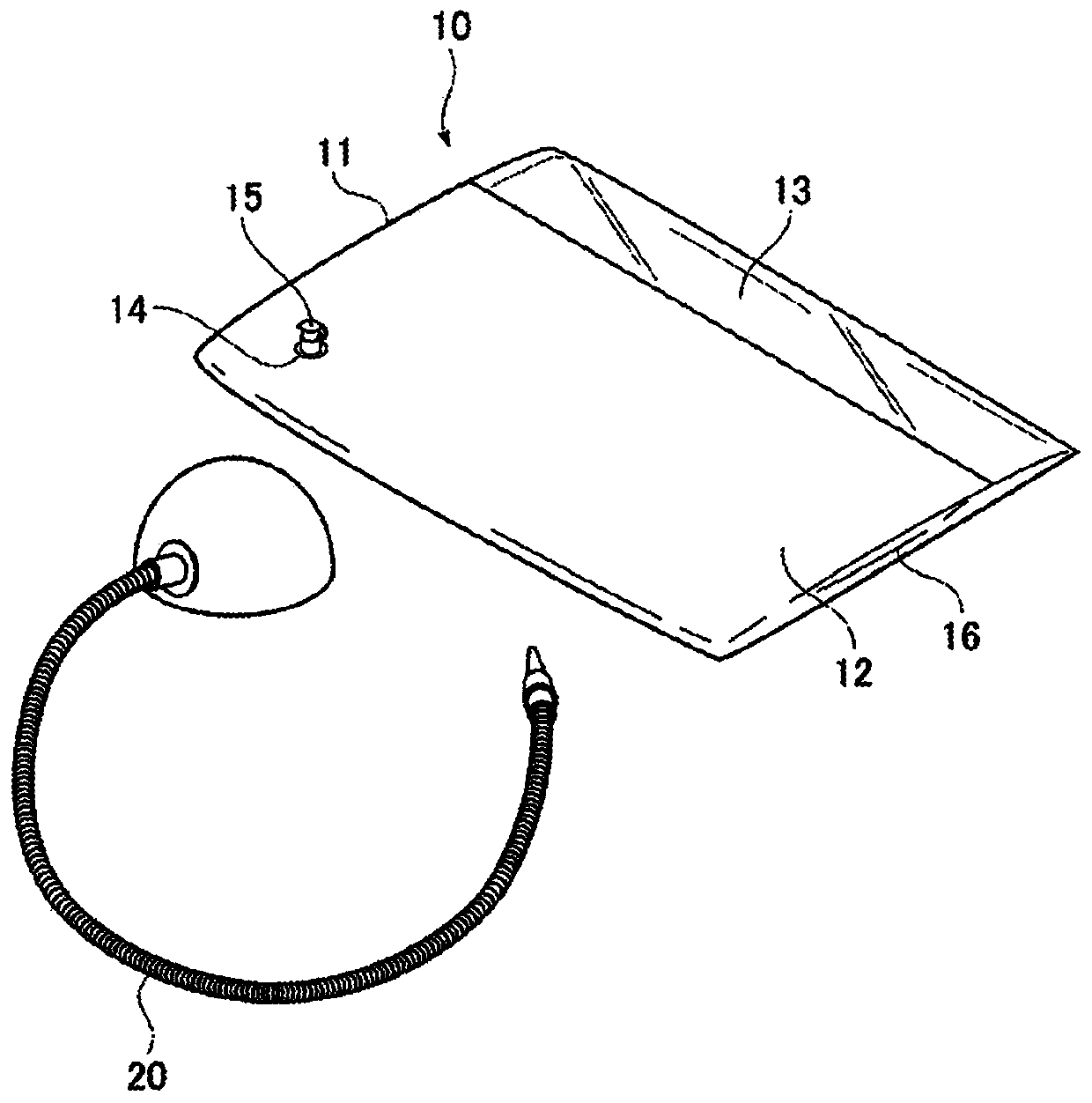
33 results about "Spasticity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An increased rigidity of muscles due to brain or spinal cord injury.
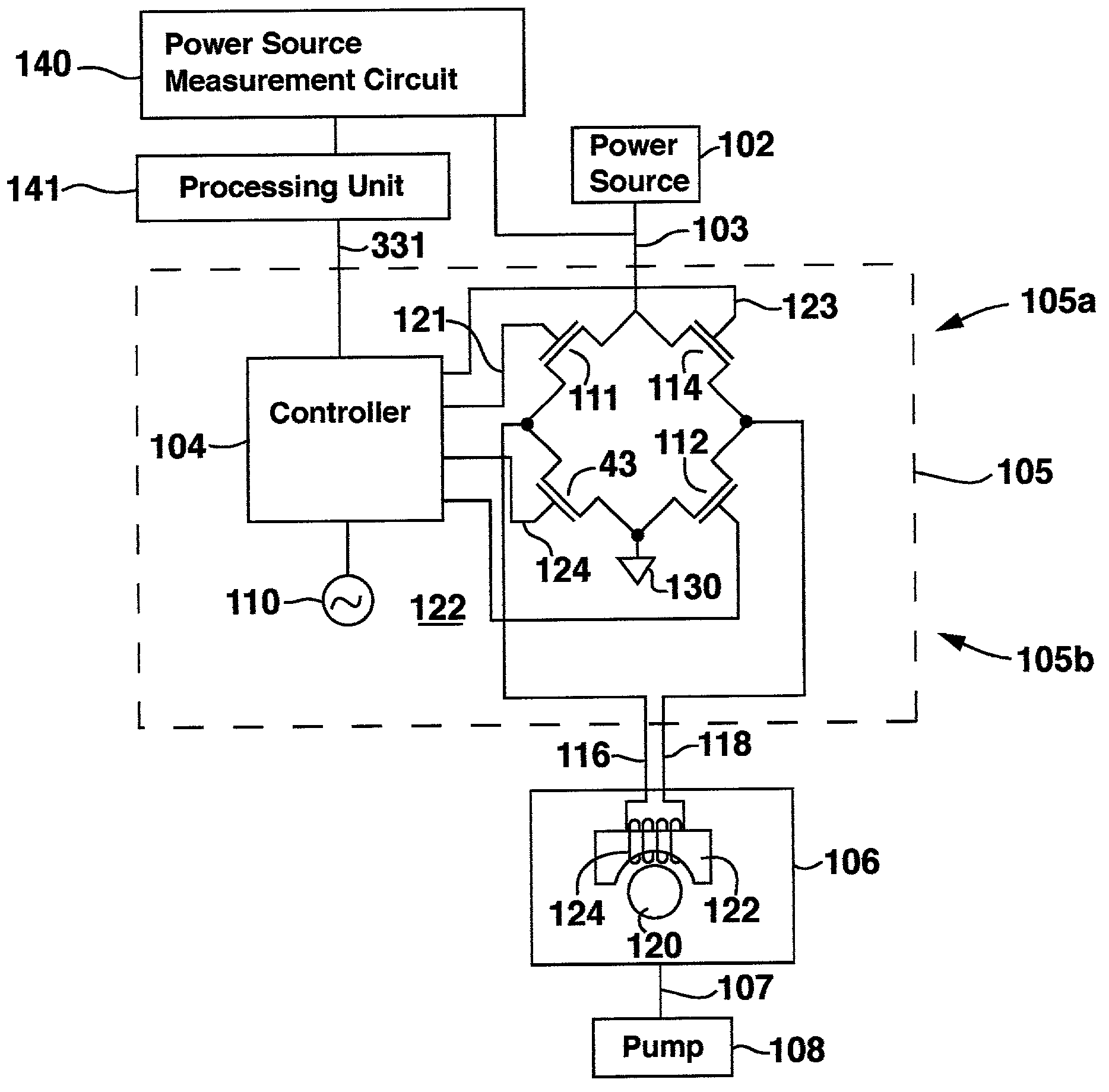
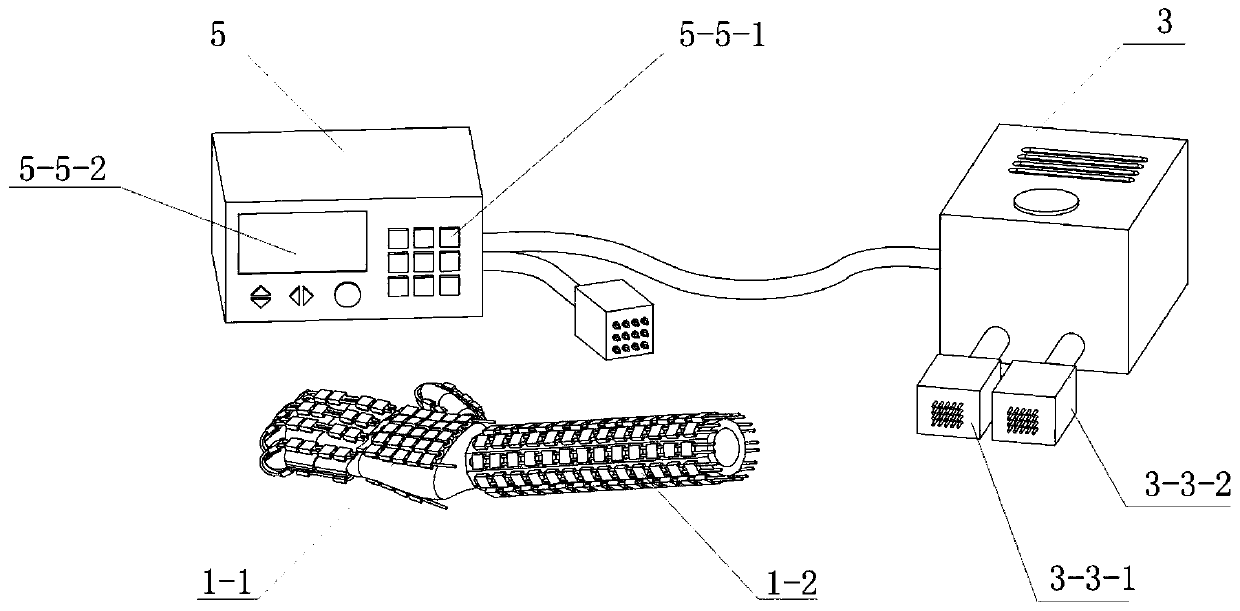
Implantable infusion device with optimized peristaltic pump motor drive
InactiveUS7122026B2Maximum service lifeAvoid wastingPharmaceutical delivery mechanismMedical devicesPeristaltic pumpPulse parameter
A medical device known as an implantable therapeutic substance delivery device is configured for implanting in humans to deliver a therapeutic substance such as pharmaceutical compositions, genetic materials, and biologics to treat a variety of medical conditions such as pain, spasticity, cancer, and many other conditions. The infusion device incorporates a stepper motor that controls the infusion flow rate during the service life of the device. The stepper motor is controlled by continuously varying electrical pulse parameters based on the continuously decreasing power source voltage during the service life of the substance delivery device. In particular the stepper motor electrical pulse parameters, especially duty cycle, are selected to efficiently compensate for decreasing battery voltage thereby optimizing the motor performance while maximizing the power source service life. The infusion device has a housing, a power source, a therapeutic substance reservoir, a therapeutic substance pump, and electronics. Many embodiments of the therapeutic substance delivery device with optimized pump motor drive and its methods of operation are possible.
Owner:MEDTRONIC INC
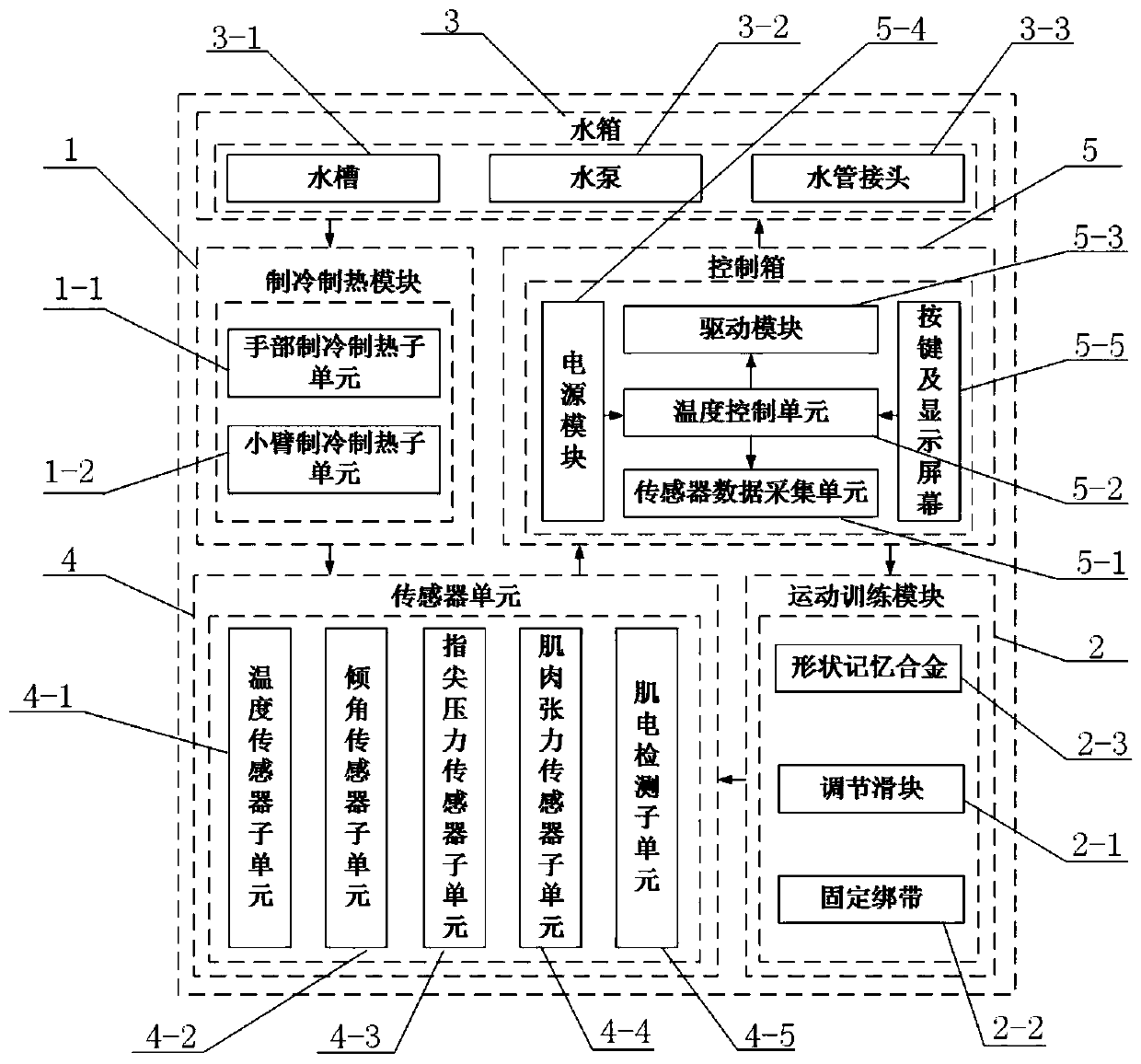
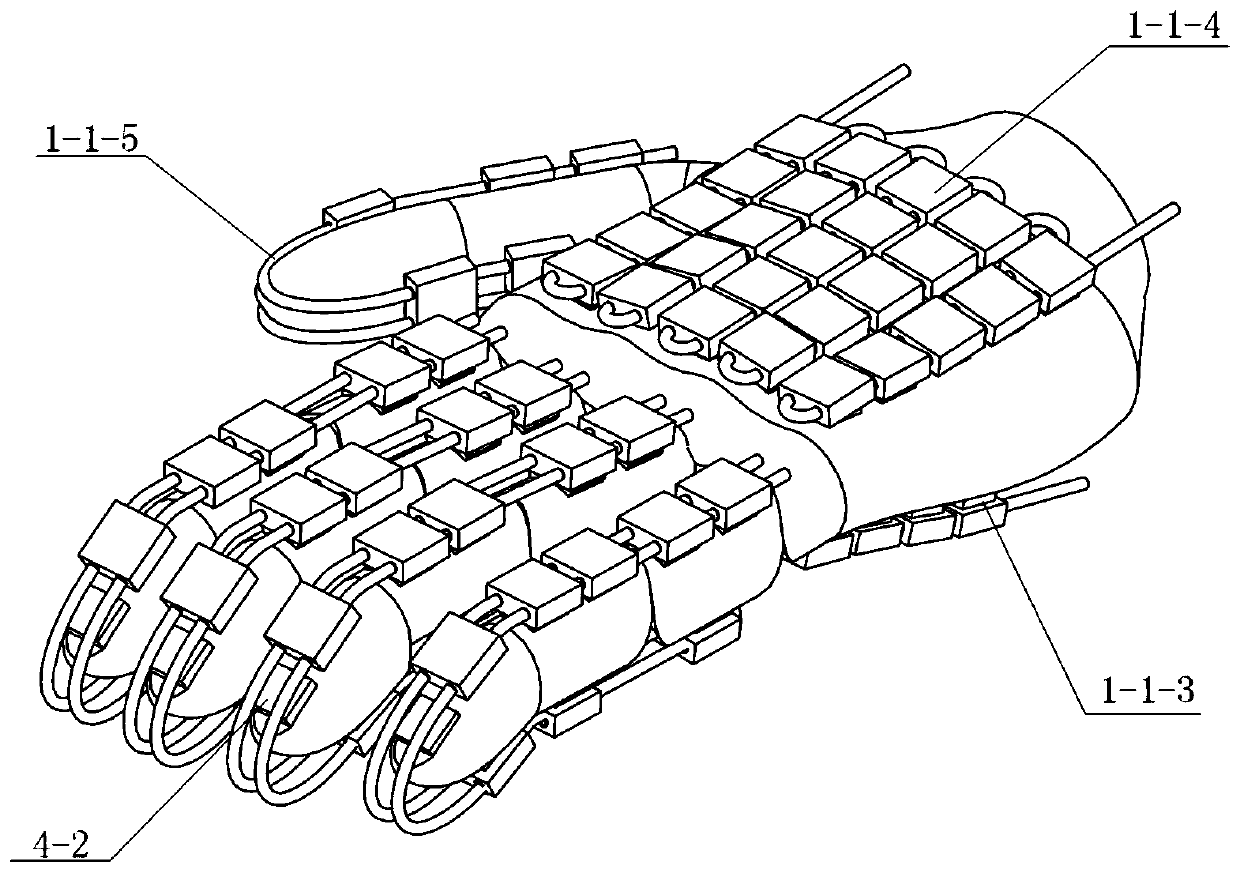
Hand function rehabilitation robot for patients with stroke and use method of hand function rehabilitation robot
ActiveCN110731879AEffective detection of spasticityEasy to carryDiagnosticsChiropractic devicesHand partsShape-memory alloy
The present invention discloses a hand function rehabilitation robot for patients with stroke and a use method of the hand function rehabilitation robot. The rehabilitation robot comprises a refrigeration and heating module, a motion training module, a water tank, a sensor unit and a control box, the refrigeration and heating module consists of a heat-conducting glove, a refrigeration sheet fixingfilm, semiconductor refrigeration sheets, a refrigerator, a flexible water pipe and a protective outer sleeve, and the motion training module comprises an adjusting slide block, a fixing bandage anda shape memory alloy sheet; and the sensor unit comprises a temperature sensor subunit, an inclination angle sensor subunit, a fingertip pressure sensor subunit, a muscle tension detection subunit anda myoelectricity detection subunit. Spasticity of a patient is evaluated to give out training parameters through multi-sensor information fusion. The robot has advantages of accurate control, compactstructure, etc., can realize cold and heat therapy and exercise training at the same time, improves rehabilitation efficiency, and can solve problems of huge system, inconvenient use, slow temperature response, etc. of traditional temperature stimulation therapy based on hydrotherapy.
