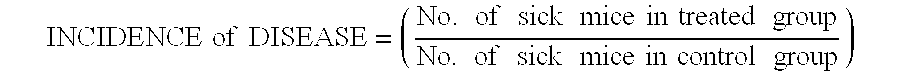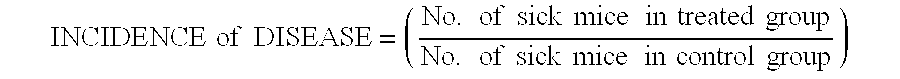Patents
Literature
36 results about "Clinically isolated syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A clinically isolated syndrome (CIS) is a clinical situation of an individual's first neurological episode, caused by inflammation or demyelination of nerve tissue. An episode may be monofocal, in which symptoms present at a single site in the central nervous system, or multifocal, in which multiple sites exhibit symptoms. CIS with enough paraclinical evidence can be considered as a clinical stage of multiple sclerosis (MS). It can also be retrospectively diagnosed as a kind of MS when more evidence is available.
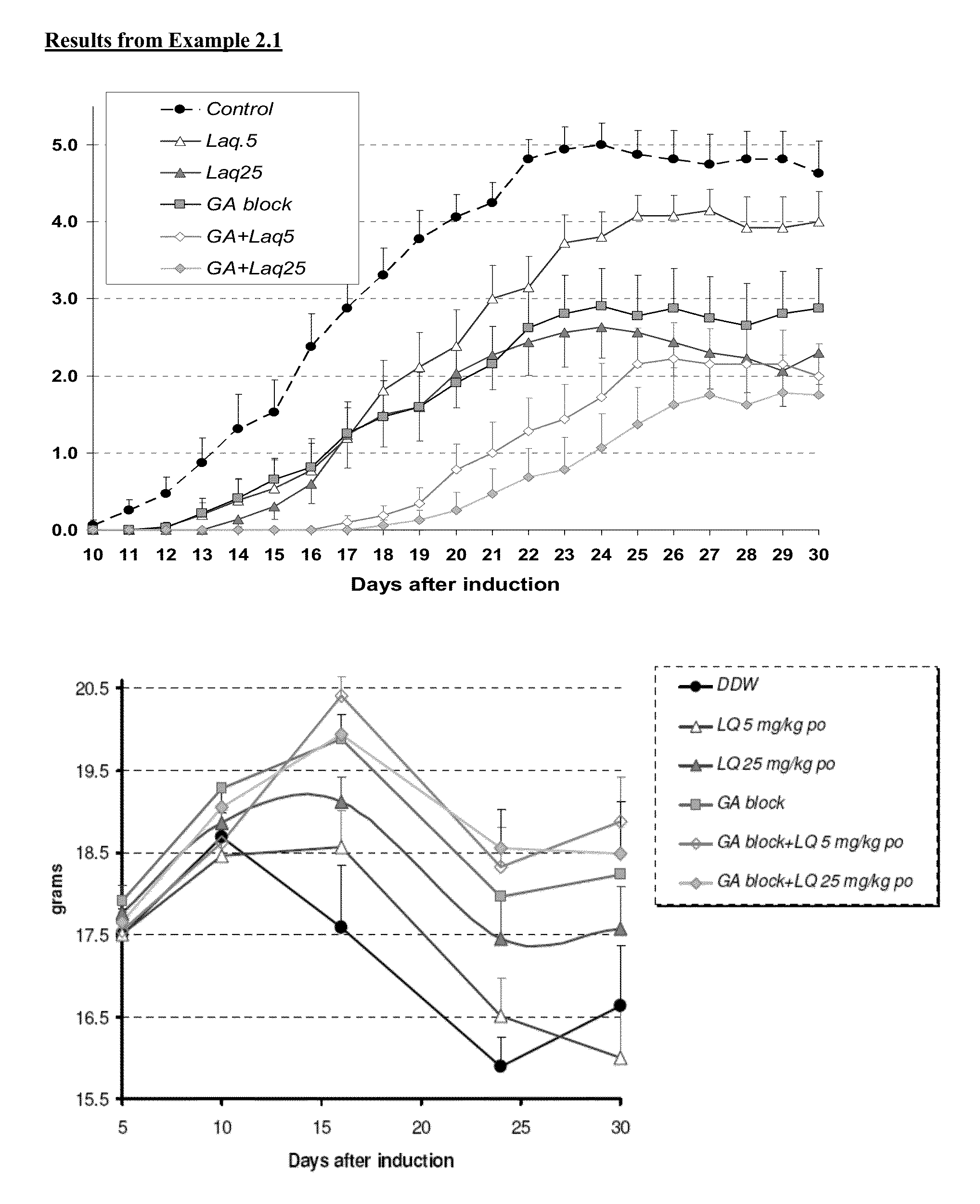
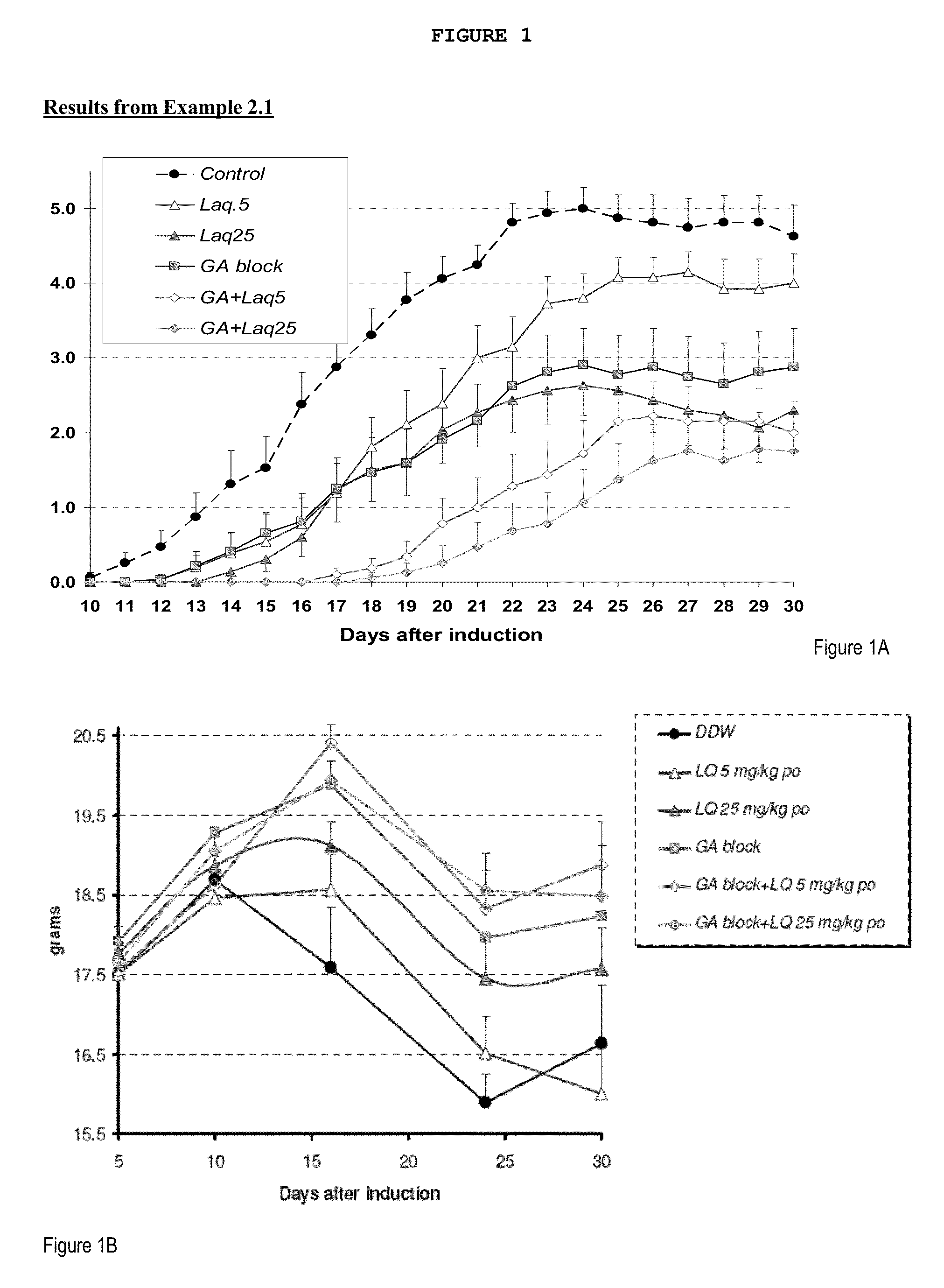
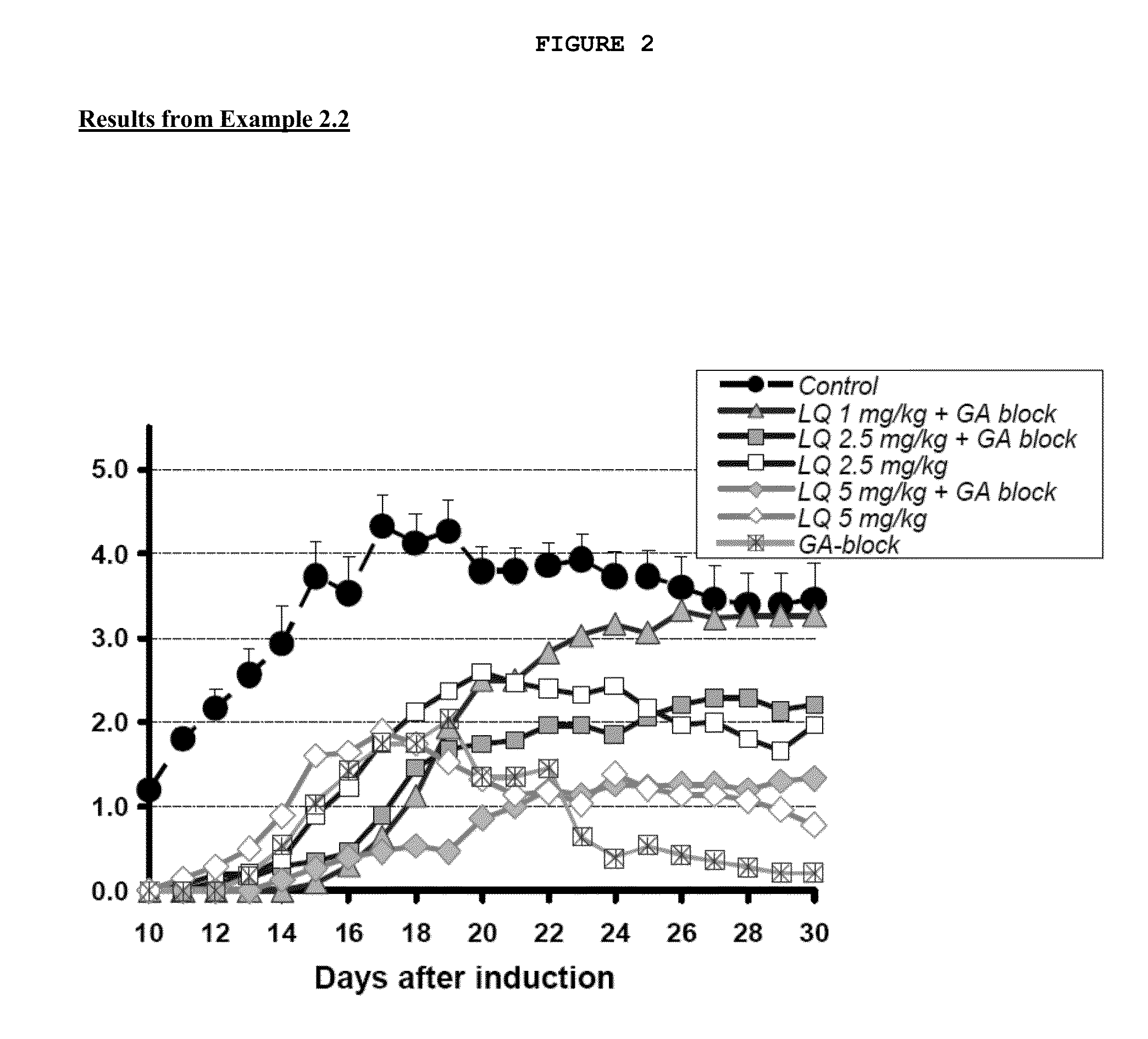
