Conditioning medicine aiming at pain and rheumatism and preparation method of conditioning medicine
A technology for rheumatism and diseases, which is applied in the field of conditioning drugs for pain and rheumatic diseases and its preparation. It can solve the problems of poor effect of treatment, eradication, conditioning and supplementation, and no long-term effect of functional factors, so as to improve the immune function of the human body. , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
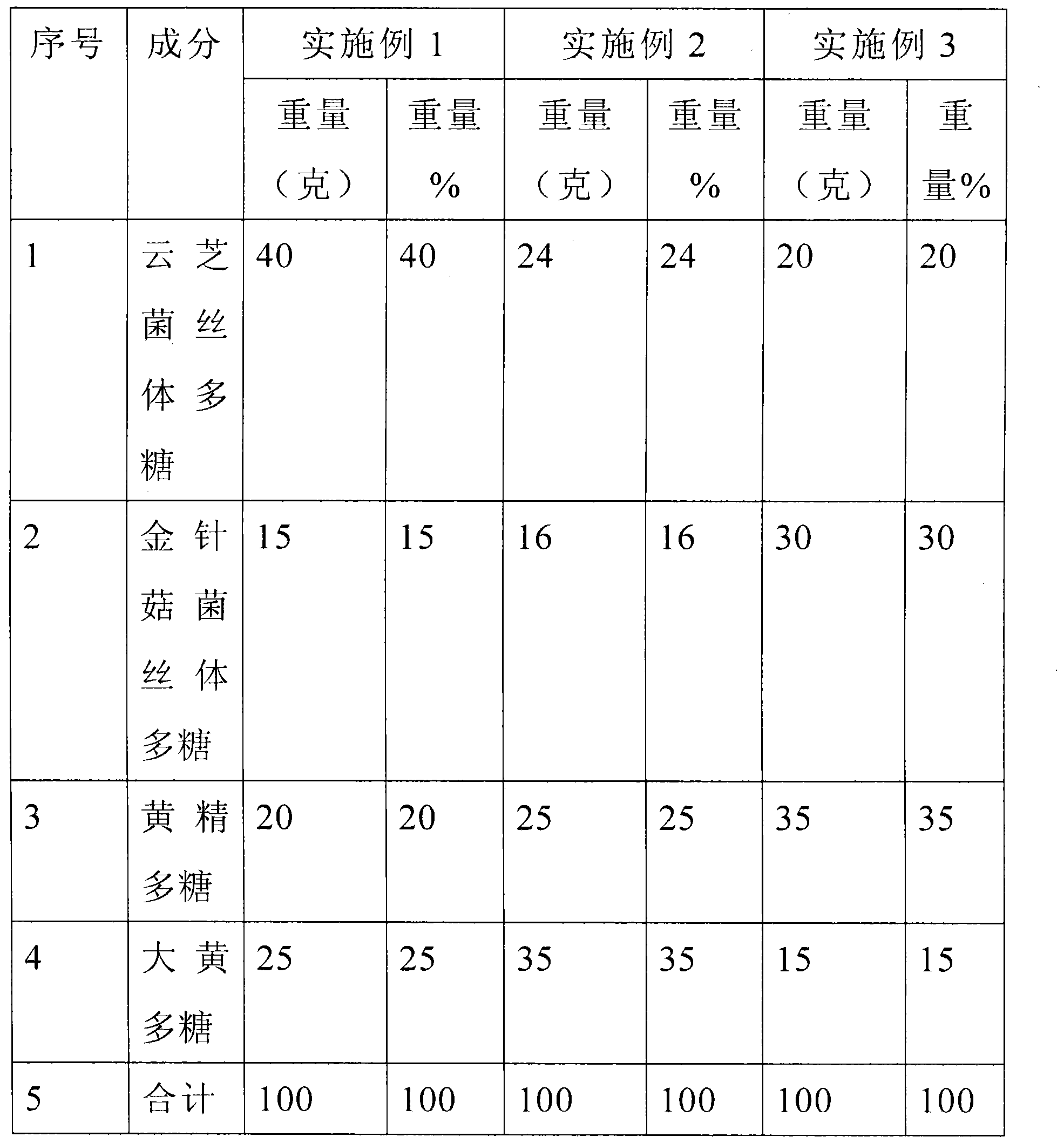
[0011] The weight percentages of various ingredients are 40 polysaccharides of versicolor mycelia, 15 polysaccharides of mycelium of Flammulina velutipes, 20 polysaccharides of rhubarb rhubarb and 25 polysaccharides of rhubarb. Its process includes the following steps. The first step is to take a portion of versicolor mycelium powder and add 20 times the amount of distilled water, heat to boil for 6 hours, stir and make up for water loss, add 15 times the amount of distilled water to the filtered residue, and repeat the extraction once , combine the filtrate extracted twice, concentrate to a small volume on a water bath, add 3% activated carbon to the concentrated solution and reflux for 1 hour, filter the filtrate to remove protein by sevag method, that is, add chloroform-n-butanol solvent to shake to remove protein, centrifuge to collect the supernatant, After removing n-butanol, the patient was bagged and dialyzed with running water for 72 hours to remove small molecule wate...
Embodiment 2
[0014] The weight percentages of various ingredients are 24 versicolor mycelium polysaccharides, 16 flammulina velutipes mycelium polysaccharides, 25 rhubarb polysaccharides and 35 rhubarb polysaccharides. Its process includes the following steps. The first step is to take a portion of versicolor mycelium powder and add 20 times the amount of distilled water, heat to boil for 6 hours, stir and make up for water loss, add 15 times the amount of distilled water to the filtered residue, and repeat the extraction once , combine the filtrate extracted twice, concentrate to a small volume on a water bath, add 3% activated carbon to the concentrated solution and reflux for 1 hour, filter the filtrate to remove protein by sevag method, that is, add chloroform-n-butanol solvent to shake to remove protein, centrifuge to collect the supernatant, After removing n-butanol, the patient was bagged and dialyzed with running water for 72 hours to remove small molecule water-soluble components s...
Embodiment 3
[0017]The weight percent of various ingredients is 20 versicolor mycelium polysaccharides, 30 flammulina velutipes mycelium polysaccharides, 35 rhubarb polysaccharides and 35 rhubarb polysaccharides. Its process includes the following steps. The first step is to take a portion of versicolor mycelium powder and add 20 times the amount of distilled water, heat to boil for 6 hours, stir and make up for water loss, add 15 times the amount of distilled water to the filtered residue, and repeat the extraction once , combine the filtrate extracted twice, concentrate to a small volume on a water bath, add 3% activated carbon to the concentrated solution and reflux for 1 hour, filter the filtrate to remove protein by sevag method, that is, add chloroform-n-butanol solvent to shake to remove protein, centrifuge to collect the supernatant, After removing n-butanol, the patient was bagged and dialyzed with running water for 72 hours to remove small molecule water-soluble components such as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com