Owner:SOUTHEAST UNIV
Baclofen solution for low-volume therapeutic delivery
A high concentration baclofen solution is provided suitable for therapeutic use in a medical setting. A high concentration solution of baclofen in multivalent physiological ion solution such as artificial cerebrospinal fluid is provided with concentrations of baclofen of 10 mg / ml. Artificial cerebrospinal fluid is particularly advantageous as a baclofen solvent. A medical package is also provided for baclofen delivery to patients suffering from spasticity.
Owner:MEYTHALER JAY M +1
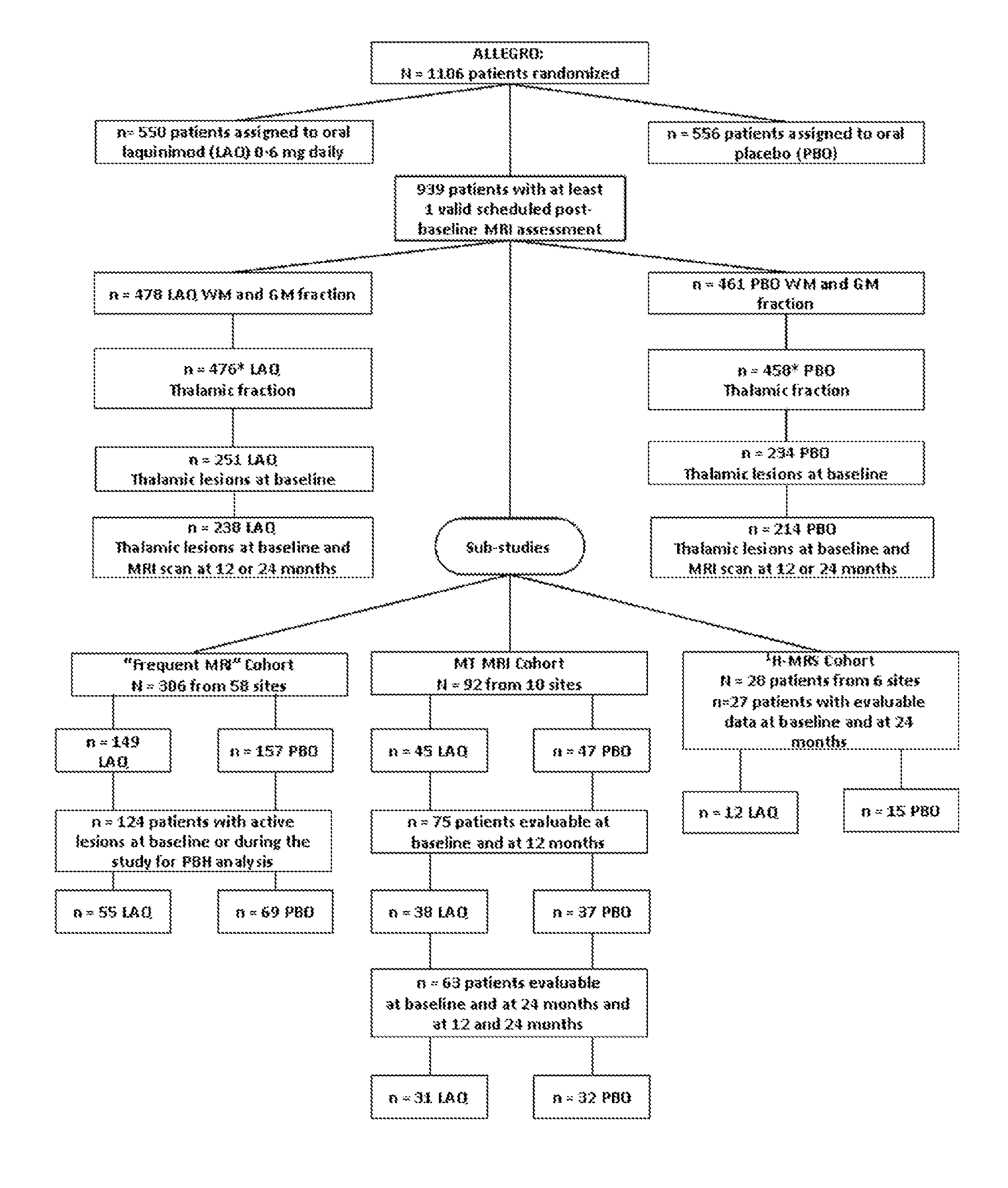
Laquinimod for reducing thalamic damage in multiple sclerosis
InactiveUS20140107154A1Inhibiting and reducing thalamic damageReduce harmBiocideNervous disorderDiseaseThalamus
This invention provides methods for inhibiting or reducing thalamic damage in a subject comprising administering to the subject an amount of laquinimod, wherein the subject is a human patient afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome who has been determined to have thalamic damage at baseline, a subject afflicted with a disease or disorder other than a form of multiple sclerosis or a clinically isolated syndrome, or a subject not afflicted with a form of multiple sclerosis or a presenting clinically isolated syndrome, and laquinimod and laquinimod pharmaceutical compositions for use thereof. This invention also provides methods for inhibiting or reducing tremor or spasticity in a subject afflicted by tremor or spasticity, comprising administering to the subject an amount of laquinimod, and laquinimod and laquinimod pharmaceutical compositions for use thereof.
Owner:TEVA PHARMA IND LTD
Spasticity measurement device
InactiveUS20140343459A1Easy to measurePrevent rotationPerson identificationSensorsHeel-and-toeMuscle contraction
A spasticity measurement apparatus includes: a leg mounting fixture including a lower leg mounting portion mounted on a lower leg of a subject for fixation, and a foot receiving portion coupled to a lower end portion of the lower leg mounting portion and having a heel receiving portion and a toe receiving portion for respectively receiving a heel and a toe of a foot leading to the lower leg, the toe receiving portion being disposed relatively rotatably around the heel receiving portion in an approaching rotation direction bringing the toe receiving portion closer to the lower leg mounting portion; a rotation inhibition apparatus inhibiting relative rotation when the foot receiving portion rotates relative to the lower leg mounting portion in the approaching direction; and a muscular contraction force measurement apparatus measuring a muscular contraction force acting in a rotation direction.
Owner:TOMEI BRACE
Modulator
InactiveUS20080262011A1Good water solubilityReduction in lipophilicityBiocideNervous disorderArylHalogen
The present invention relates to a compound of formula I, or a pharmaceutically acceptable salt thereof. Formula (I), wherein R1 and R2 are each independently H or alkyl; Y is an alkyl group. CONR3R4, COOR5SO2NR16R17, NHSO2R18 or CN; X is an aryl or heteroaryl group, each of which may be optionally substituted with one or more substituents selected from (CH2)mZ where Z is halogen, OH, CN, alkyl, alkoxy, NO2, CF3, CONR6R7, CN, NR8R9, COOR10 or NHCOR11 and m is 0 to 3; R3 to R11 are each independently H, alkyl or aryl, wherein said alkyl and aryl groups are optionally substituted by one or more substituents selected from halogen, OH, CN, alkyl, alkoxy, NO2, CF3, CONR12R13, CN, NH2, COOR14, NHCOR15, and CN; R12 to R18 are each independently H or alkyl, more preferably H or Me; n is 1 to 6; wherein the compound is other than 3′,5′-dimethyl-4-(1,1-dimethylheptyl)-1,1′-biphenyl-2-ol. Further aspects of the invention related to the use of such compounds in the preparation of a medicament for the treatment of a muscular disorder, a gastrointestinal disorder, or for controlling spasticity or tremors.
Owner:UCL BUSINESS PLC
Application of butylphthalide in preparation of medicament for treating bronchial asthma
InactiveCN102793696AGood for asthmaReduce adverse reactionsOrganic active ingredientsRespiratory disorderTreatment effectButylphthalide
The invention provides novel medical application of butylphthalide, i.e. the application of the butylphthalide in the preparation of a medicament for treating the bronchial asthma. The experimental research on the spasmolysis of the butylphthalide on the isolated tracheal smooth muscle of a guinea pig and the influence of the butylphthalide on the incubation period of asthma of the guinea pig shows that the butylphthalide in the concentration range of 3.68*10-6 to 3.68*10-4g / mL has obvious spasmolysis on the tracheal smooth muscle of the guinea pig in the isolated spasticity caused by 5*10-6g / mL of acetylcholine or histamine. On the basis of continuously applying the butylphthalide to the guinea pig which is sensitive for the acetylcholine and the histamine for 10 days, a mixture of the acetylcholine and the histamine is atomized and sucked and the incubation period of asthma is observed. A result shows that 30 mg / kg and 90 mg / kg of butylphthalide has an effect of relieving the tracheospasm of the guinea pig, which is caused by the acetylcholine and the histamine. Therefore, the butylphthalide has a treatment effect on the asthma guinea pig and the treatment effect of the butylphthalide on the asthma guinea pig is related to the effect of inhibiting Ca2+ inner flow and expanded tracheal smooth muscle of the butylphthalide.
Owner:GANSU UNIV OF CHINESE MEDICINE
Modulator
InactiveUS7696382B2Good water solubilityReduction in lipophilicityBiocideNervous disorderArylSpasticity
The present invention relates to a compound of formula (I), or a pharmaceutically acceptable salt thereof, wherein Z is OR1 or NR1R2 wherein each of R1 and R2 is independently H, or a hydrocarbyl group; X is an alkylene, alkenylene, or alkynylene group, each of which may be optionally substituted by one or more substituents selected from alkyl, COOH, CO2-alkyl, alkenyl, CN, NH2, hydroxy, halo, alkoxy, CF3 and nitro; Y is a polar functional group selected from OH, NO2, CN, COR3, COOR3, NR3R4, CONR3R4, SO3H, SO2—R3, SO2NR3R4 and CF3, where each of R3 and R4 is independently H or a hydrocarbyl group; A is an aryl or heteroaryl group, each of which may be optionally substituted; and B is (CH2)n where n is 0, 1, 2, 3, 4 or 5; with the proviso that: (i) when A is phenyl, n is 0, and Z is OH, X—Y is other than meta-C≡—C—(CH2)2CO2H, meta-C≡—C—(CH2)2OH, meta-C≡C—(CH2)2CO2Me, meta-(CH2)4CO2H, ortho-CH2CO2H, ortho-(CH2)2CO2H and ortho-(CH2)4CO2H; and (ii) when A is phenyl, n is 0, and Z is OMe, X—Y is other than meta-C≡C—(CH2)4OH. Further aspects of the invention relate to the use of such compounds in the preparation of a medicament for the treatment of a muscular disorder, a gastrointestinal disorder, or for controlling spasticity or tremors.
Owner:UCL BUSINESS PLC
Motion delay and tremor quantitatively detecting device and detecting method
PendingCN108664147ANo harmNo side effectsInput/output processes for data processingHandwritingTremor amplitude
The present invention discloses a motion delay and tremor quantitatively detecting device and detecting method, and relates to the field of medical instruments. The motion delay and tremor quantitatively detecting method and device disclosed by the present invention are implemented through cooperation of software in combination with hardware electronic devices. According to the scheme of the present invention, fine pen data input by a subject by using an electronic pen is captured and analyzed through deep learning and big data methods, and the motion time, the median motion speed, the acceleration maximum value, the tremor frequency, the tremor amplitude, and the position (x,y) of the pen during handwriting of the subject, a motion trajectory within 1 cm from the plane written by using the electronic writing input device, dynamics indicators such as the speed and the acceleration, and other data are obtained, so that a solution of an auxiliary detection and medication effect trackingmethod for quantifiable objective indicators of the motion delay and tremor (especially in the early stages) is provided from the perspective of typical motion delay and tremor core symptoms such as the tremor, the motion delay, the spasticity and the like.
Owner:上海磐度信息科技有限公司
Electrical producing creams
InactiveUS20080069903A1Improve metabolic activityRelieve painBiocideNervous disorderSinusitisDisease
New electrical producing creams are provided. In one embodiment, the electrical producing creams comprise menthol and camphor, preferably provided as part of a base gel, supplemented with potassium and a source of oxygen. The most preferred base gel is sold under the name SOMBRA. In another embodiment, the electrical producing creams comprise potassium and a source of oxygen supplemented with optional active and inactive ingredients. The most preferred source of oxygen is a chlorite (e.g., sodium chlorite) and / or spirulina. The electrical producing creams provide high metabolic activities and sustain those activities over prolonged periods of time, thus being useful for treating a large variety of ailments, including diabetic neuropathy, post hepatic neuralgia, scleroderma, psoriasis, strain, spasticity, headaches, neuropathy secondary to drugs, peripheral neuropathy, leg pain, muscle cramps, muscle aches and pains, bruise, sinusitis, sprain, arthritis, joint pain (arthralgia), and edema.