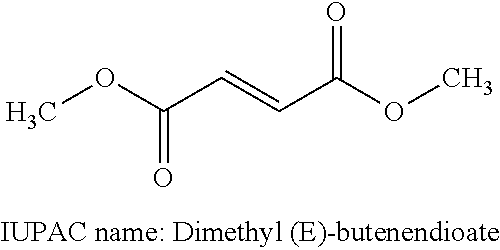
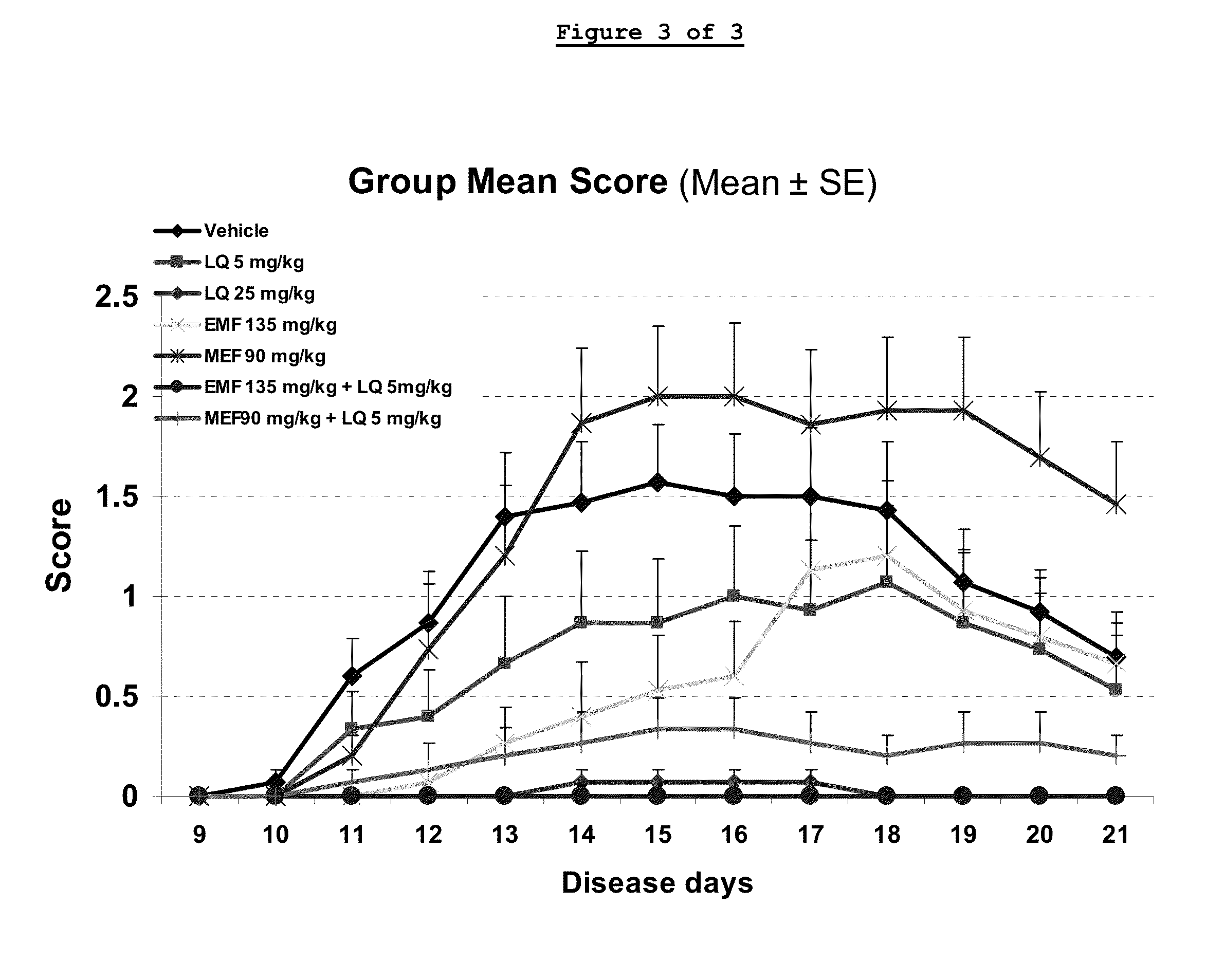
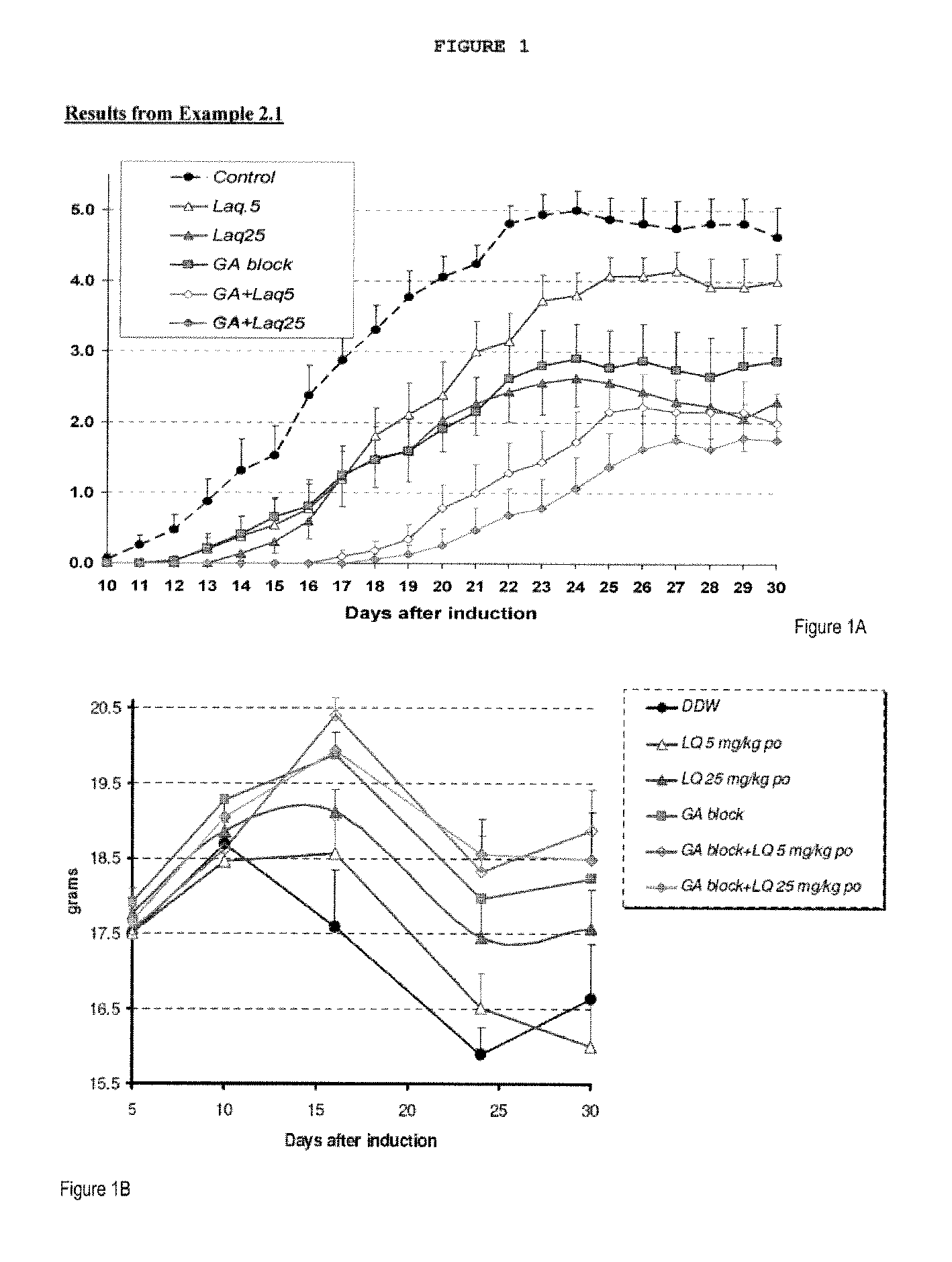
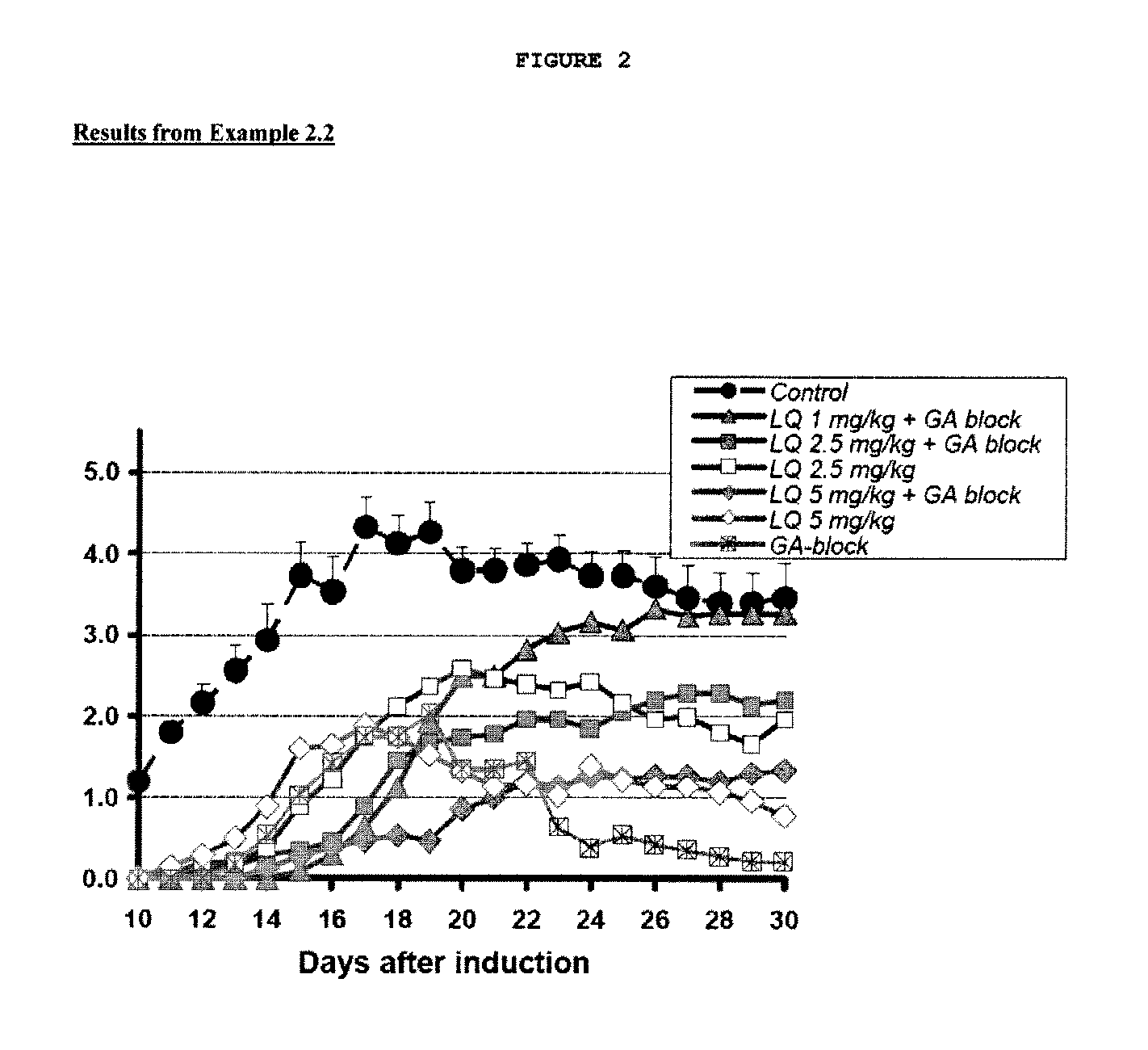
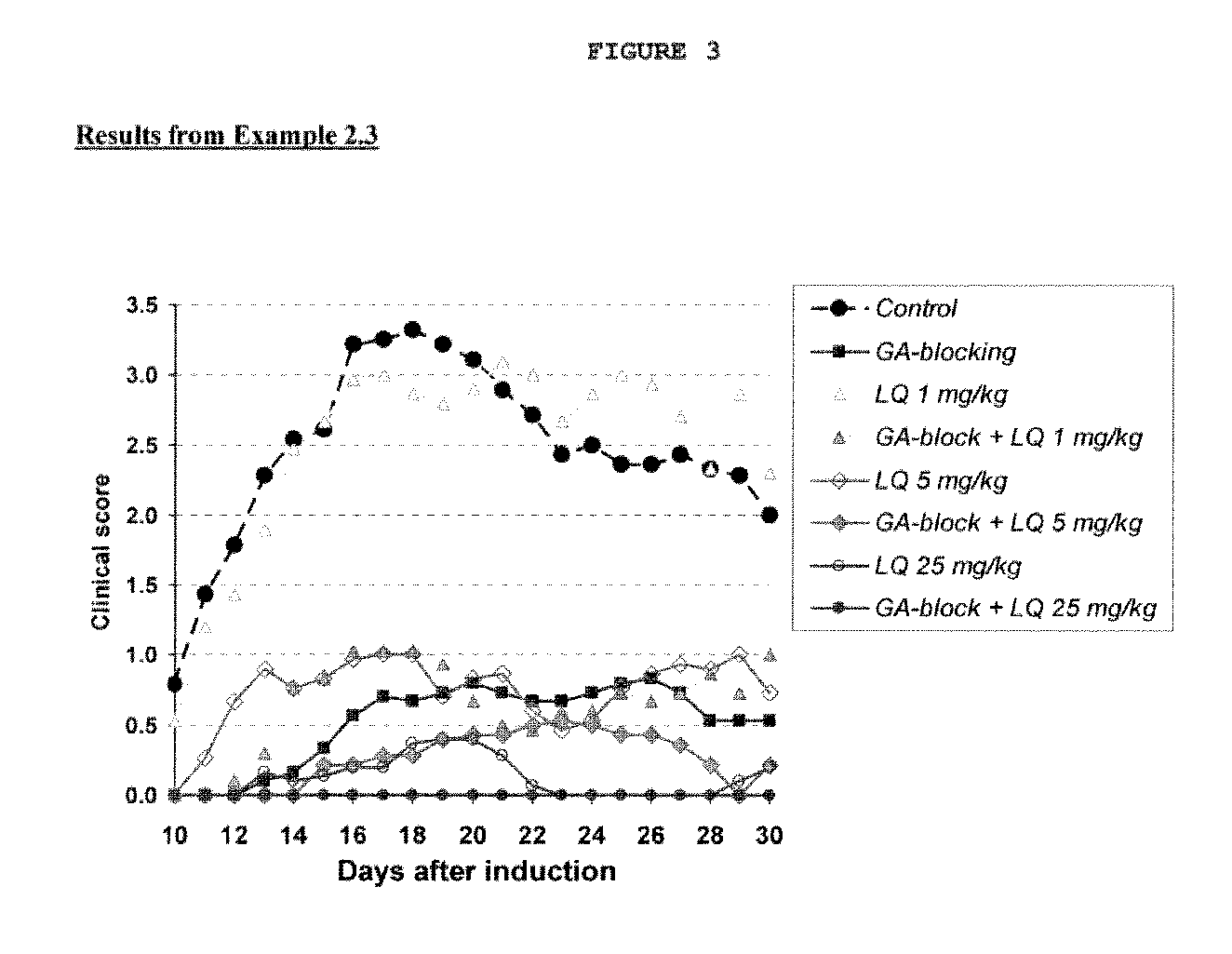
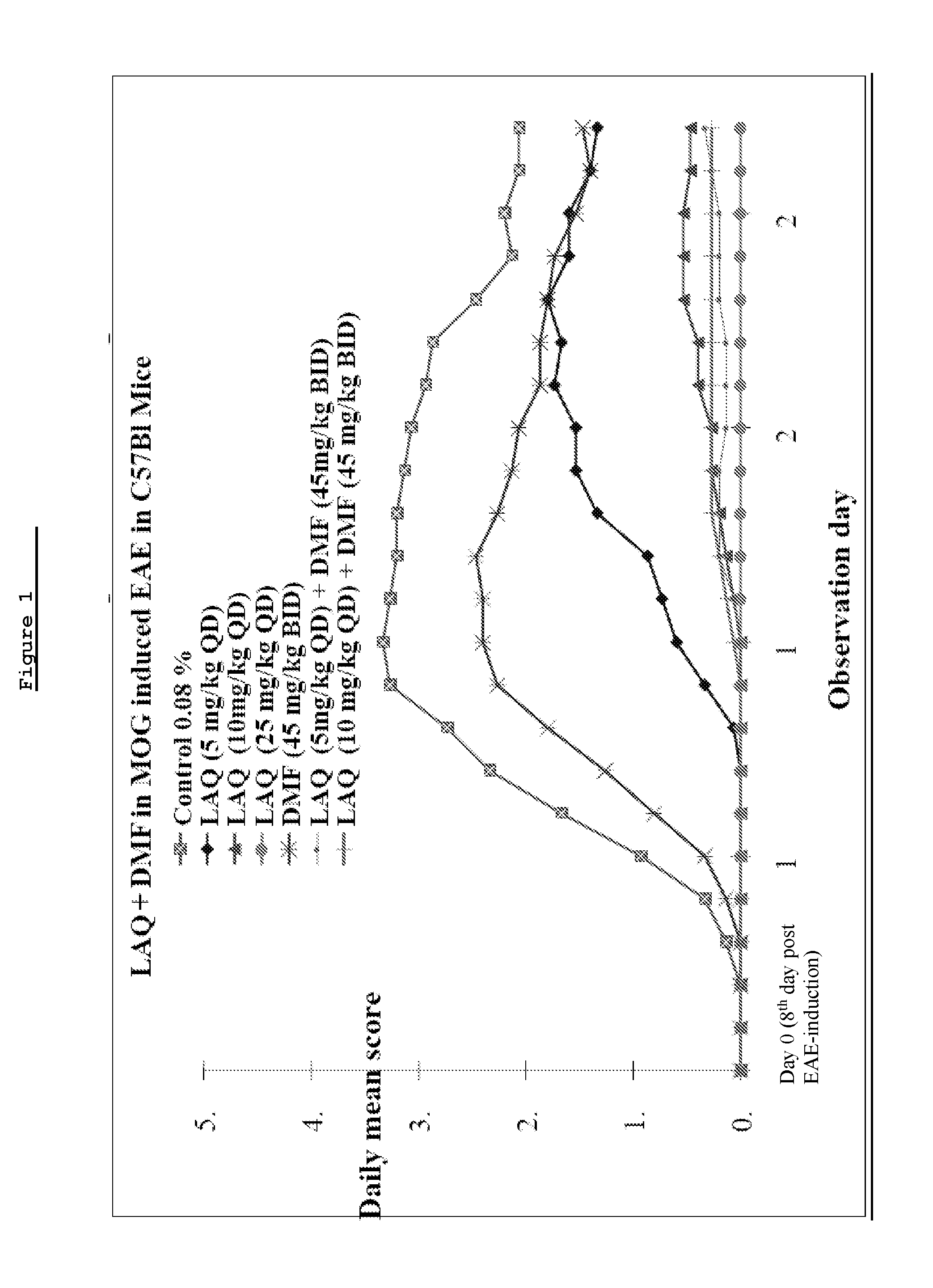
Treatment of multiple sclerosis with combination of laquinimod and glatiramer acetate
InactiveUS20130029916A1Effective treatmentPowder deliverySenses disorderLaquinimodGlatiramer acetate
This invention provides a method of treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the patient laquinimod as an add-on therapy to or in combination with glatiramer acetate. This invention also provides a package and a pharmaceutical composition comprising laquinimod and glatiramer acetate for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with glatiramer