Owner:MORGAN CLYDE E
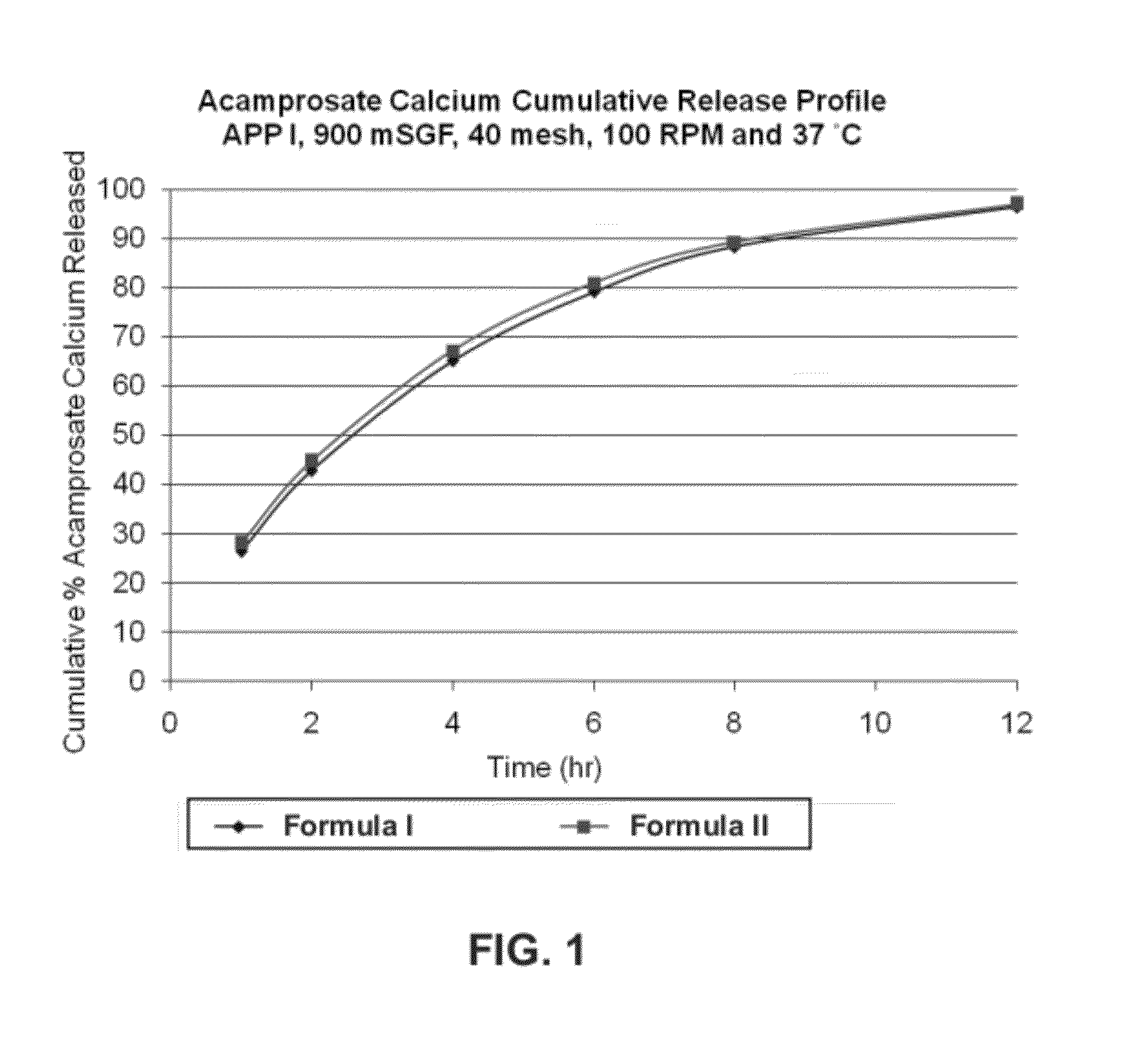
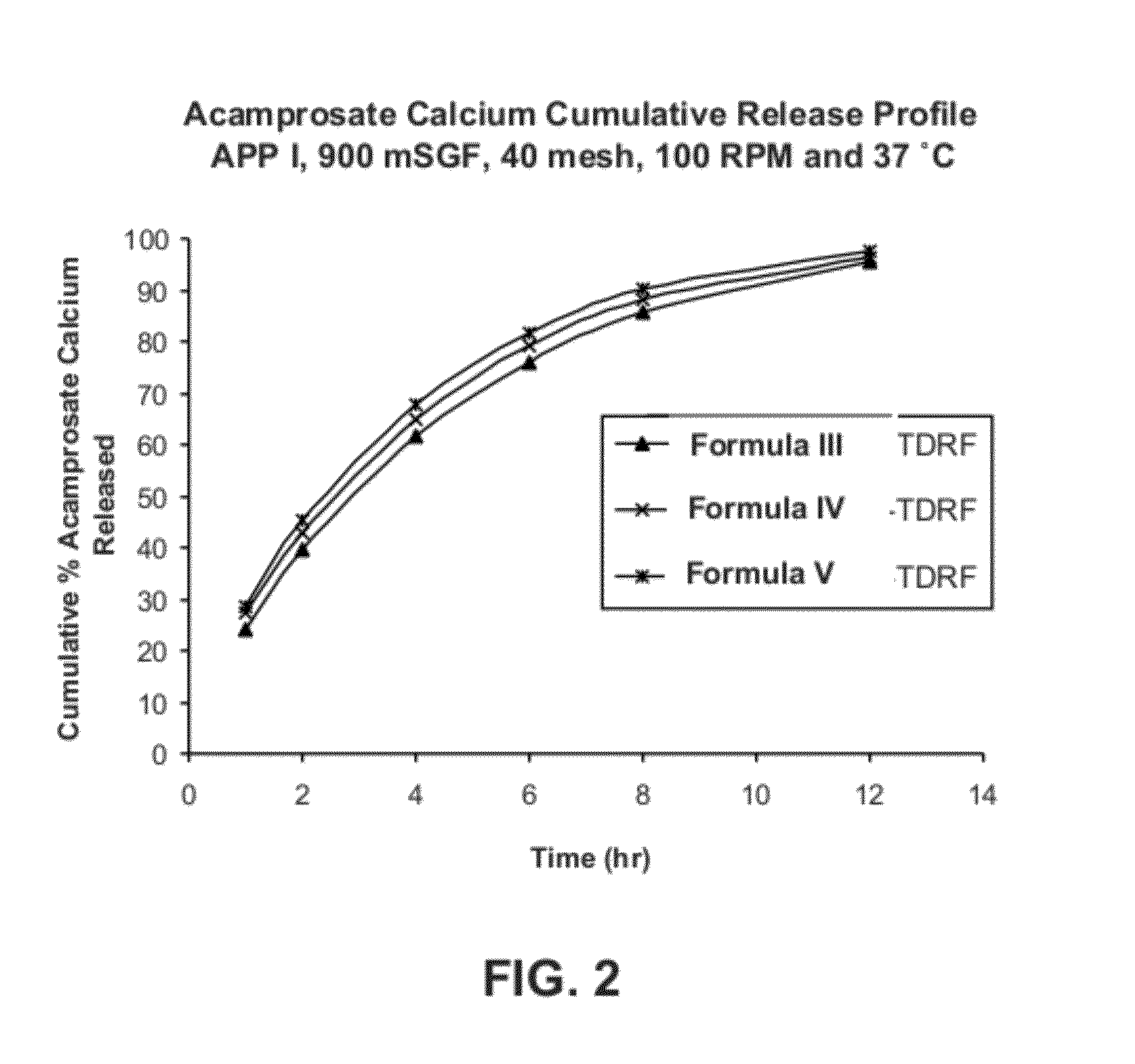
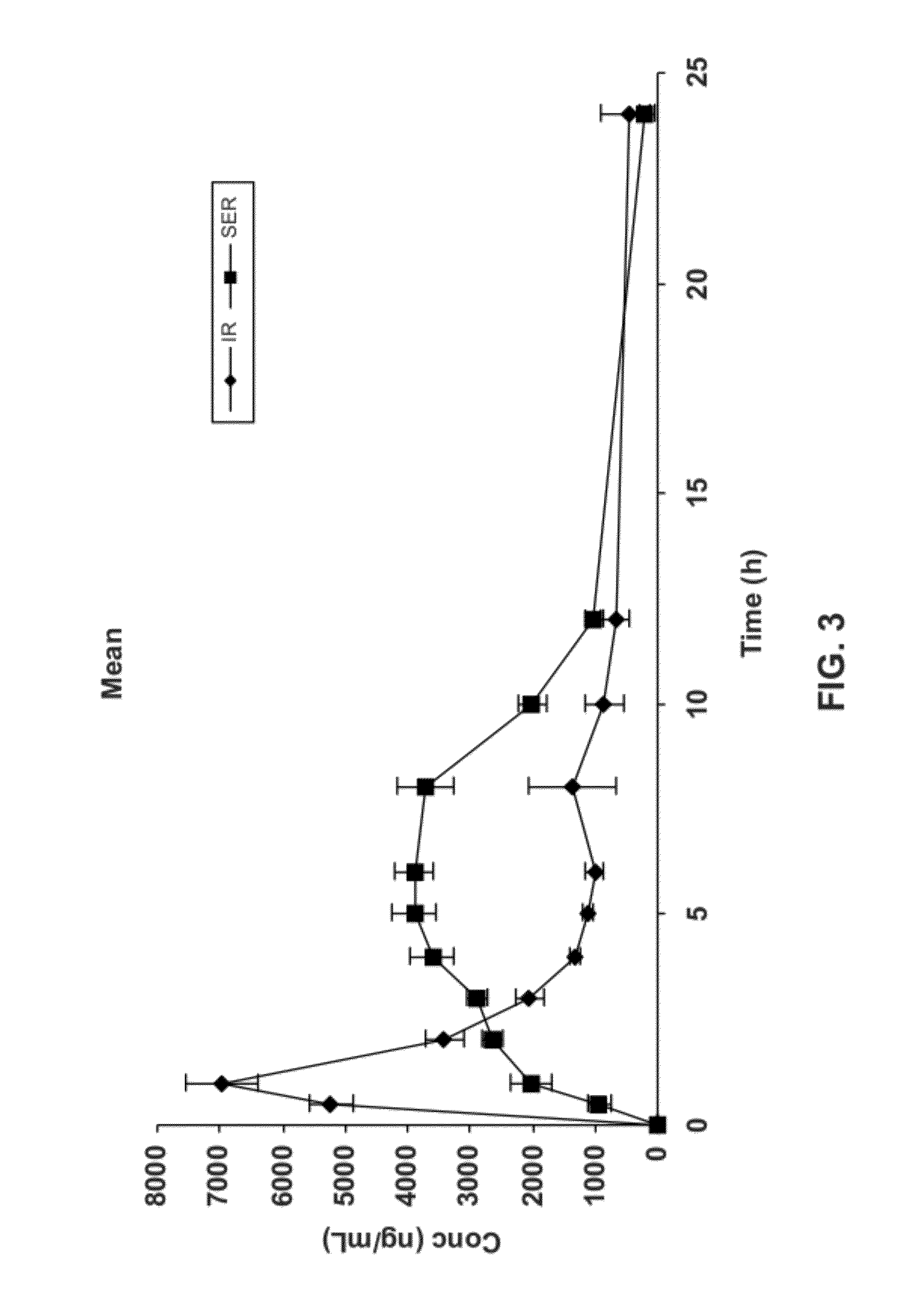
Gastric retentive dosage forms for extended release of acamprosate into the upper gastrointestinal tract
Gastric retentive dosage forms for sustained release of acamprosate are described which may allow once- or twice-daily dosing for both acute and long-term treatment of a disorder including alcohol dependence, tinnitus, sleep apnea, Parkinson's disease, levodopa-induced dyskinesias in Parkinson's disease, Alzheimer's disease, Huntington's disease, Amyotrophic lateral sclerosis, Cortical spreading depression, migraine, schizophrenia, anxiety, tardive dyskinesia, spasticity, multiple sclerosis, various types pain, or binge eating. Methods of treatment using the dosage forms and methods of making the dosage forms are also described.
Owner:DEPOMED SYST INC
Skin scraping plate and manufacturing method thereof
InactiveCN104921911AGood treatment effectEasy to useSuction-kneading massageTreatment effectJoint arthralgia
The invention relates to the technical field of medical health-care equipment, and particularly relates to a skin scraping plate used for alleviating rheumatic pain, arthralgia, and numbness spasm, and a manufacturing method thereof. The skin scraping plate is made of liquidambar formosana and is obtained through the immersion of eucommia, buck grass, Gaultheria yunnanensis, and liquor. The skin scraping plate is made of liquidambar formosana, the liquidambar formosana itself has the effects of dispelling wind and eliminating dampness, and dredging collaterals and activating blood circulation, and the skin scraping plate is soaked by the eucommia, buck grass, and Gaultheria yunnanensis, the above three plants have the treatment effects on wind-cold-dampness arthralgia, tendon and vessel spasm pain, and traumatic injury, so that the skin scraping plate has good treatment effects on rheumatic pain, arthralgia, and numbness spasm. The skin scraping plate is simple in use and can directly scrap the ache or discomfort part, and compared with a skin scraping device made of horns and jade, the skin scraping plate provided has the characteristic of excellent treatment effects. In addition, the skin scraping plate is made of wood, so that the skin scraping plate is low in cost and can meet the use demands of a lot of consumers.