acetate in treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and glatiramer acetate in the preparation of a combination for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
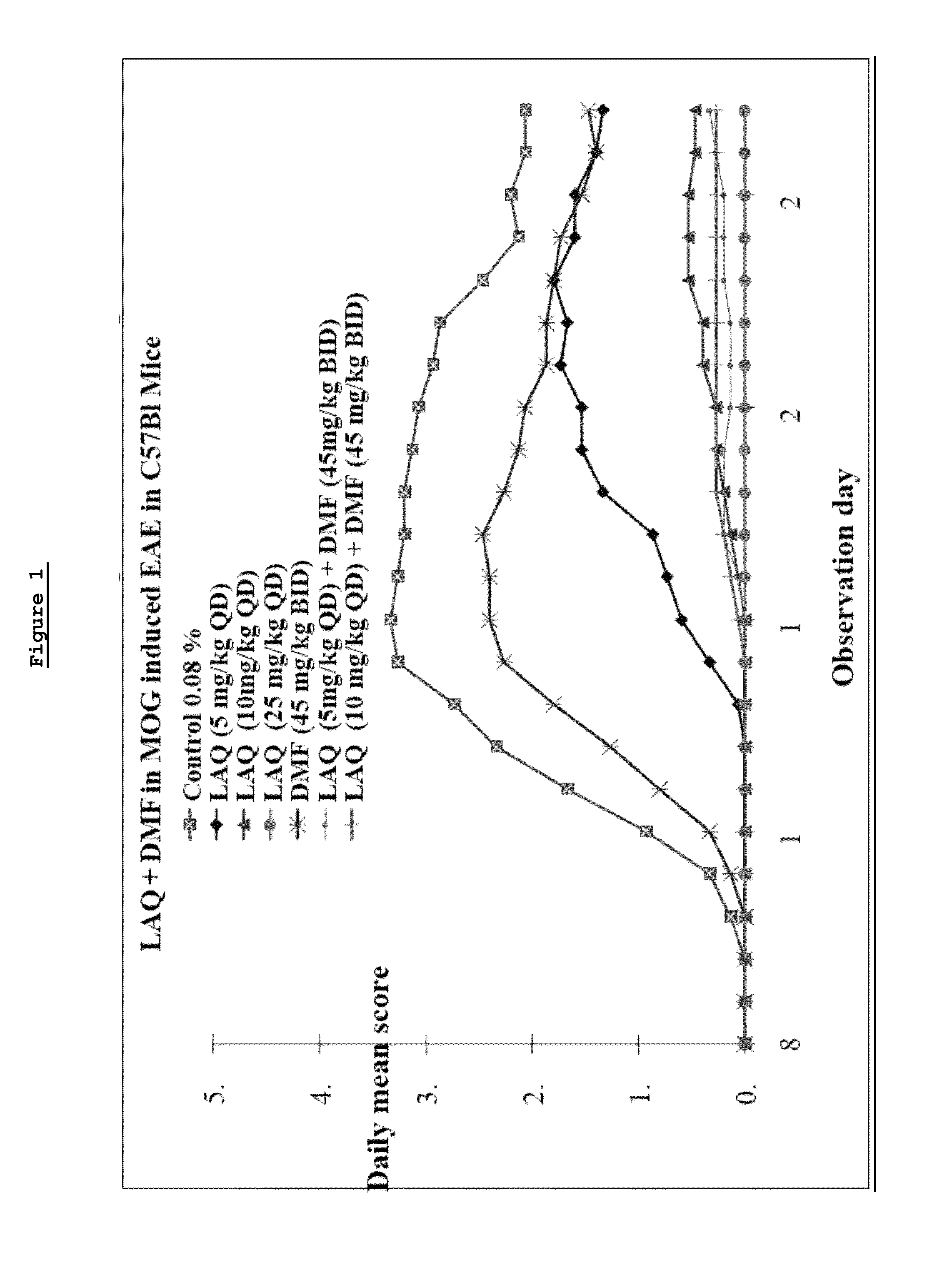
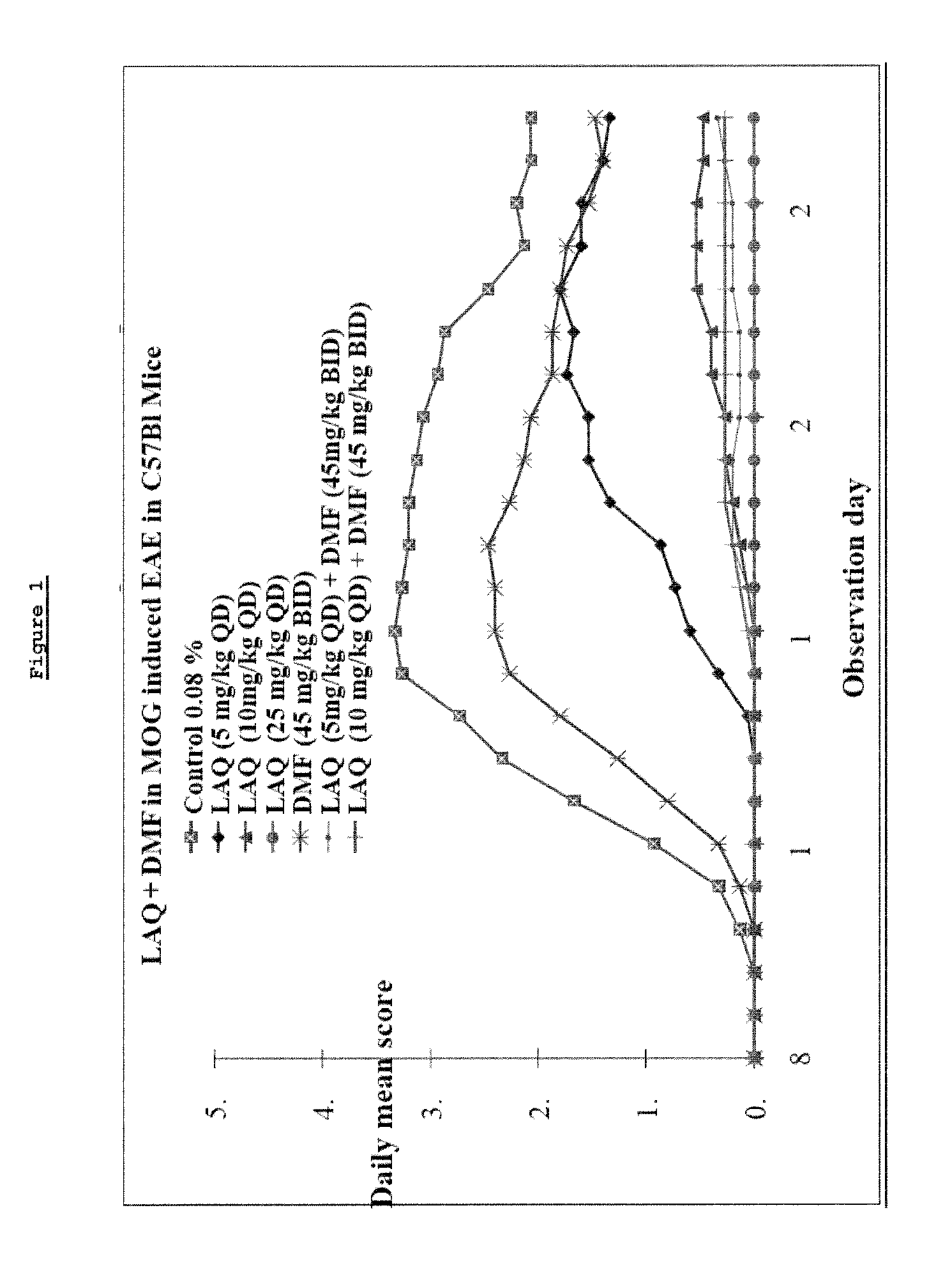
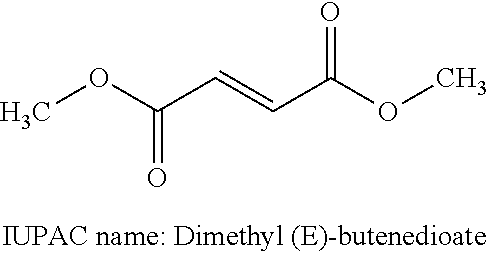
Treatment of multiple sclerosis with combination of laquinimod and dimethyl fumarate
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with DMF. This invention also provides a package comprising laquinimod and DMF for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with DMF in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides a pharmaceutical composition comprising laquinimod and DMF for use in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and DMF in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