Owner:GUIYANG MINGCAOTANG GREEN SCI & TECH DEV CENT
Topical therapy for the treatment of migranes, muscle sprains, muscle spasms, spasticity and related conditions
ActiveUS20120114741A1Quick treatmentEliminate side effectsBiocideNervous disorderTopical treatmentSerotonin Agonist
The invention is directed to topical formulations and methods of treating a migraines and / or cluster headaches, muscle sprains, muscle spasms, spasticity, tension headaches, tension related migraines and related conditions associated with muscle tension and pain with a therapeutically effective amount of an ergot alkaloid, skeletal muscle relaxant, serotonin agonist, combinations thereof, pharmaceutically acceptable salt thereof, prodrugs thereof or derivative thereof.
Owner:DEF LLC
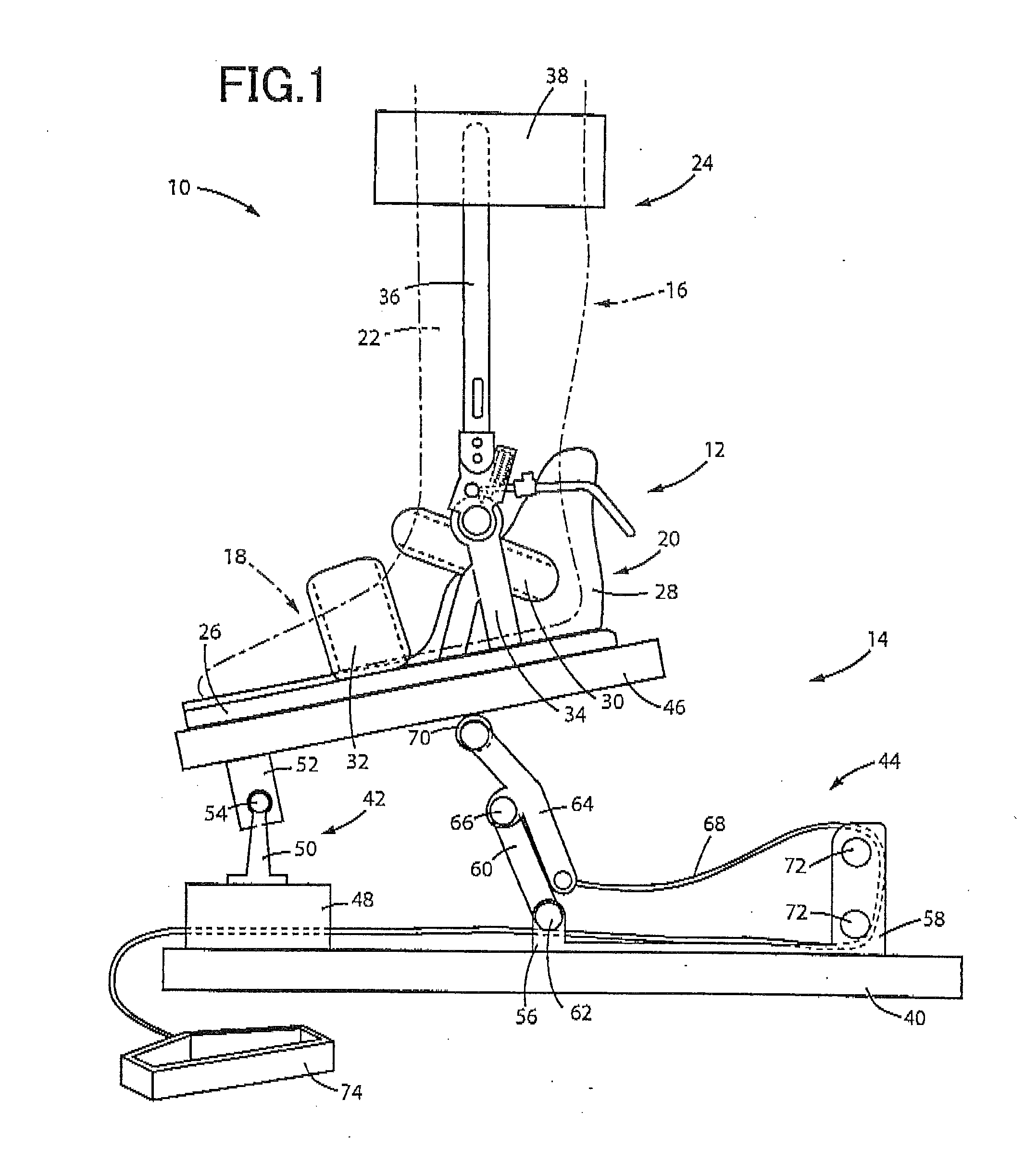
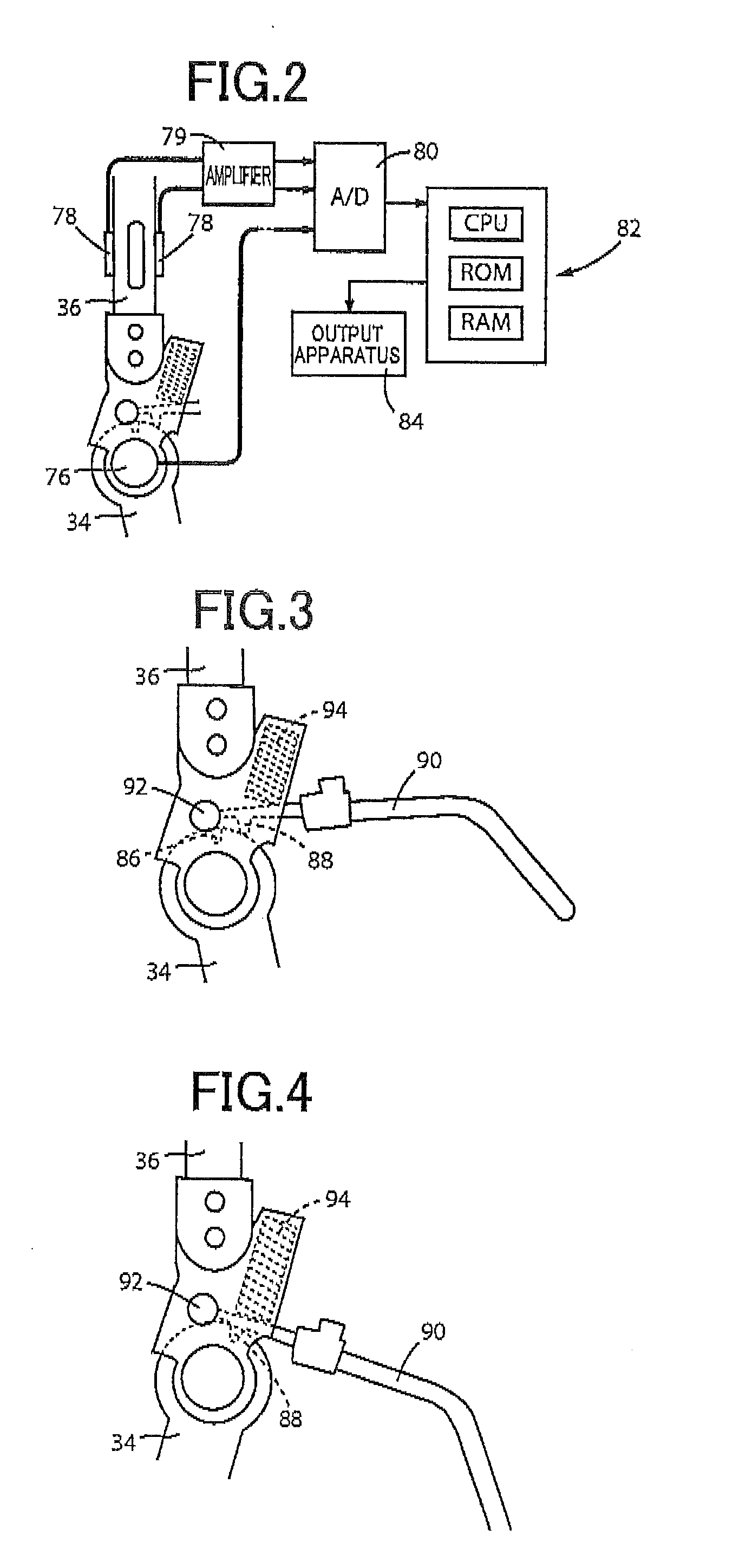
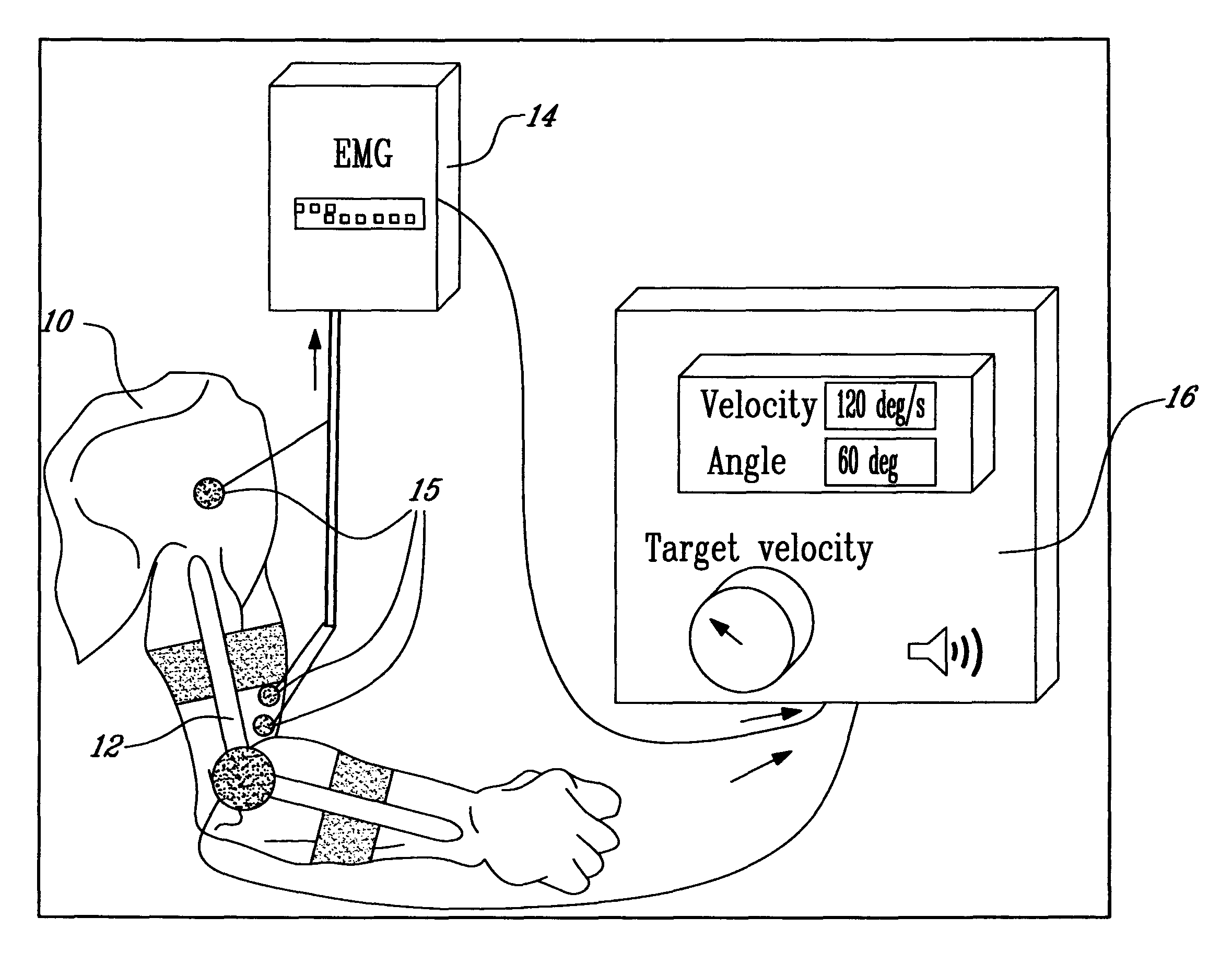
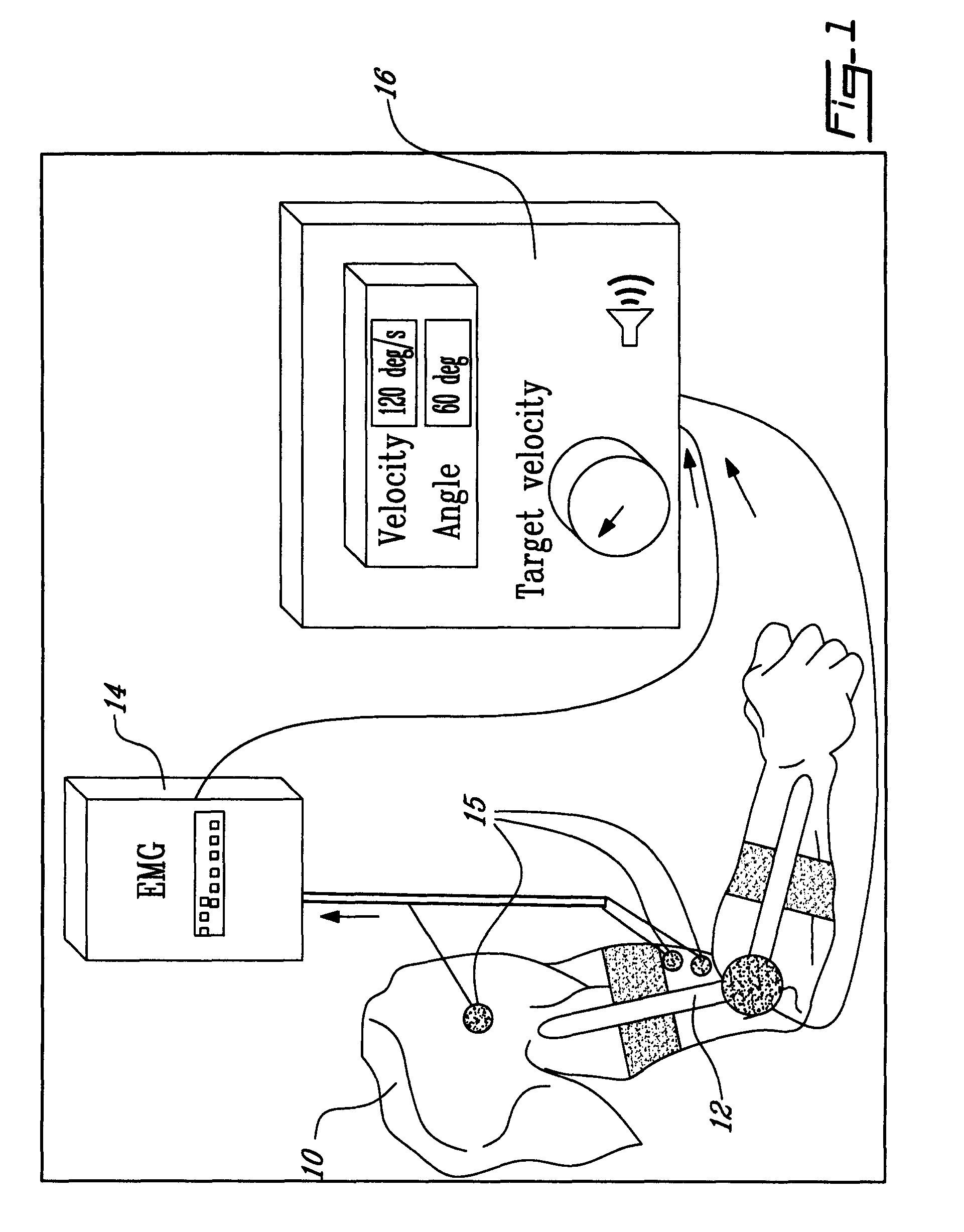
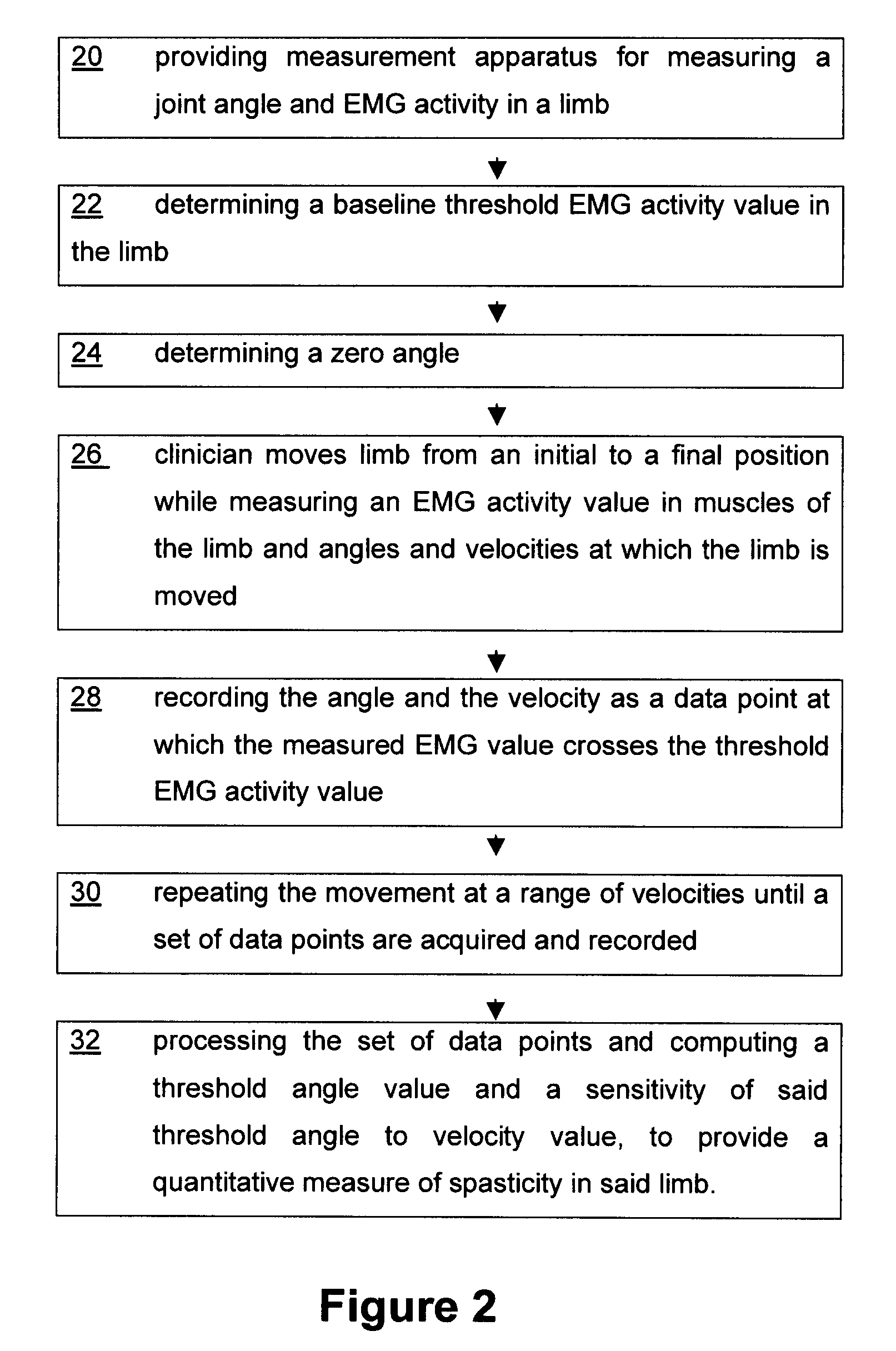
Method and apparatus for determining spasticity
Owner:MCGILL UNIV +1
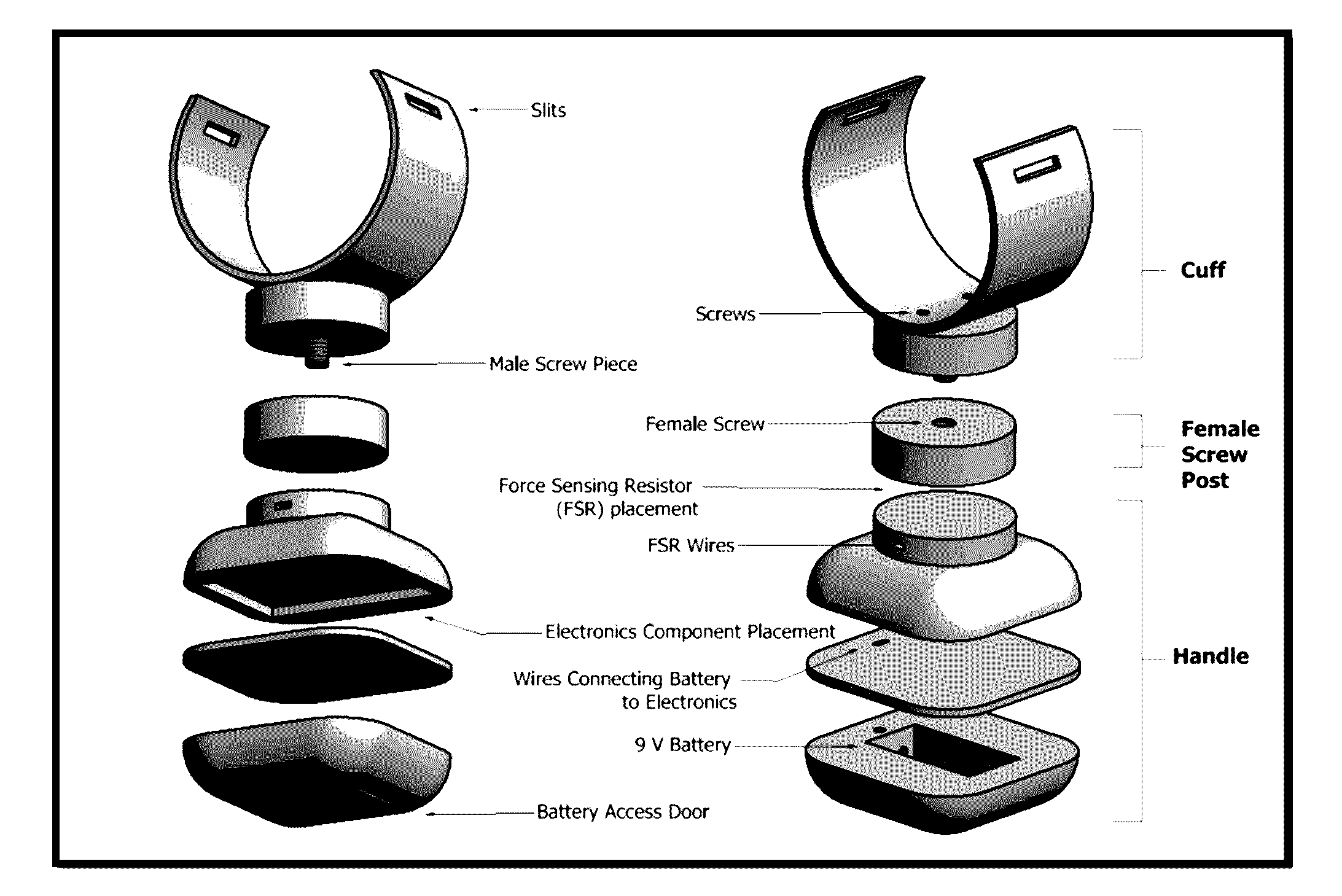
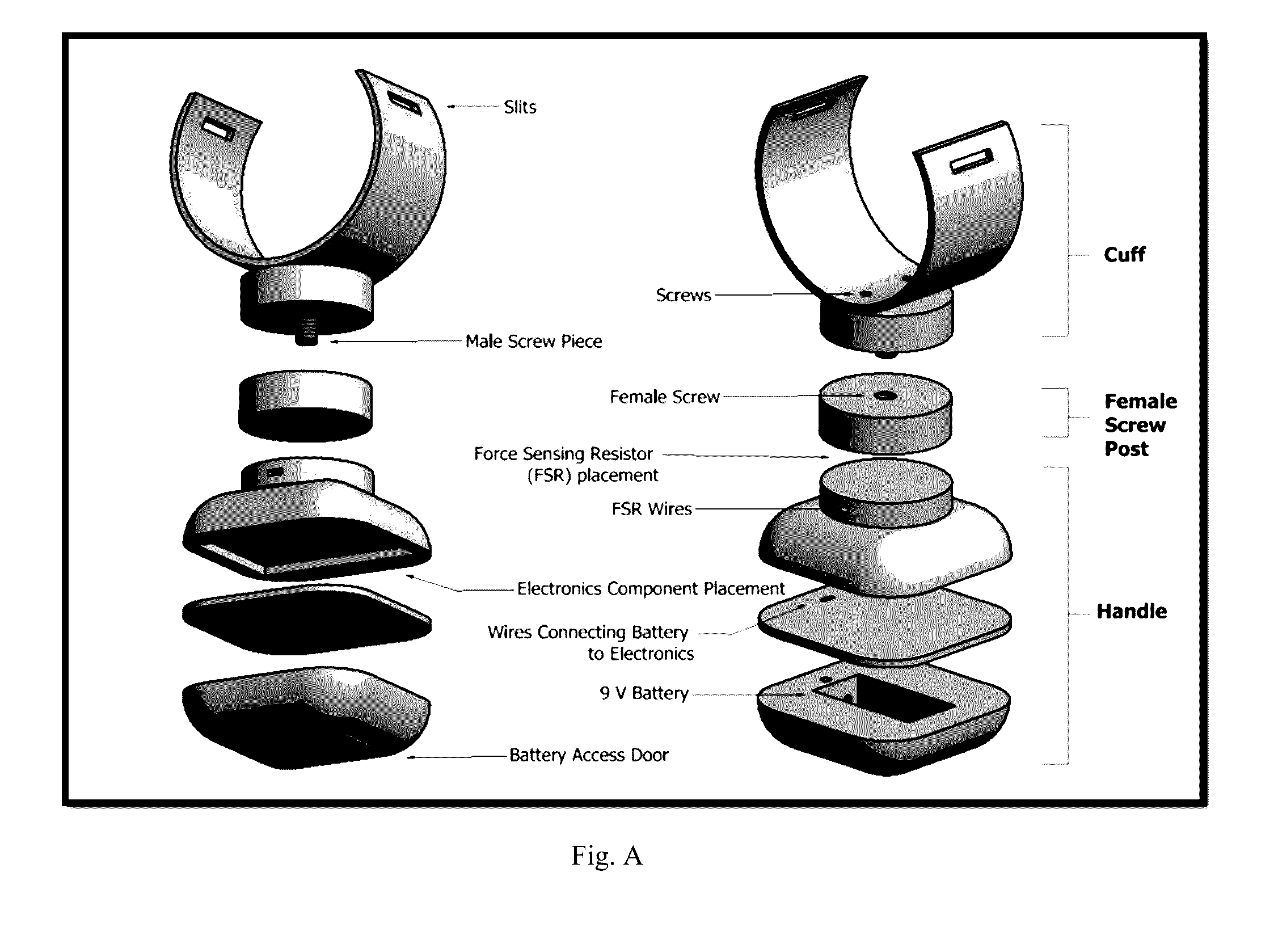
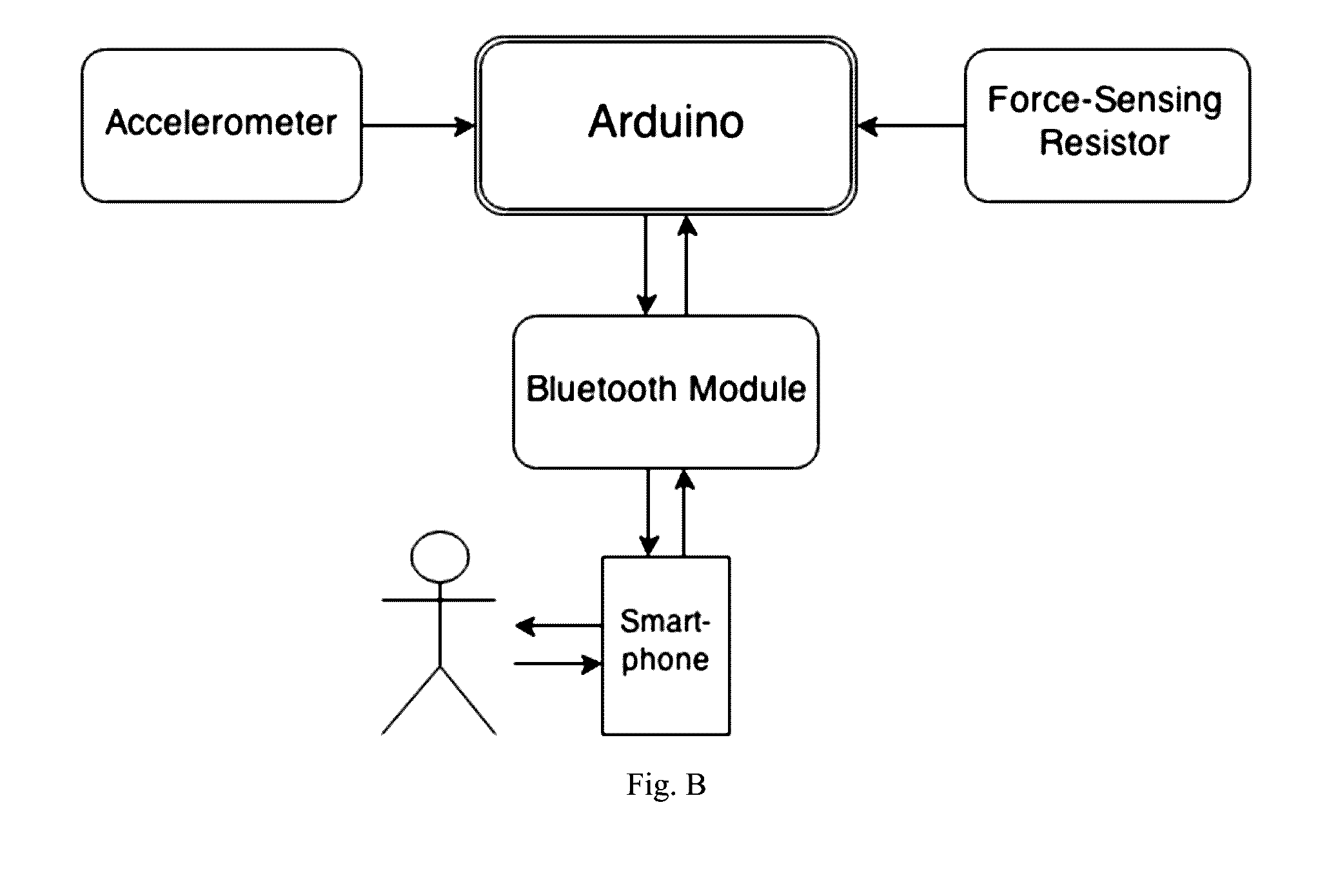
Spasticity quantification device
InactiveUS20160317066A1Accurate and repeatable mannerAccurately and precisely quantify spasticityLocal control/monitoringStrain gaugeAccelerometerForce-sensing resistor
The present invention relates to a portable devise that is able to quantify spasticity. In one aspect, the invention allows clinicians to objectively quantify spasticity in an accurate and repeatable manner. The device is designed to accommodate for different limb sizes and includes an accelerometer and a force sensing resistor to obtain quantitative data. The device further includes a data acquisition module where the data collected can be processed and sent to an output device.
Owner:ACCELERATED REHABILITATION TECH LLC
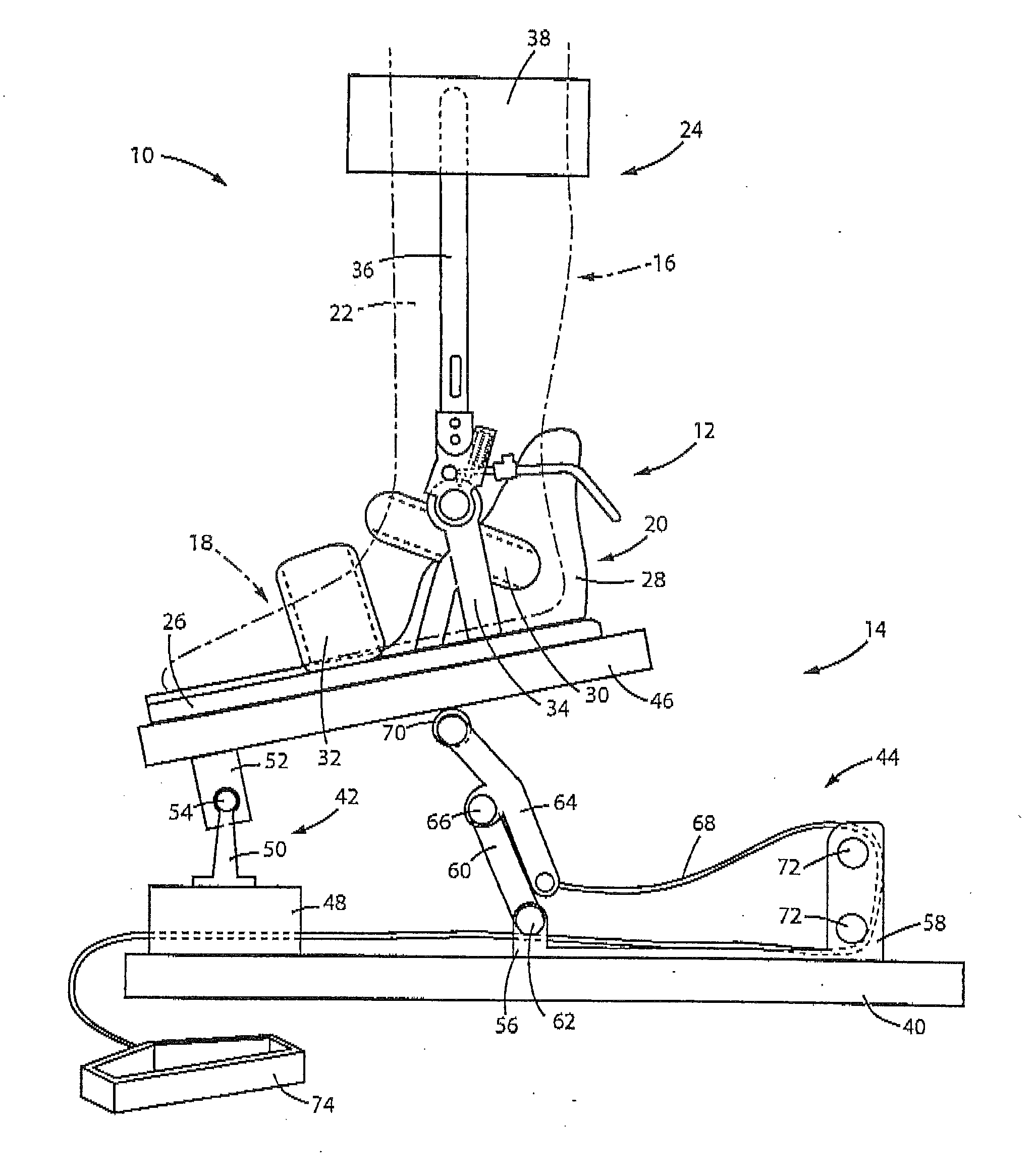
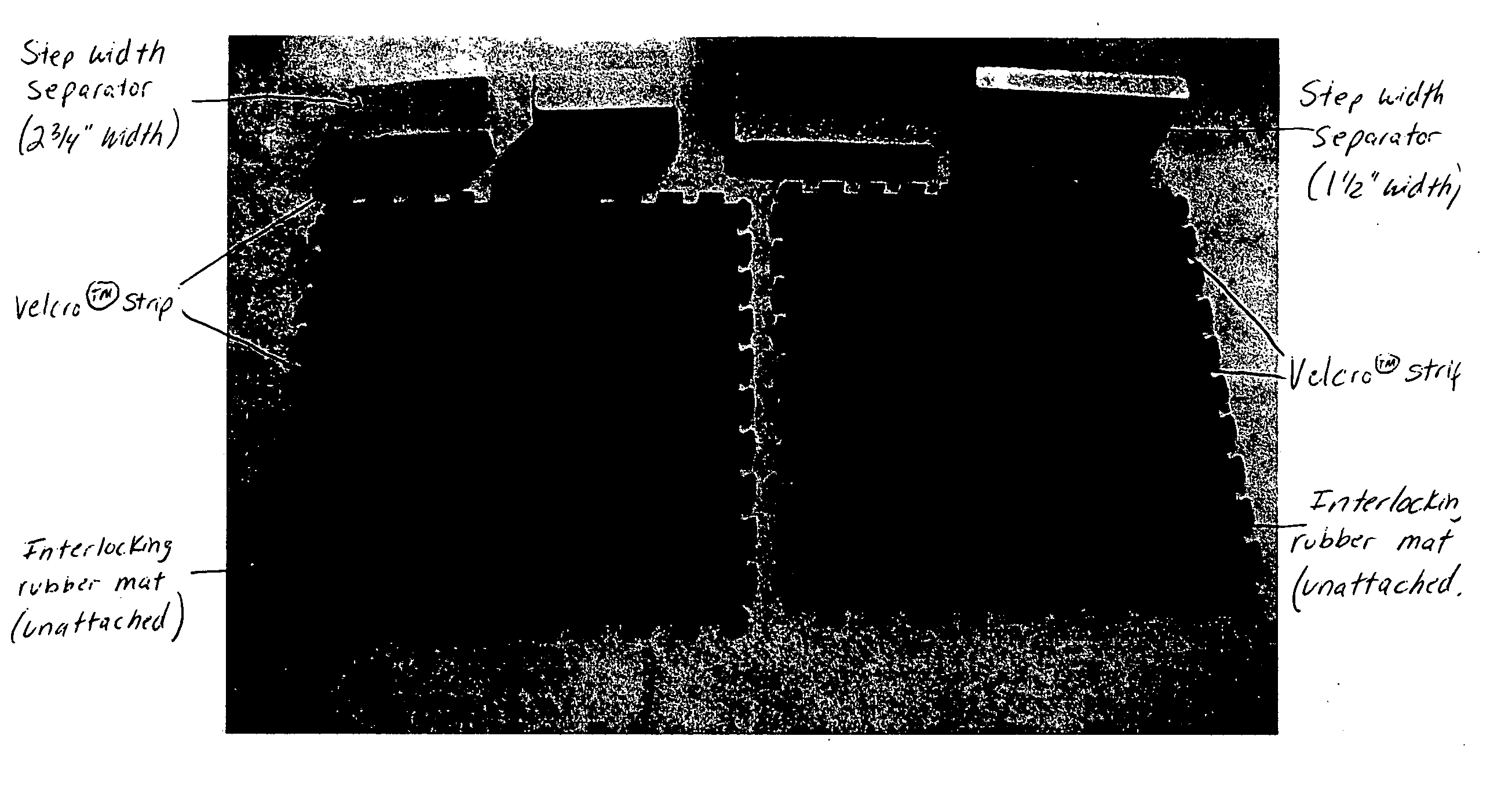
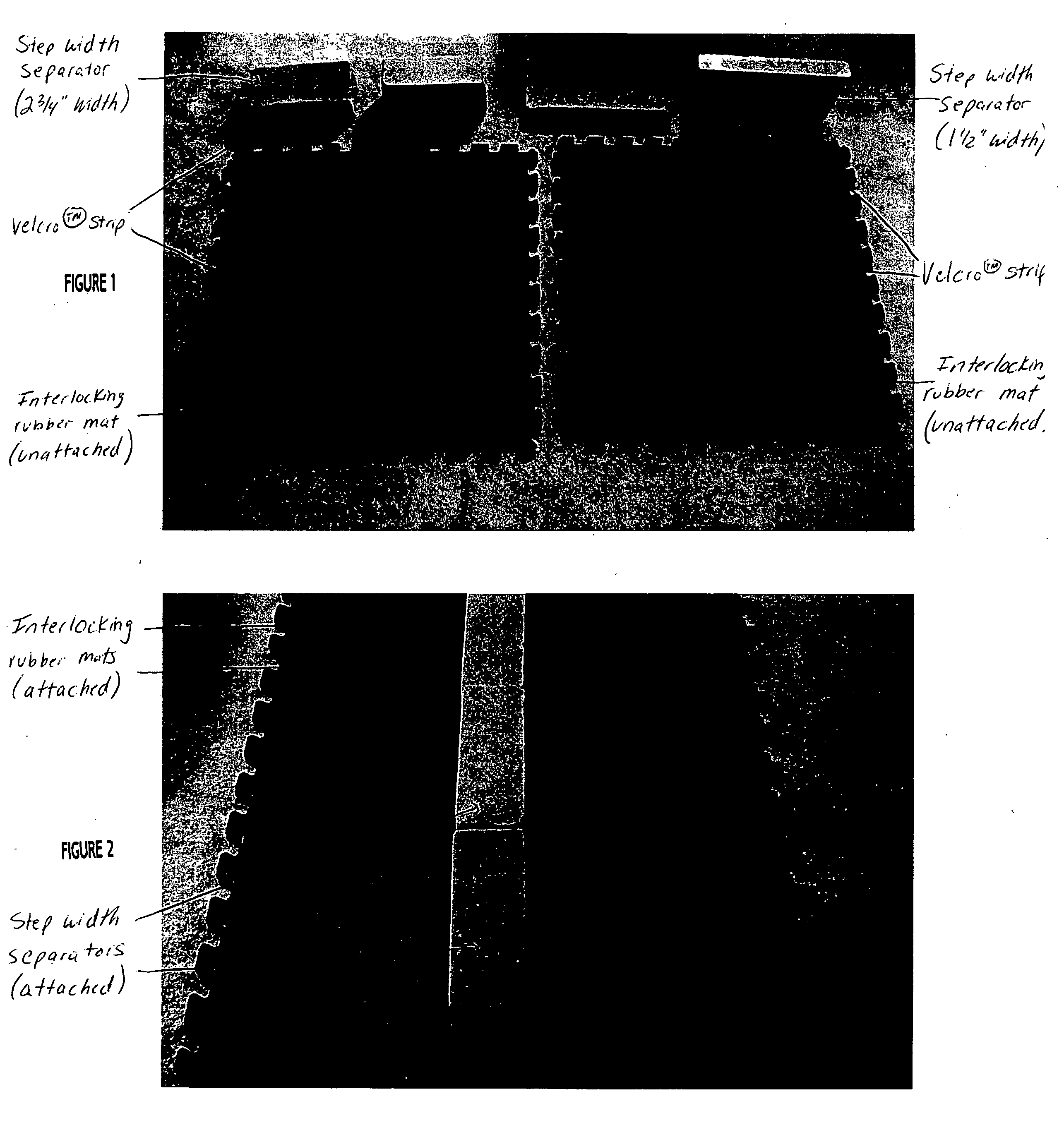
Step width increaser
The apparatus provides a proper step width for patients during gait training with neurological disorders, such as spasticity or paraparesis of the lower extremities. The apparatus provides proprioceptive feedback through the ankles during gait training with an assistive device (cane, walker, or parallel bars). The apparatus can be adjusted to different step widths depending on the patient and the physical therapist's recommendation. The result would be an improvement in the patient's gait pattern over time (with less risk of injury to the patient) and an improvement in the method used by the physical therapist for gait training for this problem.
Owner:CHINCHILLO ROBERT LINCOLN
Compound and method for reducing appetite, fatigue and pain
InactiveUS20180169172A1Reduce painReduce spasticityOrganic active ingredientsSuppositories deliveryNutritionPharmacometrics
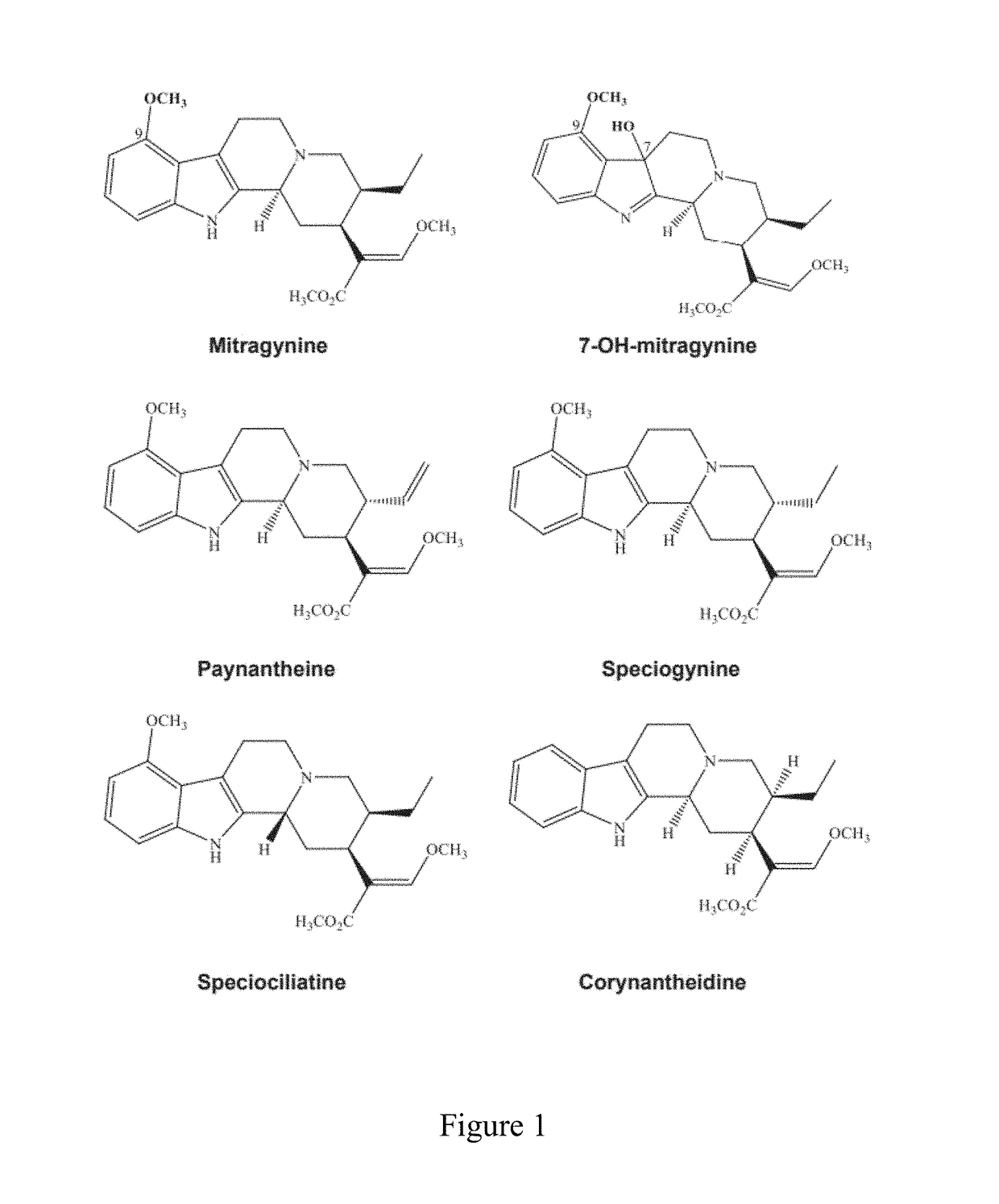
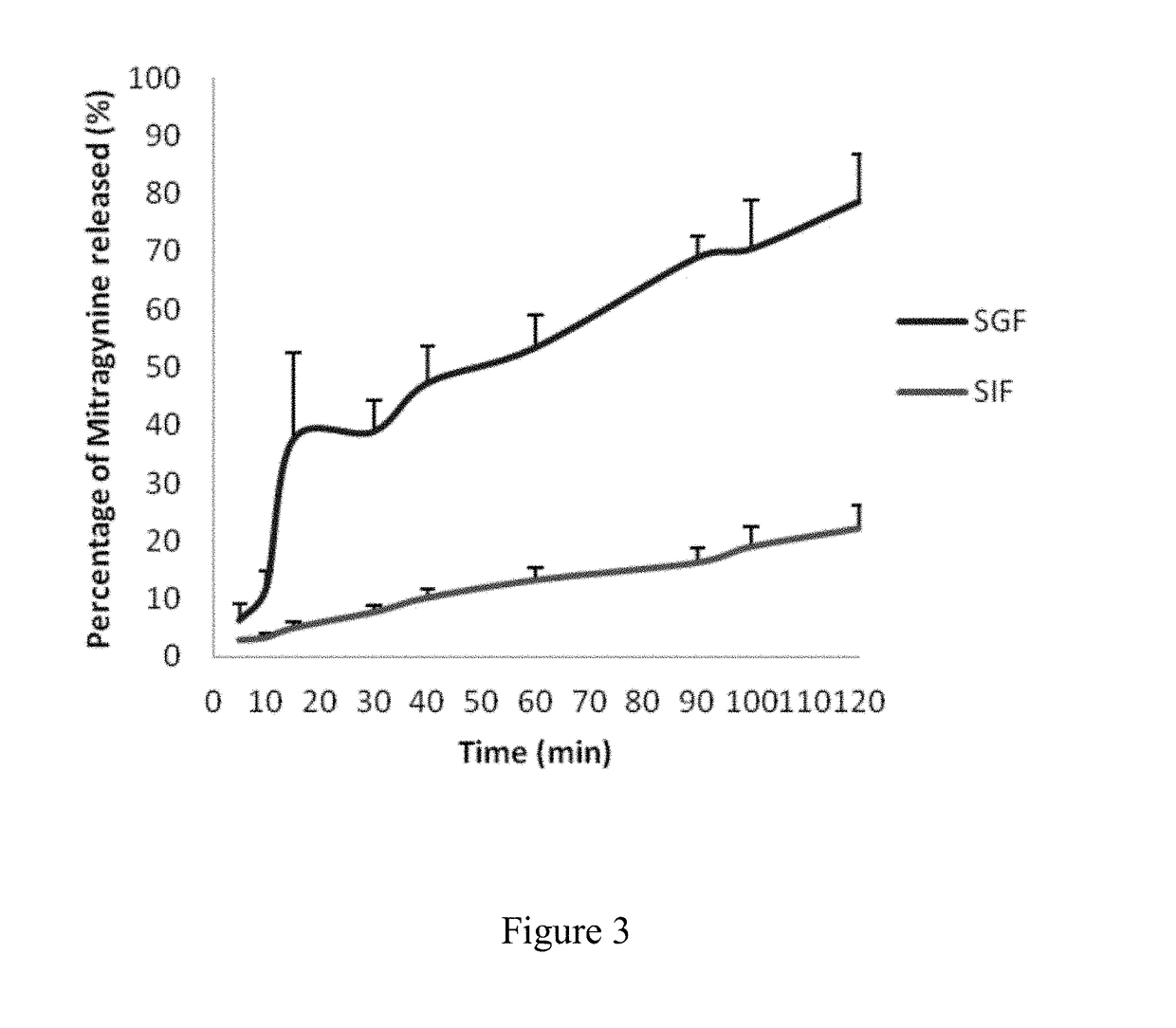
The disclosed invention generally relates to pharmaceutical and nutraceutical compounds and methods for reducing appetite, muscle fatigue and spasticity, enhancing athletic performance, and treating pain associated with cancer, trauma, medical procedure, and neurological diseases and disorders in subjects in need thereof. The disclosed invention further relates to Kratom compounds where said compound contains at least some pharmacologically inactive component.