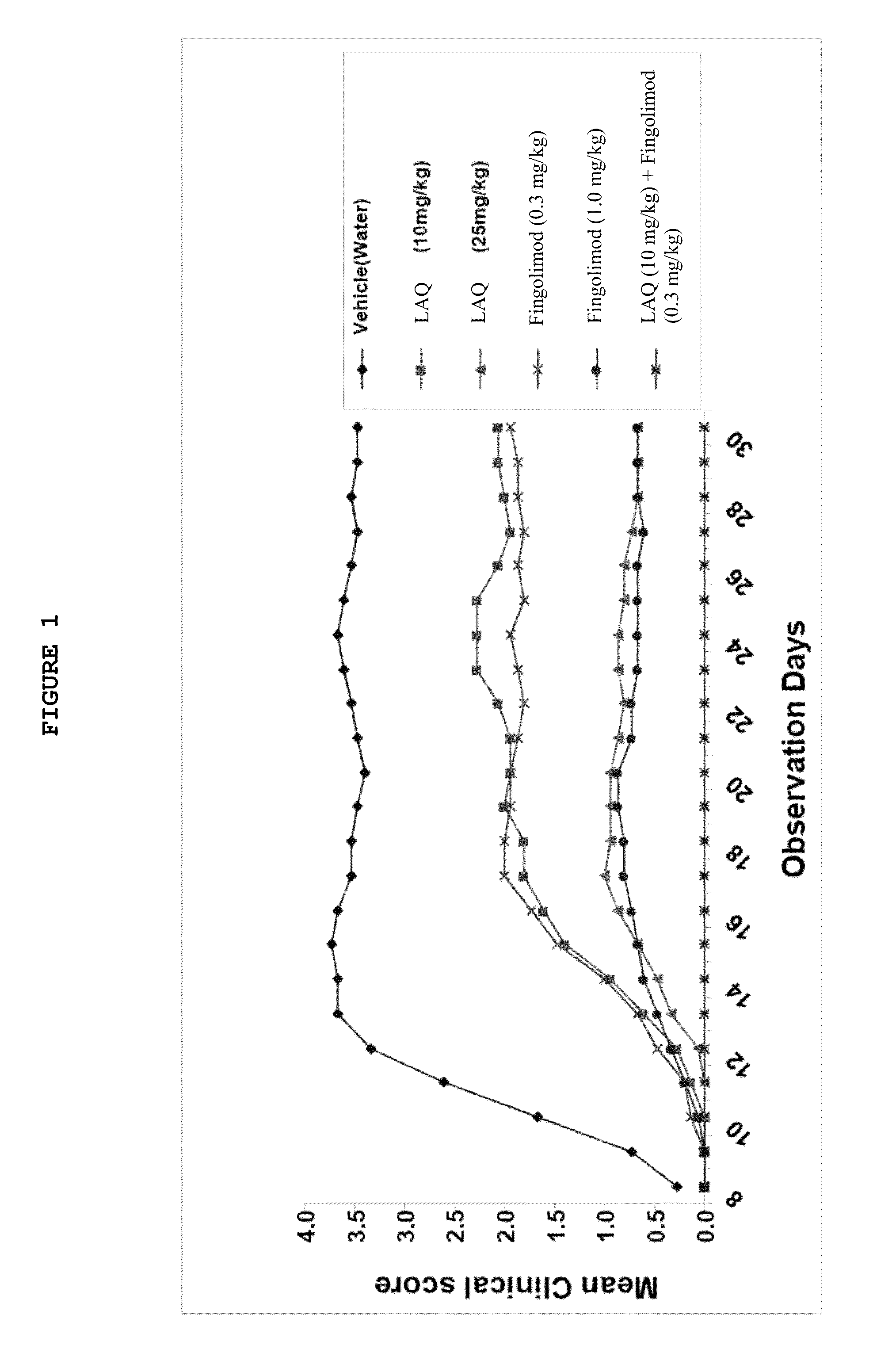
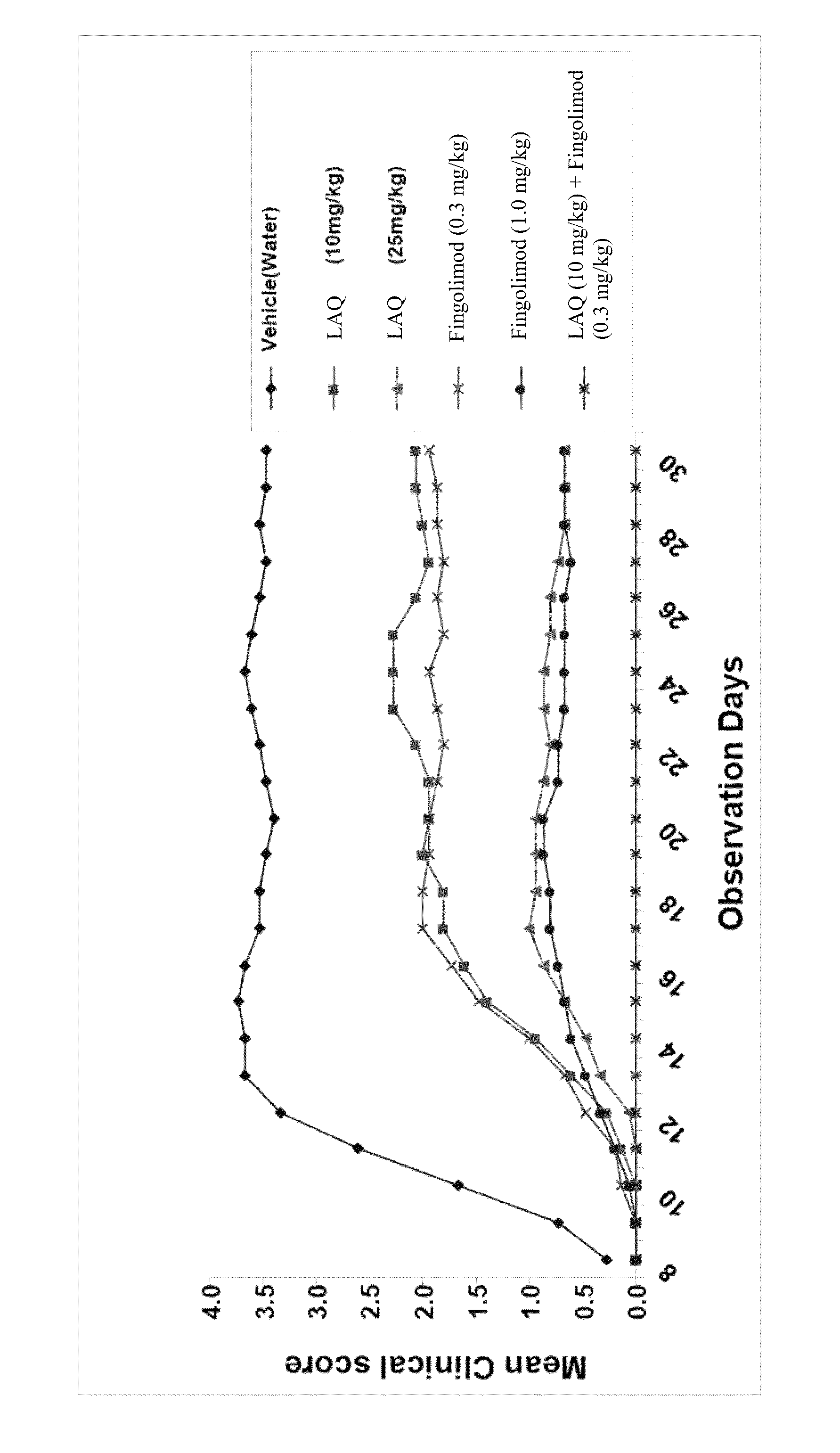
Treatment Of Multiple Sclerosis With Combination Of Laquinimod And Fingolimod
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with fingolimod. This invention also provides a package and a pharmaceutical composition comprising laquinimod and fingolimod for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with fingolimod in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and fingolimod in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Use of high dose laquinimod for treating multiple sclerosis
InactiveUS20130303569A1Extension of timeReducing brain atrophyBiocideNervous disorderHigh dosesLaquinimod
Disclosed herein are methods of treating a human patient afflicted with multiple sclerosis (MS) or presenting a clinically isolated syndrome (CIS), for treating a human subject by providing neuroprotection, and of treating a human patient afflicted with MS or presenting a CIS by increasing the time to confirmed disease progression and / or confirmed relapse or reducing brain atrophy, comprising orally administering a daily dose of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof. Also disclosed is a pharmaceutical oral unit dosage form of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier for use in treating a human patient afflicted with MS or presenting a CIS, in treating a human subject by providing neuroprotection, or in treating a human patient afflicted with MS or presenting a CIS by increasing the time to confirmed disease progression and / or confirmed relapse or reducing brain atrophy.