Owner:KARIMAN ALEXANDER
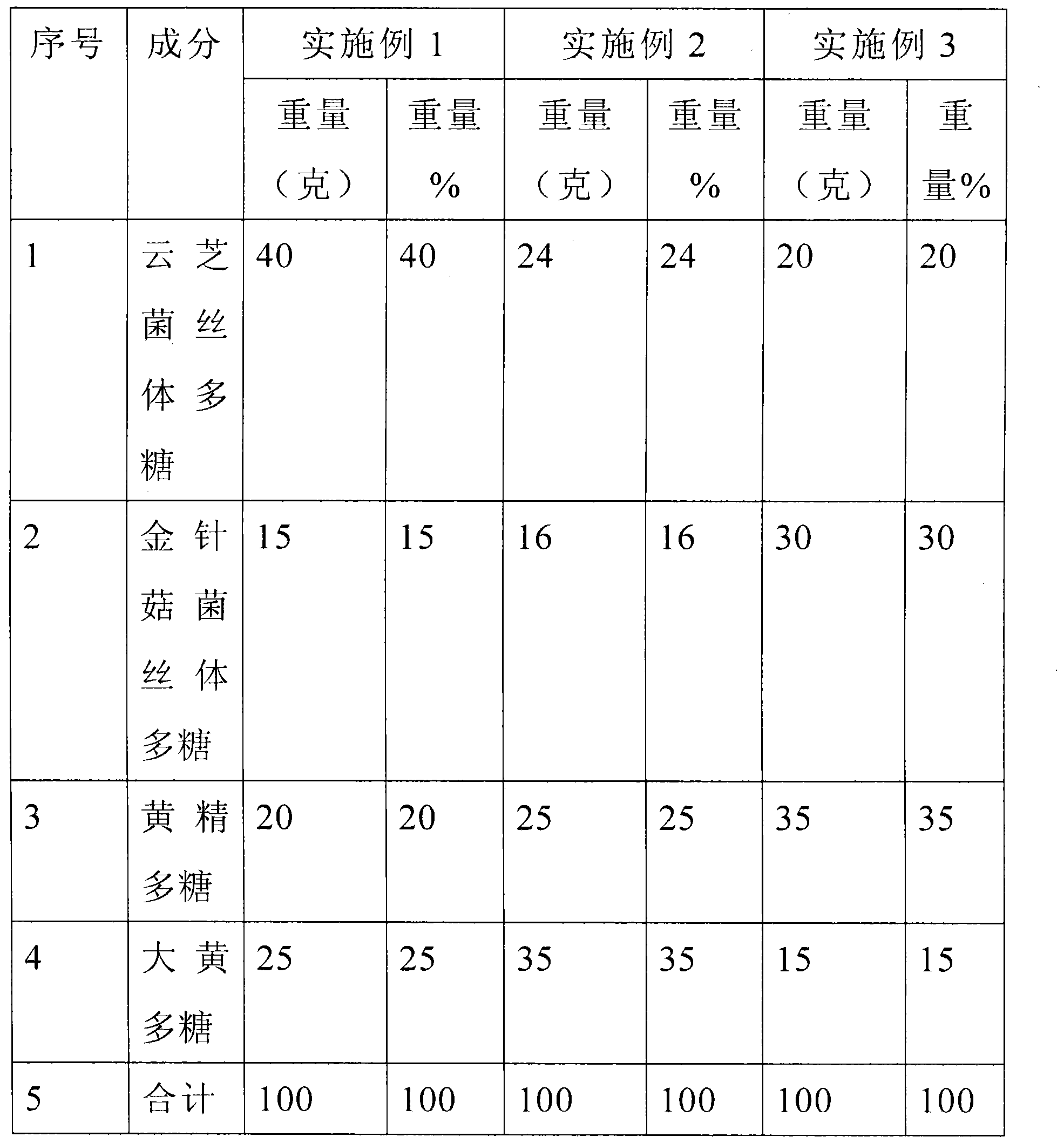
Conditioning medicine aiming at pain and rheumatism and preparation method of conditioning medicine
InactiveCN104324046AEnhance immune functionLow costOrganic active ingredientsNervous disorderAntineoplastic ImmunotherapeuticSide effect
The invention discloses a conditioning medicine aiming at pain and rheumatism and a preparation method of the conditioning medicine. The conditioning medicine consists of the following components in percentage by weight: 20-40 percent of coriolus versicolor mycelium polysaccharide, 15-30 percent of needle mushroom mycelium polysaccharide, 20-35 percent of polygahatous polysaccharide and 15-35 percent of rheum officinale polysaccharide. The preparation method comprises the following steps: respectively extracting the coriolus versicolor mycelium polysaccharide, needle mushroom mycelium polysaccharide, polygahatous polysaccharide and rheum officinale polysaccharide, and preparing according to the ratio. The conditioning medicine disclosed by the invention is low in cost and does not have any toxic or side effect, can achieve the effects of improving the human immunologic function, relieving swelling and pain, diminishing inflammation, expelling wind and eliminating dampness and diminishing inflammation and relieving pain and has obvious effects on traumatic injury, lumbar muscle degeneration, sciatica, prosopalgia, facial palsy and spasticity, rheumatoid arthritis, antitumor immunological therapy, internal and external hemorrhoids and colitis. The conditioning medicine is taken by adults 2-3 times per day, 1-2 grains of the medicine are taken each time, and the medicine can be taken for a long time.
Owner:李志成
Flexor spasticity drafting device after apoplectic hemiplegia
InactiveCN112006895AAvoid secondary damageAvoid pullingChiropractic devicesFractureEngineeringSpasticity
The invention relates to the technical field of medical rehabilitation equipment, in particular to a flexor spasticity drafting device after apoplectic hemiplegia. The device includes: a forearm assembly, wherein the forearm assembly comprises a forearm connecting pipe, a first ball joint and a forearm protector, and the forearm connecting pipe is connected to the forearm protector through the first ball joint; a big arm assembly, wherein the big arm assembly comprises a big arm connecting pipe, a second ball joint and a big arm protector, and the big arm connecting pipe is connected to the big arm protector through the second ball joint. The forearm connecting pipe is hinged to the big arm connecting pipe through a rotating shaft; elastic pieces are respectively arranged in the forearm connecting pipe and the big arm connecting pipe; and the forearm connecting pipe and the big arm connecting pipe are jointly hinged to a control assembly for drafting the big arm and the forearm. The drafting device is high in flexibility and good in body protection, the technical purpose of flexor traction is achieved, and secondary damage of a mechanical rigid structure to a patient is overcome.
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF TCM
Baclofen solution for low-volume therapeutic delivery
A high concentration baclofen solution is provided suitable for therapeutic use in a medical setting. A high concentration solution of baclofen in multivalent physiological ion solution such as artificial cerebrospinal fluid is provided with concentrations of baclofen of 10 mg / ml. Artificial cerebrospinal fluid is particularly advantageous as a baclofen solvent. A medical package is also provided for baclofen delivery to patients suffering from spasticity.
Owner:WAYNE STATE UNIV
Method for treating colonic viscerosensitivity and spasticity
There is provided a method of reducing pain associated with colonic viscerosensitivity and spasticity induced during a colonic examination chosen from colonic endoscopy, barium / air contrast colonic radiography and virtual colonoscopy of a non-sedated patient.
Owner:GICARE PHARMA INC
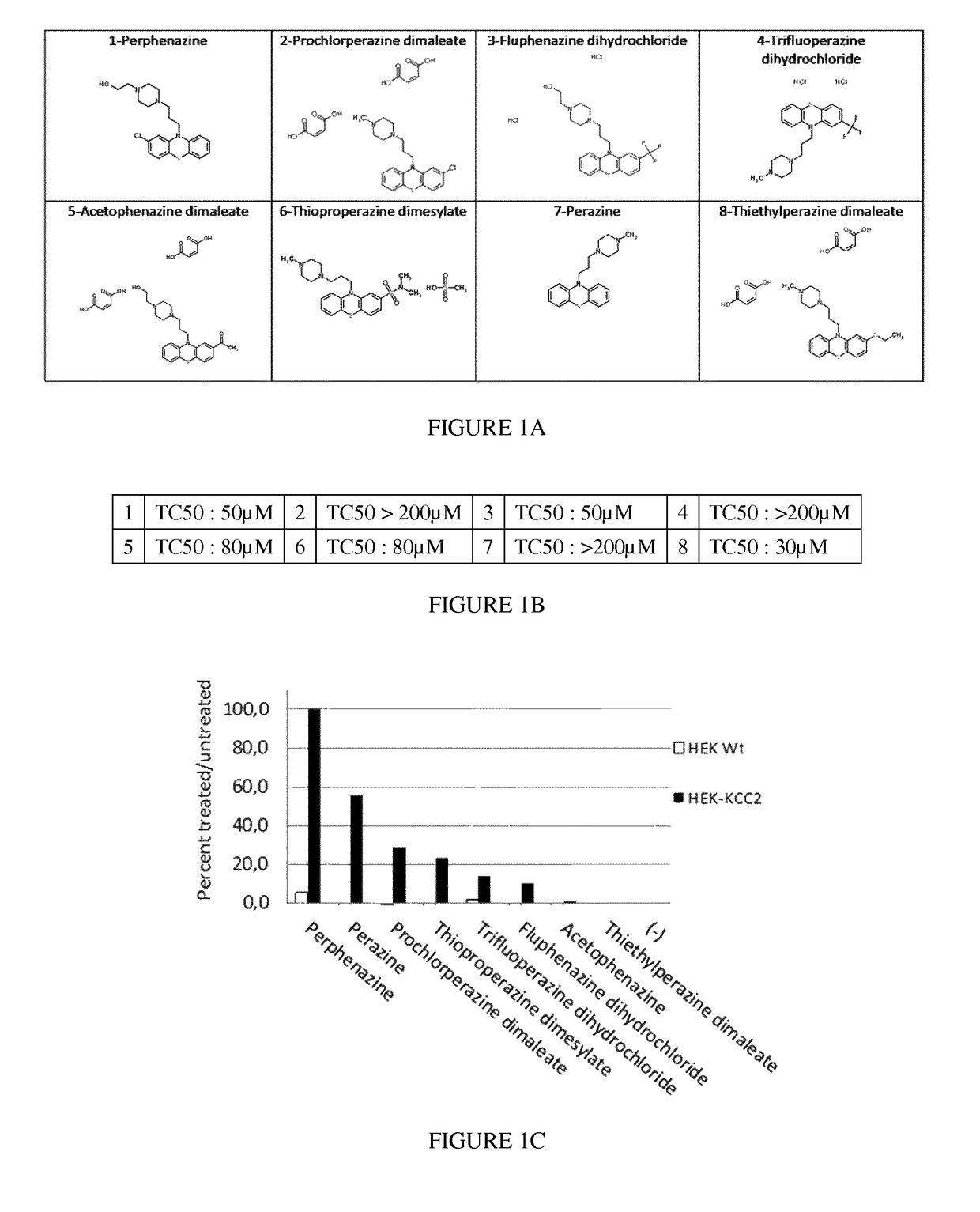
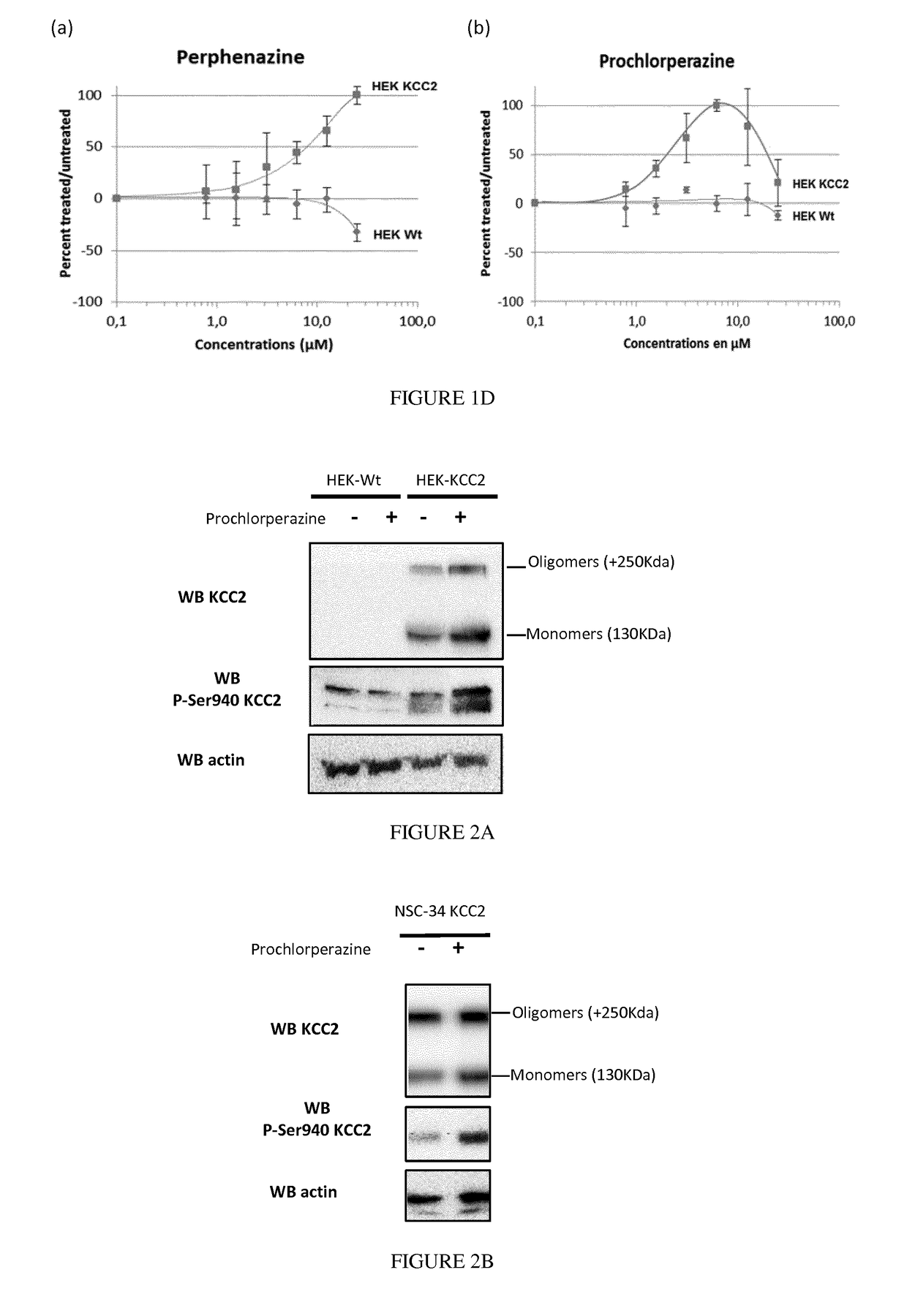
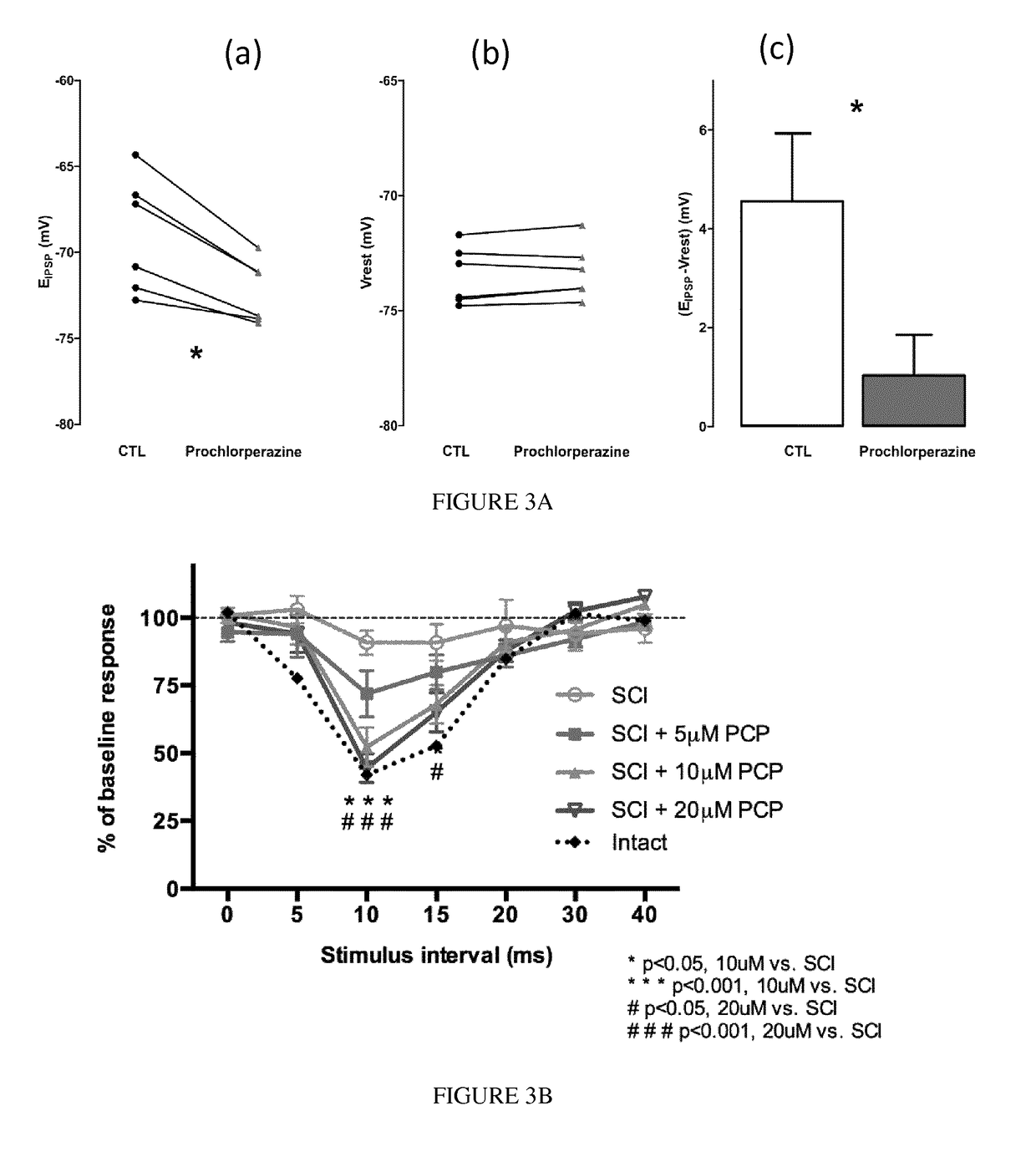
Piperazine phenothiazine derivatives for treating spasticity
InactiveUS9814727B2Reduce concentrationAvoid painOrganic active ingredientsNervous disorderPhenothiazine derivativeTraumatic injury
The present invention relates to piperazine phenothiazine derivatives useful as therapeutic agents for treating spasticity, particularly following an ischemia or traumatic injury, or compression syndrome. The invention further relates to a pharmaceutical composition comprising a compound of the invention for treating spasticity.