Owner:TEVA PHARMA IND LTD
Rituximab induction therapy followed by glatiramer acetate therapy
InactiveUS20140271630A1Organic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
The present invention provides a method of treating a subject afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome comprising periodic administration of an amount of rituximab at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject, wherein the amounts are effective to treat the subject. The present invention also provides a method of treating a subject afflicted with an immune disease, comprising periodic administration of an amount of rituximab at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject wherein the amounts are effective to treat the subject, and wherein the immune disease is an autoimmune disease, an arthritic condition, a demyelinating disease, an inflammatory disease, multiple sclerosis, relapsing-remitting multiple sclerosis, diabetes mellitus, psoriasis, rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, or systemic lupus erythematosus.
Owner:TEVA PHARMA IND LTD
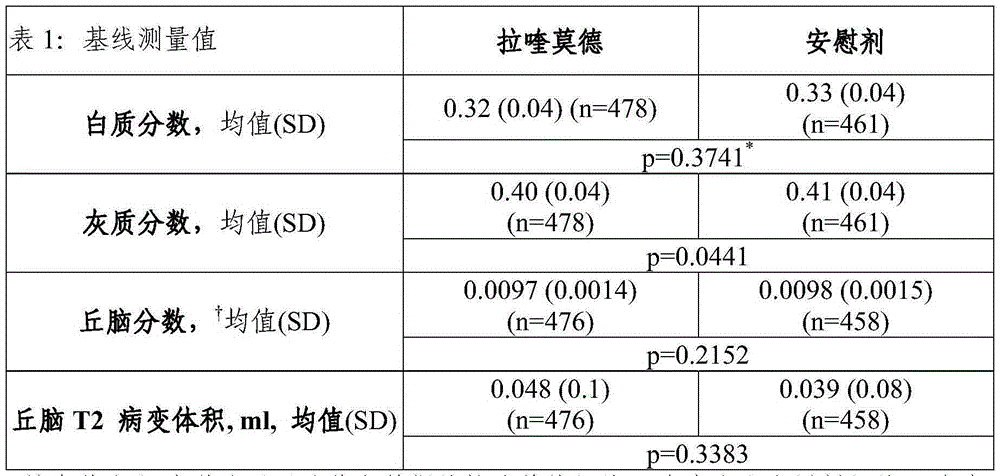
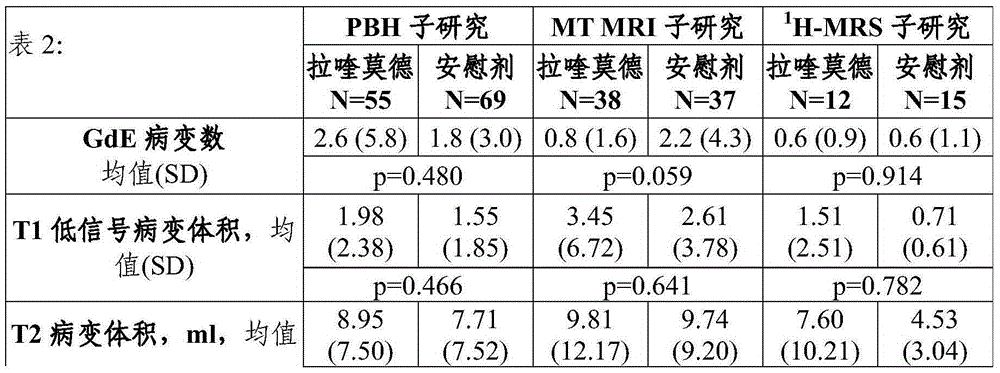
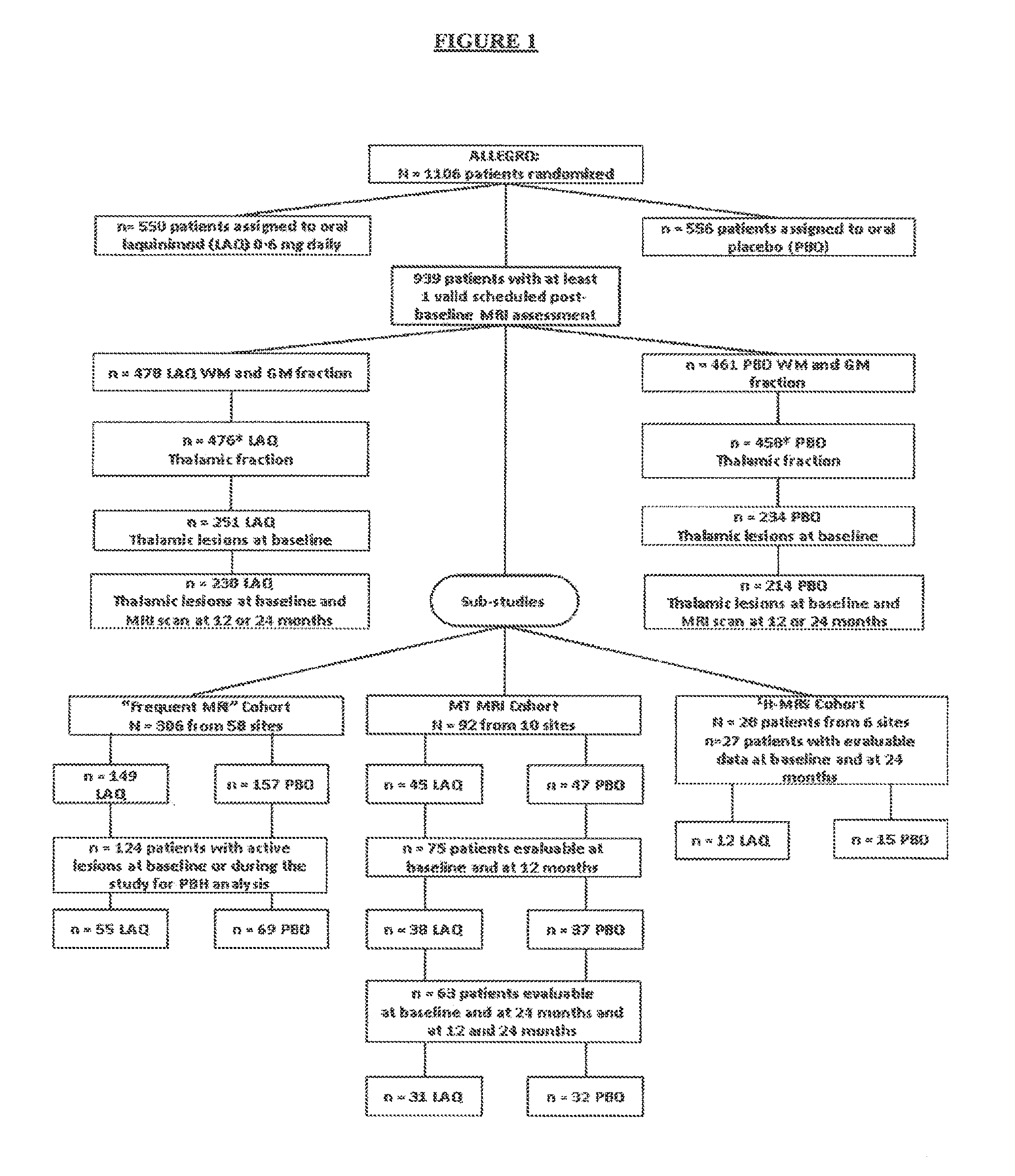
Laquinimod for reducing thalamic damage in multiple sclerosis
InactiveUS20140107154A1Inhibiting and reducing thalamic damageReduce harmBiocideNervous disorderDiseaseThalamus
This invention provides methods for inhibiting or reducing thalamic damage in a subject comprising administering to the subject an amount of laquinimod, wherein the subject is a human patient afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome who has been determined to have thalamic damage at baseline, a subject afflicted with a disease or disorder other than a form of multiple sclerosis or a clinically isolated syndrome, or a subject not afflicted with a form of multiple sclerosis or a presenting clinically isolated syndrome, and laquinimod and laquinimod pharmaceutical compositions for use thereof. This invention also provides methods for inhibiting or reducing tremor or spasticity in a subject afflicted by tremor or spasticity, comprising administering to the subject an amount of laquinimod, and laquinimod and laquinimod pharmaceutical compositions for use thereof.
Owner:TEVA PHARMA IND LTD
Method for treating multiple sclerosis
InactiveUS20100172869A1Maximize efficacyReduce adverse side effectsNervous disorderPeptide/protein ingredientsDosing FrequencyInterferon alpha
Methods for treating multiple sclerosis (MS) and clinically isolated syndromes suggestive of MS are provided. The methods comprise administering a therapeutically effective dose of interferon-beta (IFN-beta) to a subject in need thereof, where the dose is administered intramuscularly with a dosing frequency of two- to three-times per week.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
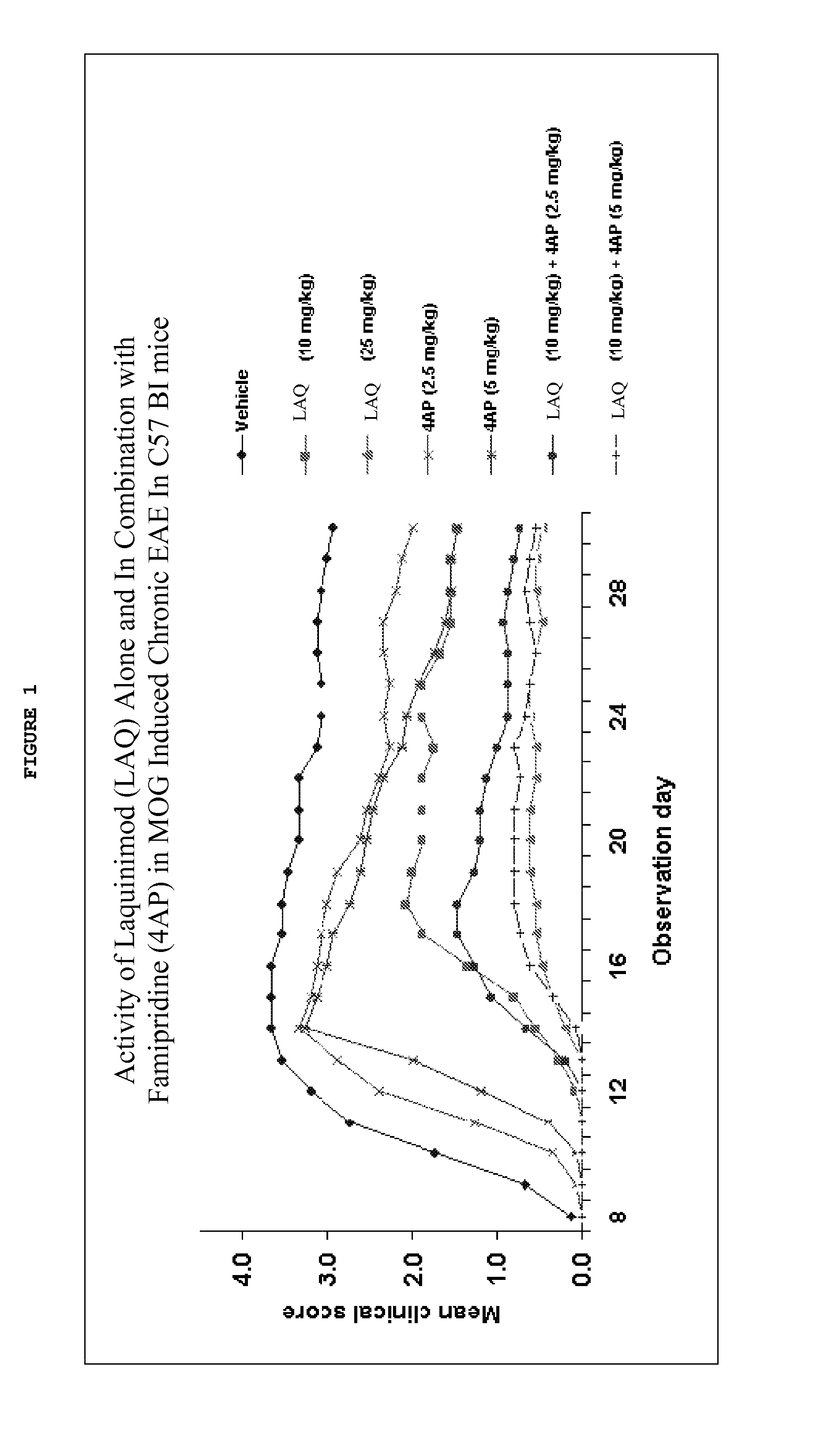
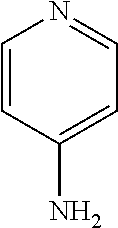
Treatment of multiple sclerosis with combination of laquinimod and fampridine
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject fampridine as an add-on therapy to or in combination with laquinimod. This invention also provides a package comprising laquinimod and fampridine for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides fampridine for use as an add-on therapy or in combination with laquinimod in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides a pharmaceutical composition comprising laquinimod and fampridine for use in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and fampridine in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
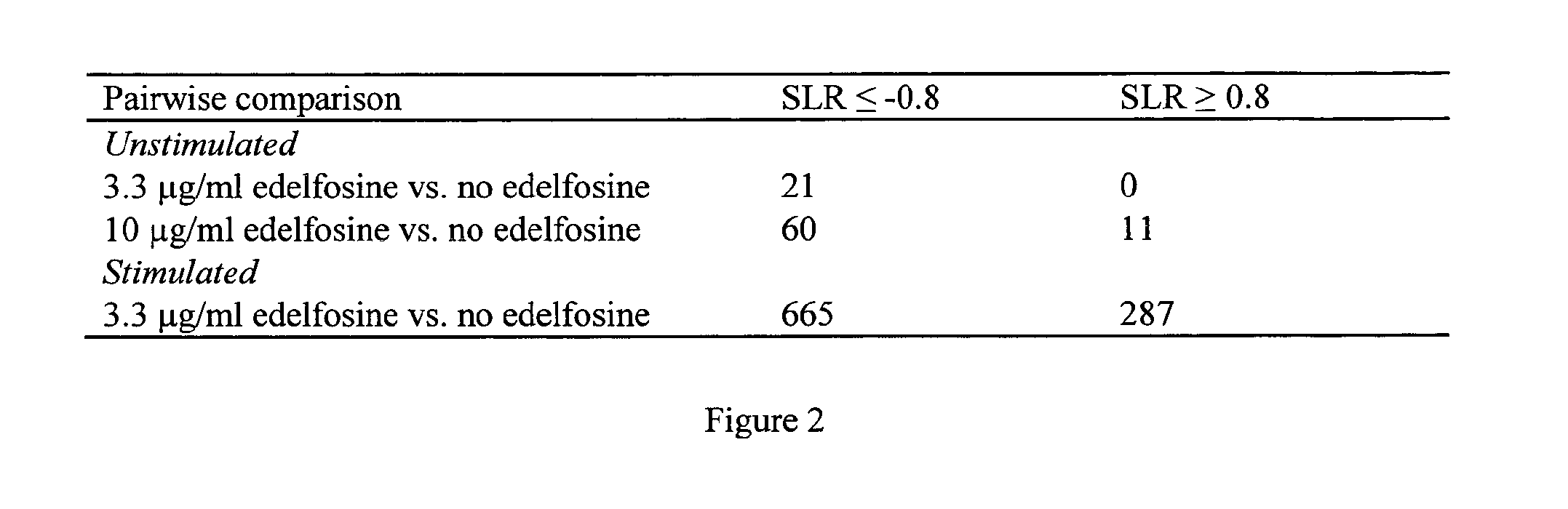
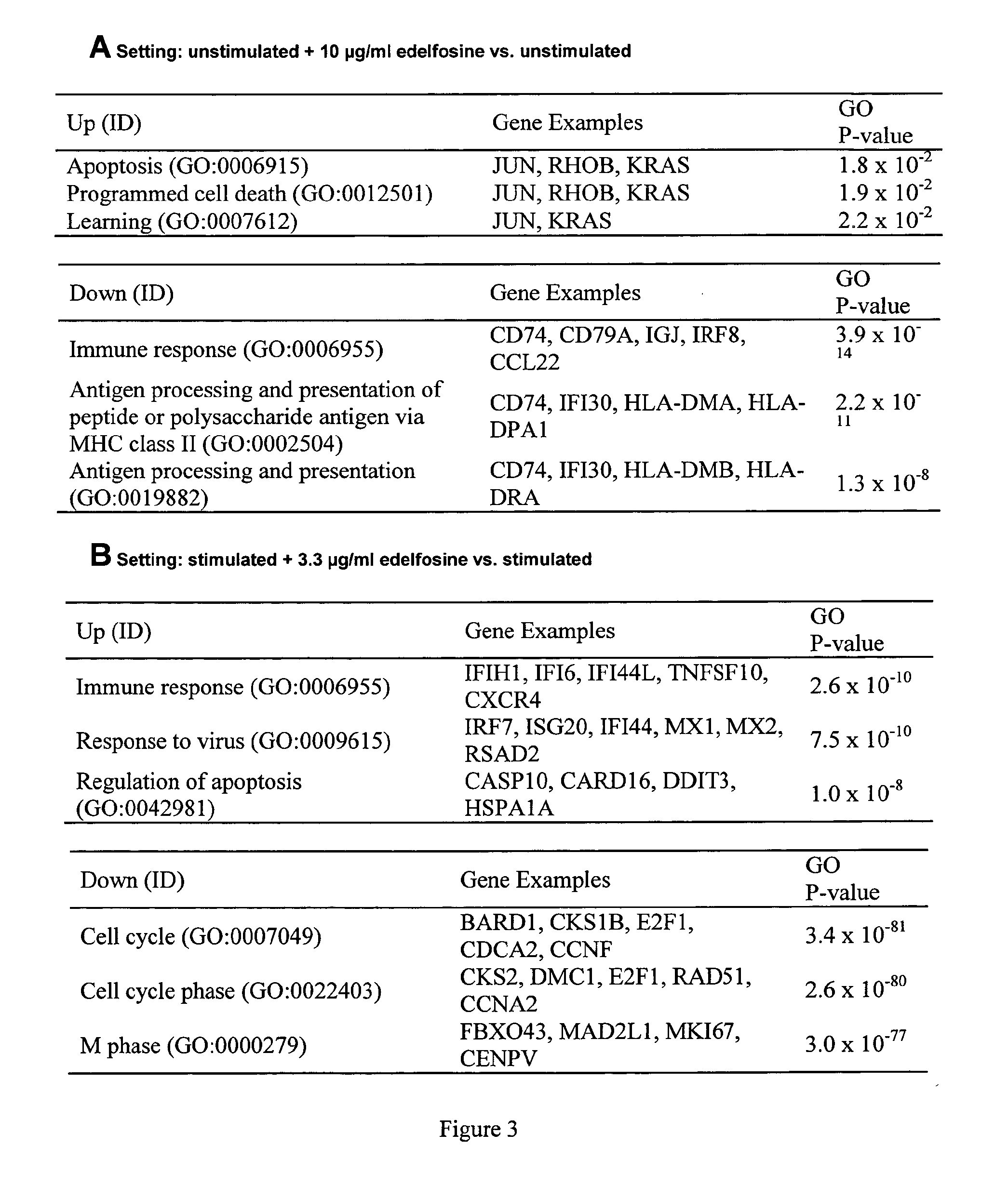
Gene expression biomarkers of laquinimod responsiveness
This invention provides a method of predicting clinical responsiveness to laquinimod therapy in a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising evaluating expression of a biomarker in the subject. This invention also provides a method of treating said subject comprising determining whether the subject is a laquinimod-responder by evaluating expression of a biomarker. Also provided is laquinimod or a pharmaceutical composition comprising laquinimod for use in treating said subject, and a therapeutic package for use in dispensing to said subject, wherein the subject has been identified as a laquinimod-responder or expression of a biomarker in the subject is up-regulated or suppressed.