Owner:UNIV DAIX MARSEILLE +2
Spastic recovery device for hemiplegic patients
ActiveCN111166627BSpasticity recoveryAchieve stretchChiropractic devicesApparatus instrumentsHead of bed
The invention discloses a spasm recovery device for hemiplegia patients, which relates to the technical field of medical devices and includes: a bed body including a bed board; The lower side of the baffle is provided with a baffle driving mechanism, which can drive the first baffle to move in the width direction of the bed; the shoulder lifting mechanism is movably arranged at the end of the bed near the head of the bed, and can drive the patient's shoulder on the affected side. The arm stretching mechanism is set above the bed board, which can drive the patient's wrist on the affected side to move to the shoulder on the other side; the device has a shoulder lifting mechanism, an arm stretching mechanism and a block that can squeeze the patient by moving. The board can easily help the patient relieve the spasticity of the trunk and arms by lifting the patient's shoulders and arms and repeatedly squeezing the patient's arms and trunk.
Owner:JIANGSU VOCATIONAL COLLEGE OF MEDICINE
Benzamide derivatives useful in the treatment of muscular disorders and pain and for controlling spasticity and tremors
ActiveUS20160304441A1Improved potency/activityHigh selectivityNervous disorderCarboxylic acid nitrile preparationDiseaseDitazole
The present invention relates to a compound of formula I, or a pharmaceutically acceptable salt thereof : (I) wherein: n is 0 or 1; R1 is selected from H, alkyl and aralkyl, wherein said alkyl and aralkyl groups may be optionally substituted by one or more OH groups; X is a group selected from —C≡C—(CH2)p—; —C(R5)═C(R6)—(CH2)q—; and —C(R5)(R6)C(R7)(R8)—(CH2)r—; where each of R5, R6, R7 and is independently II or alkyl, and each of p, q and r is independently 1, 2, 3, 4 or 5; Y is a group selected from: CN; COOR2; CONR3R4; SO2NR9R10; NR12COR13; NR14SO2R15; and a heterocyclic group selected from oxadiazolyl, thiazolyl, isothiazolyl, oxazolyl, iso-oxazolyl, pyrazolyl and imidazolyl; where each of R2, R3 and R4 is independently H or alkyl; or R3 and R4 are linked, together with the nitrogen to which they are attached, to form a 5 or 6-membered heterocycloalkyl or heterocycloalkenyl group, said heterocycloalkyl or heterocycloalkenyl group optionally containing one or more further groups selected from O, N, CO and S, and where each of R9, R10, R11, R12, R13, R14 and R15 is independently H or alkyl. Further aspects of the invention relate to the use of such compounds in the preparation of a medicament for the treatment of a muscular disorder, pain, or for controlling spasticity or tremors, for example, spasticity in MS.
Owner:UCL BUSINESS PLC
Benzamide derivatives useful in the treatment of muscular disorders and pain and for controlling spasticity and tremors
ActiveUS9908843B2Improved potency/activityHigh selectivityNervous disorderCarboxylic acid nitrile preparationDiseaseDitazole
The present invention relates to a compound of formula I, or a pharmaceutically acceptable salt thereof: (I) wherein: n is 0 or 1; R1 is selected from H, alkyl and aralkyl, wherein said alkyl and aralkyl groups may be optionally substituted by one or more OH groups; X is a group selected from —C≡C—(CH2)p—; —C(R5)═C(R6)—(CH2)q—; and —C(R5)(R6)C(R7)(R8)—(CH2)r-; where each of R5, R6, R7 and R8 is independently H or alkyl, and each of p, q and r is independently 1, 2, 3, 4 or 5; Y is a group selected from: CN; COOR2; CONR3R4; SO2NR9R10; NR12COR13; NR14SO2R15; and a heterocyclic group selected from oxadiazolyl, thiazolyl, iso-thiazolyl, oxazolyl, iso-oxazolyl, pyrazolyl and imidazolyl; where each of R2, R3 and R4 is independently H or alkyl; or R3 and R4 are linked, together with the nitrogen to which they are attached, to form a 5 or 6-membered heterocycloalkyl or heterocycloalkenyl group, said heterocycloalkyl or heterocycloalkenyl group optionally containing one or more further groups selected from O, N, CO and S, and where each of R9, R10, R11, R12, R13, R14 and R15 is independently H or alkyl. Further aspects of the invention relate to the use of such compounds in the preparation of a medicament for the treatment of a muscular disorder, pain, or for controlling spasticity or tremors, for example, spasticity in MS.
Owner:UCL BUSINESS PLC
Skeletal muscle augmentation utilizing muscle-derived progenitor compositions, and treatments thereof
The present invention provides muscle-derived progenitor cells that show long-term survival following transplantation into body tissues and which can augment soft tissue following introduction (e.g. via injection, transplantation, or implantation) into a site of soft tissue. Also provided are methods of isolating muscle-derived progenitor cells, and methods of genetically modifying the cells for gene transfer therapy. The invention further provides methods of using compositions comprising muscle-derived progenitor cells for the augmentation and bulking of mammalian, including human, soft tissues in the treatment of various cosmetic or functional conditions, including malformation, injury, weakness, disease, or dysfunction. The invention also relates to novel uses of muscle-derived progenitor cells for the treatment of cosmetic or functional conditions, including, but not limited to skeletal muscle weakness, muscular dystrophy, muscle atrophy, spasticity, myoclonus and myalgia. The invention also relates to the novel use of MDCs for the increase of skeletal muscle mass in athletes or other organisms in need of greater than average skeletal muscle mass.
Owner:UNIVERSITY OF PITTSBURGH +1
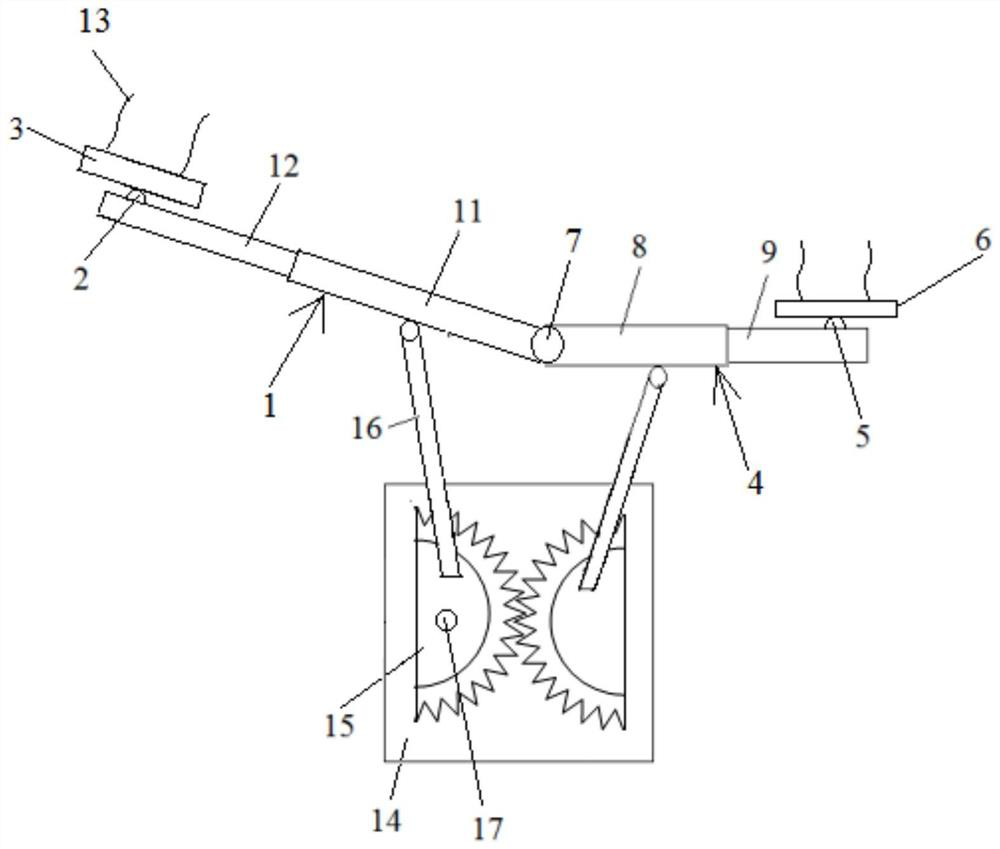
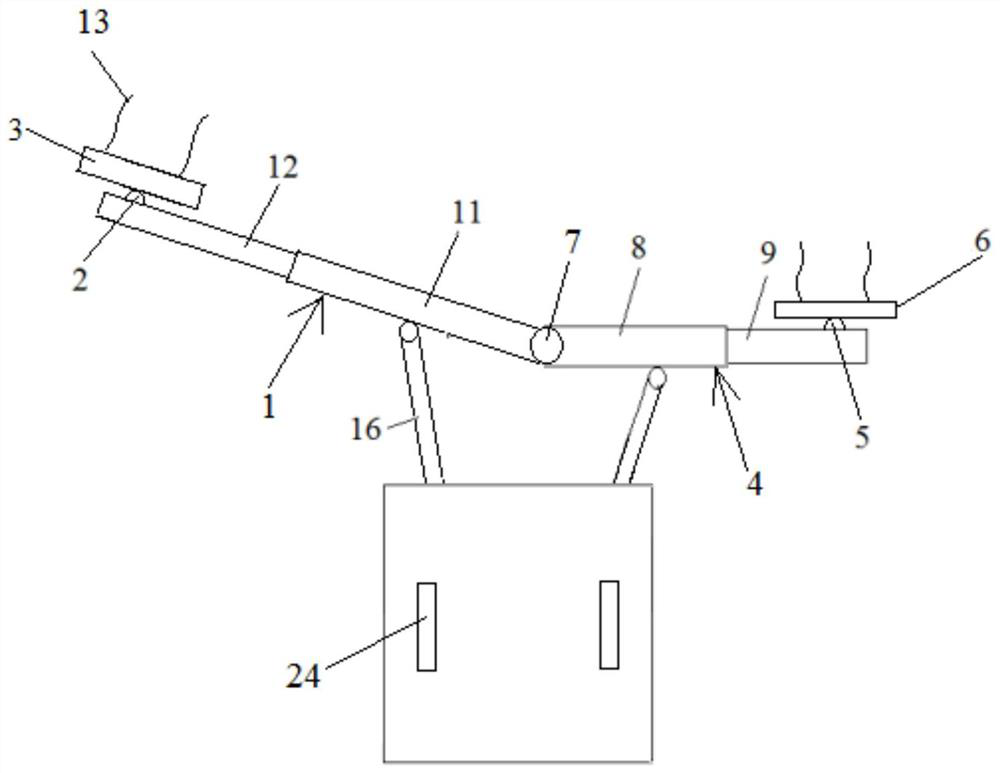
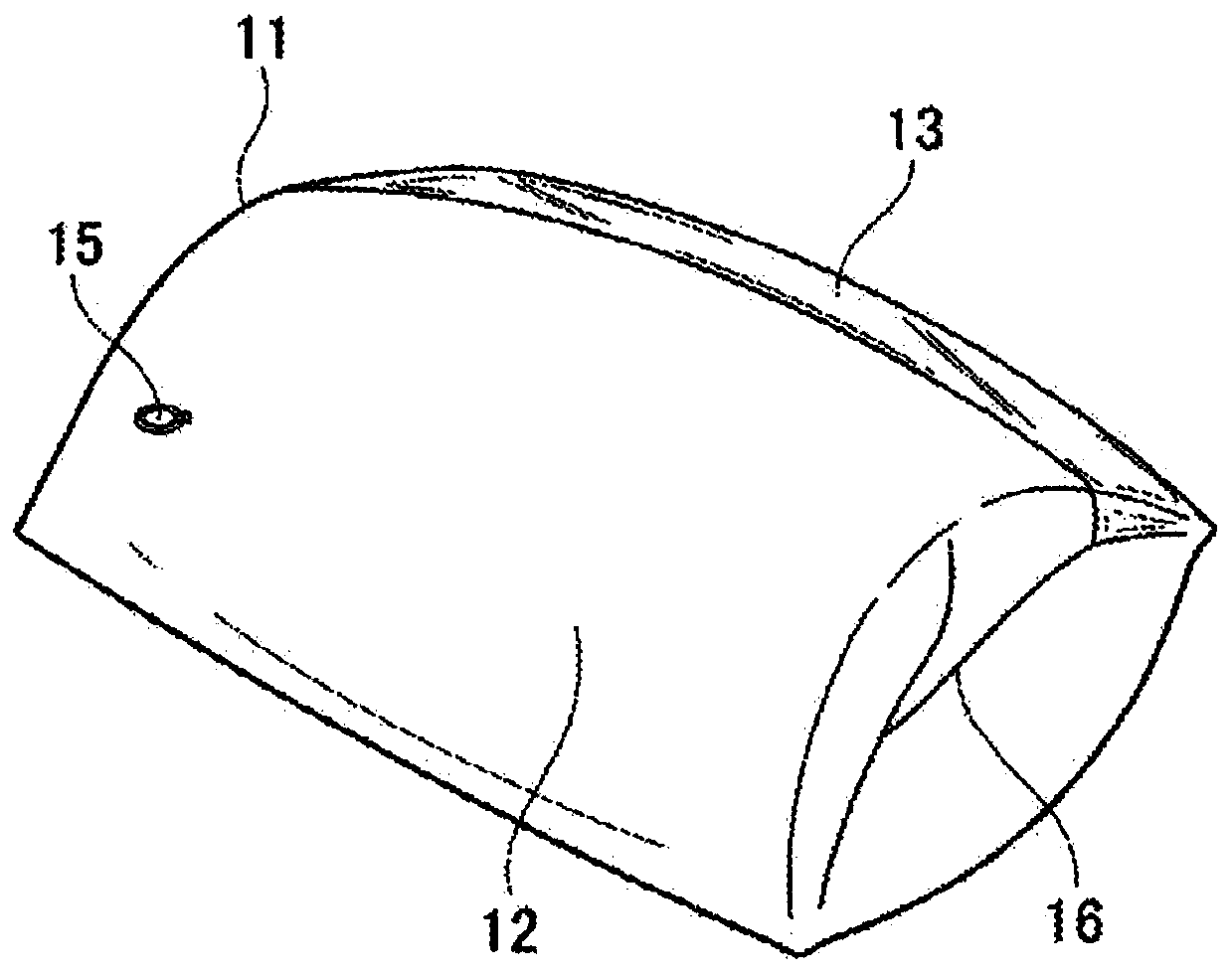
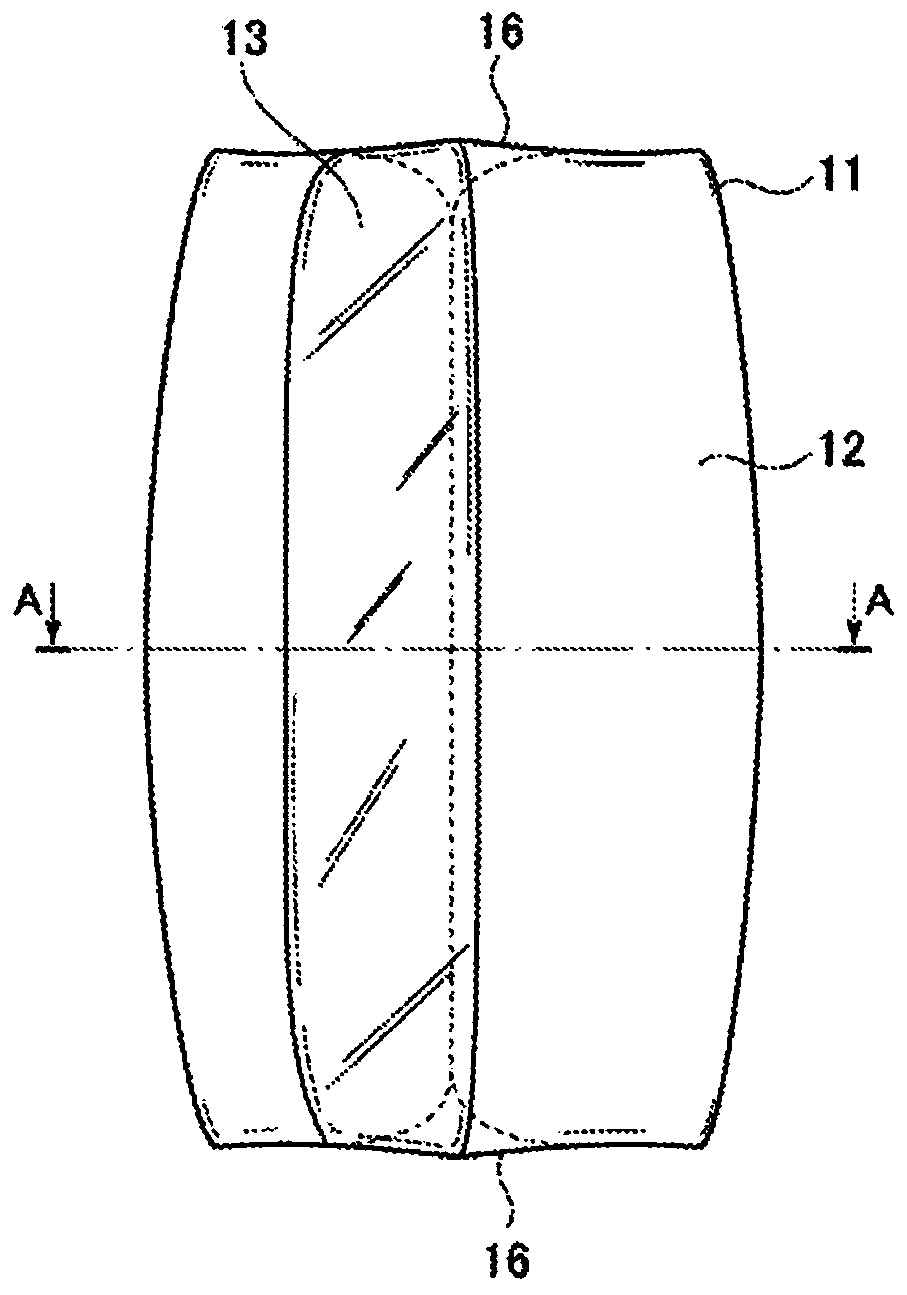
Air pressurization equipment
Provided is air pressurization equipment that is easily installed on the body, has excellent stability during use, and is for spasticity prevention. Equipment that is to be installed on a wrist or a limb that includes a wrist is an airtight airbag that is configured from a cylindrical bag body 11 that expands or contracts as a result of supply or expulsion of air. The bag body 11 is formed from asynthetic resin sheet. A pressurization part that communicates with open parts 16 that are on either side is formed at an inside center hole in the bag body 11. When the equipment is installed, a limbis inserted from an open part 16 of the expanded cylindrical bag body 11, the inside center hole in the cylindrical bag body 11 is made to contact the body, and the equipment is moved in the insertion or removal direction while being rotated, is held in a desired position, and, in that state, pressurizes and constrains an affected area.
Owner:A EL CO LTD
Use of 1H-quinazoline-2,4-diones
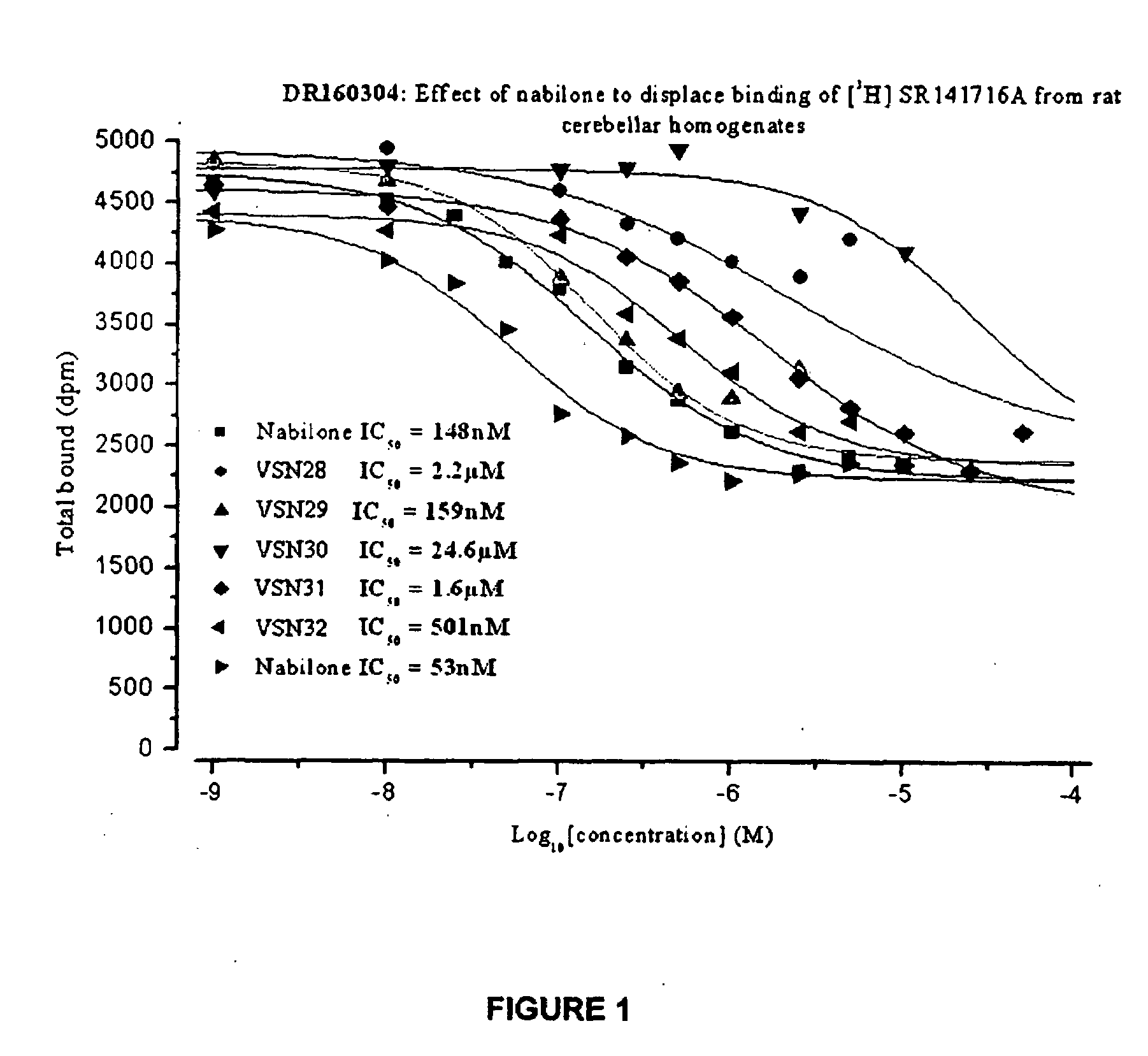
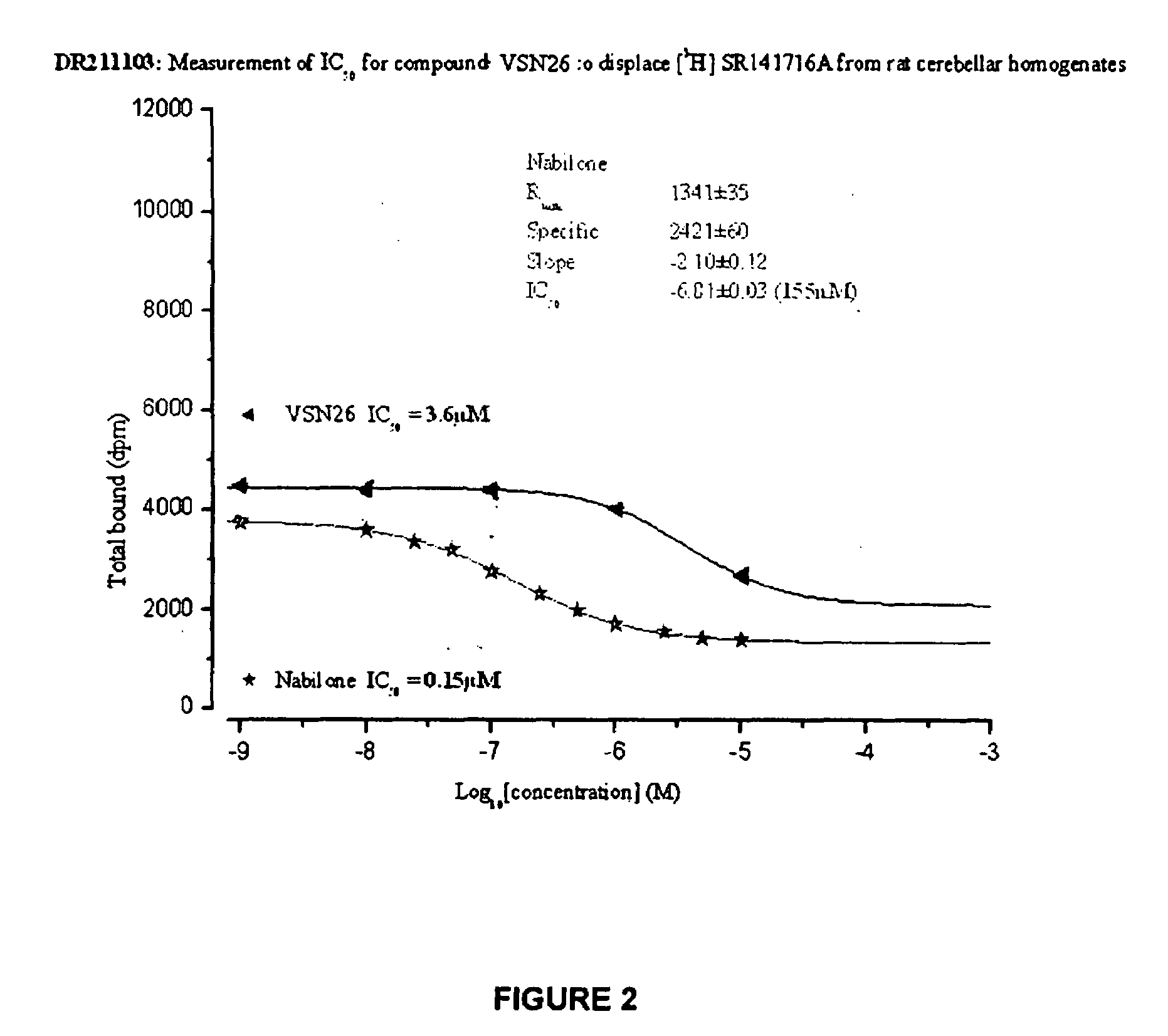
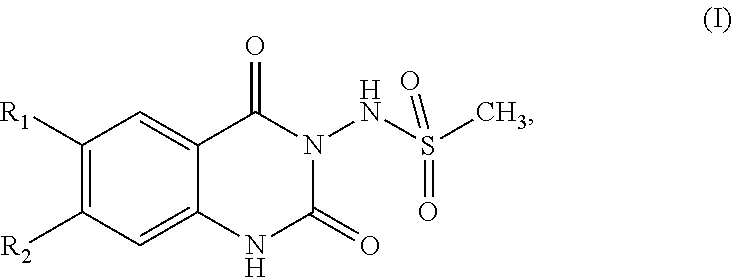
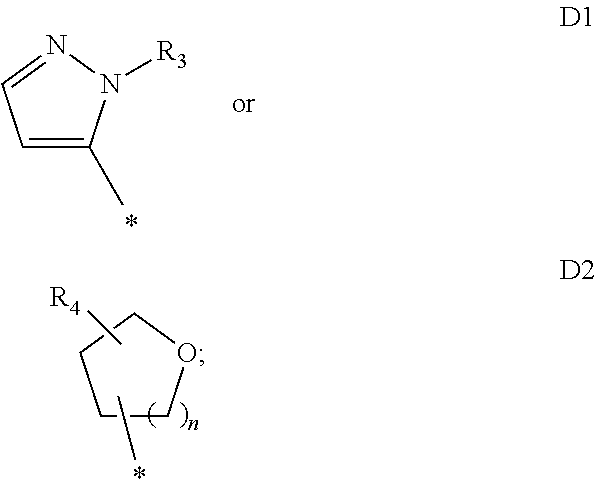
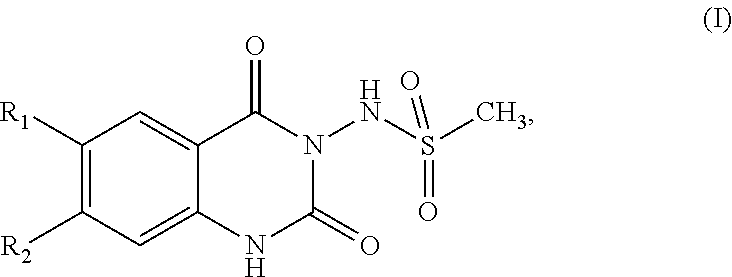
InactiveUS20130096145A1Potent reduction of spasticityPotent reduction of rigidityBiocideOrganic active ingredientsSpasticityQuinazoline
The invention concerns the use of competitive AMPA receptor antagonists for the treatment, prevention or delay of progression of spasticity.
Owner:NOVARTIS AG
Baclofen solution for low-volume therapeutic delivery
A high concentration baclofen solution is provided suitable for therapeutic use in a medical setting. A high concentration solution of baclofen in multivalent physiological ion solution such as artificial cerebrospinal fluid is provided with concentrations of baclofen of 10 mg / ml. Artificial cerebrospinal fluid is particularly advantageous as a baclofen solvent. A medical package is also provided for baclofen delivery to patients suffering from spasticity.
Owner:WAYNE STATE UNIV
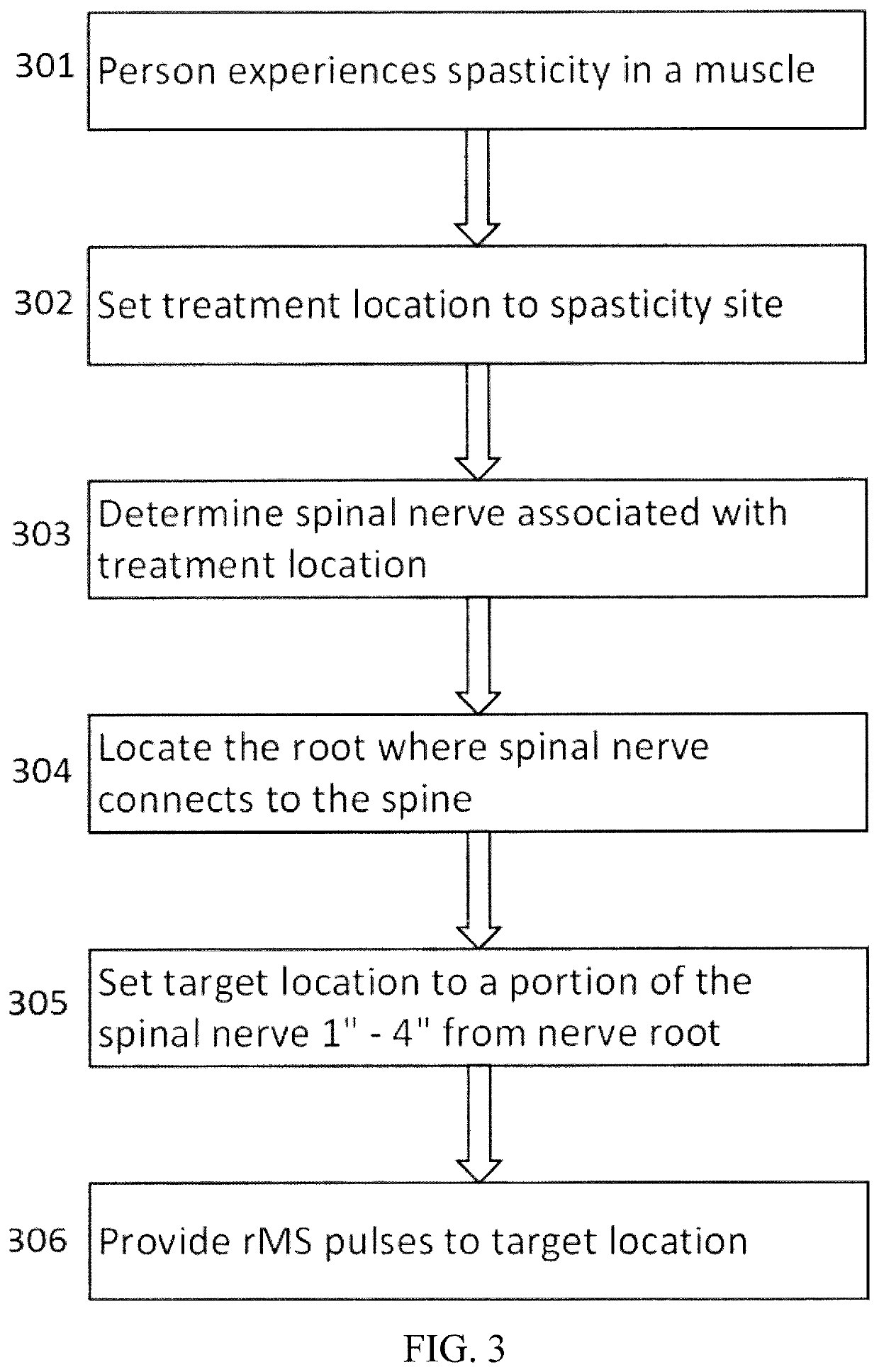
Systems and methods for spasticity treatment using spinal nerve magnetic stimulation
ActiveUS10661094B2Facilitate communicationSpread the wordElectrotherapyMagnetotherapy using coils/electromagnetsSpinal nerveTreatment use
Described are methods, devices, and systems for a novel, easy to use treatment for spasticity that does not involve medication. Methods and devices herein use low-frequency repetitive magnetic fields to enhance communication in the spinal nerve, thereby allowing improved relaxation, control, and coordination in a muscle.
Owner:WAVE NEUROSCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com