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES +1
Diagnosis of multiple sclerosis in human and animal subjects
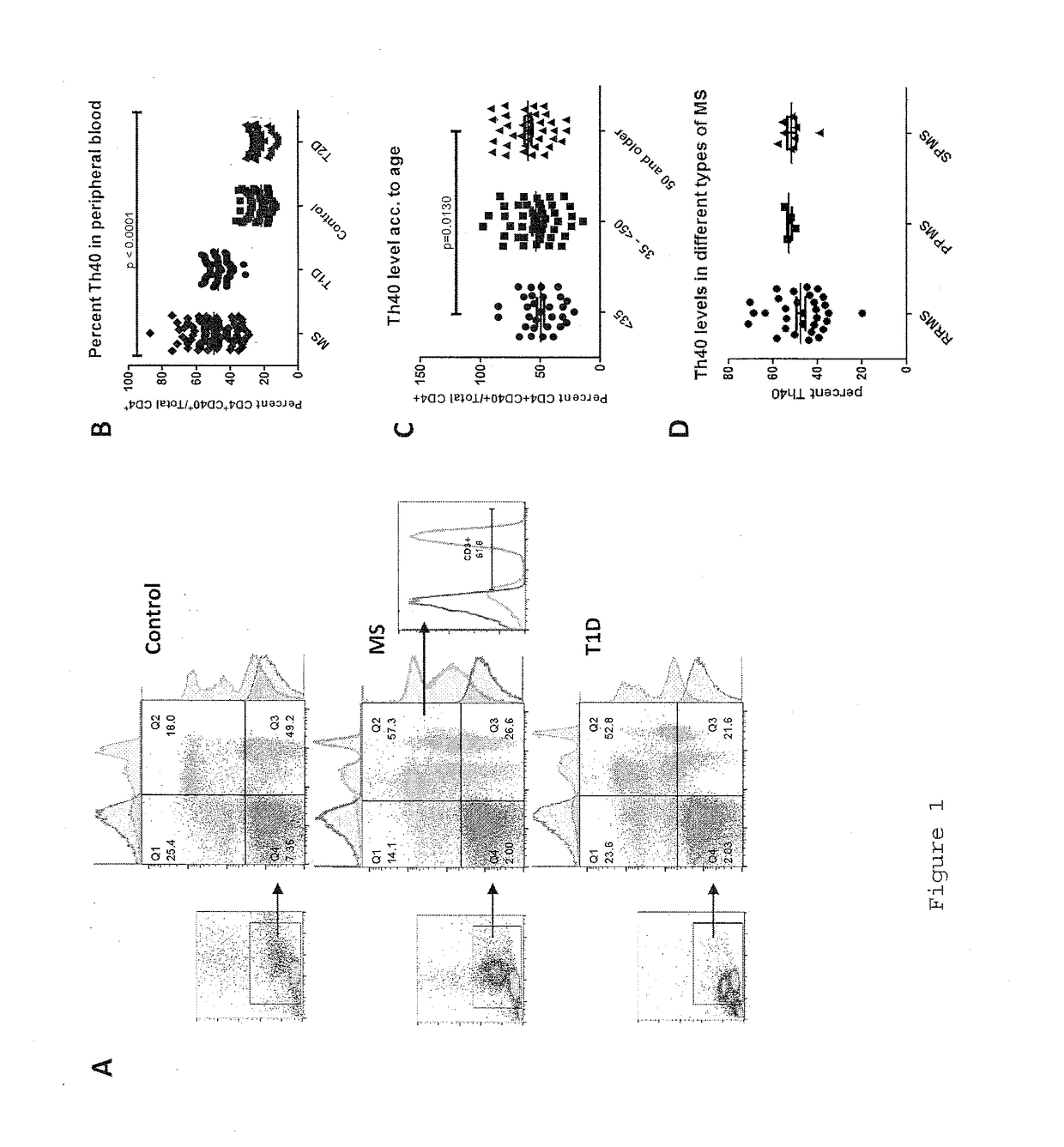
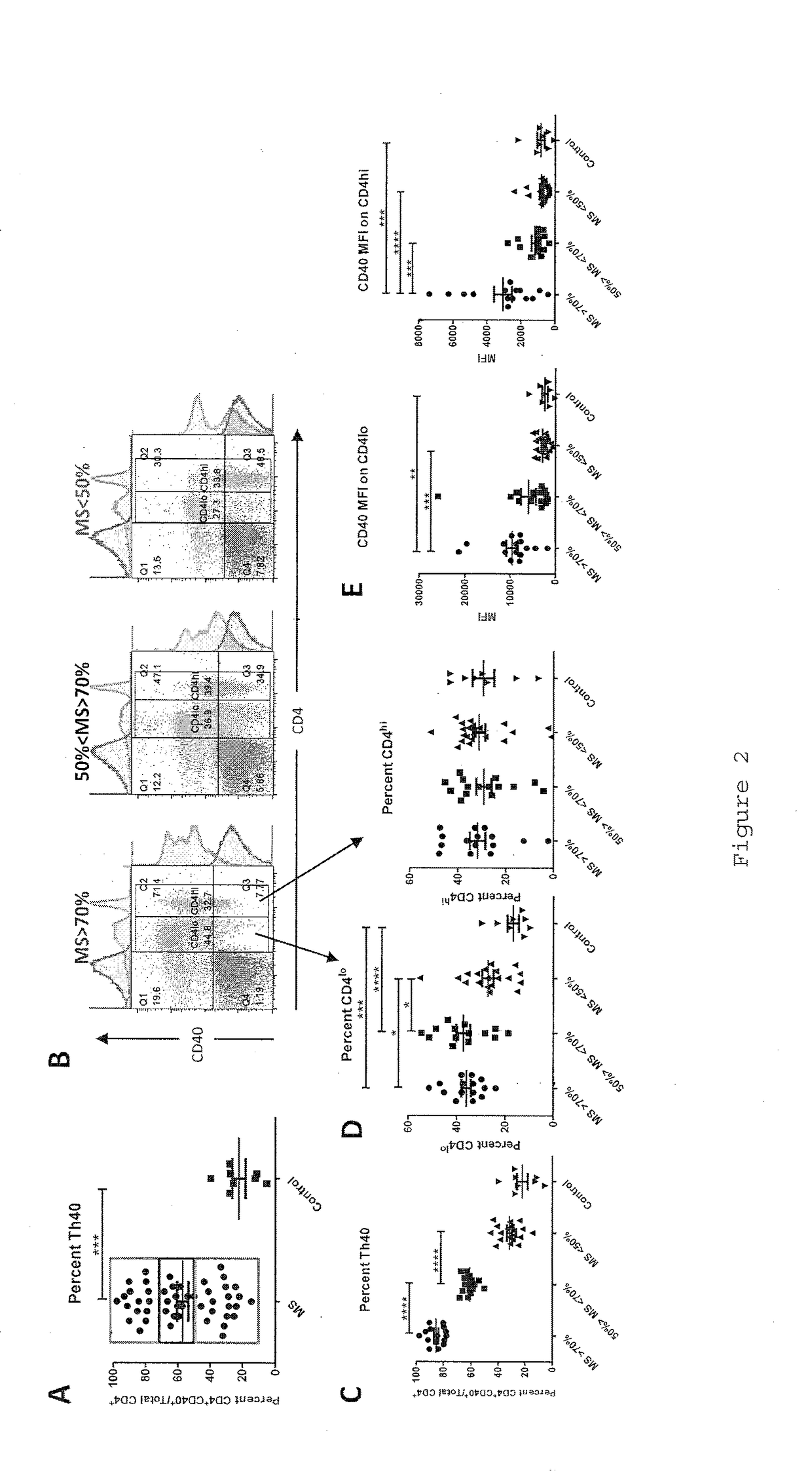
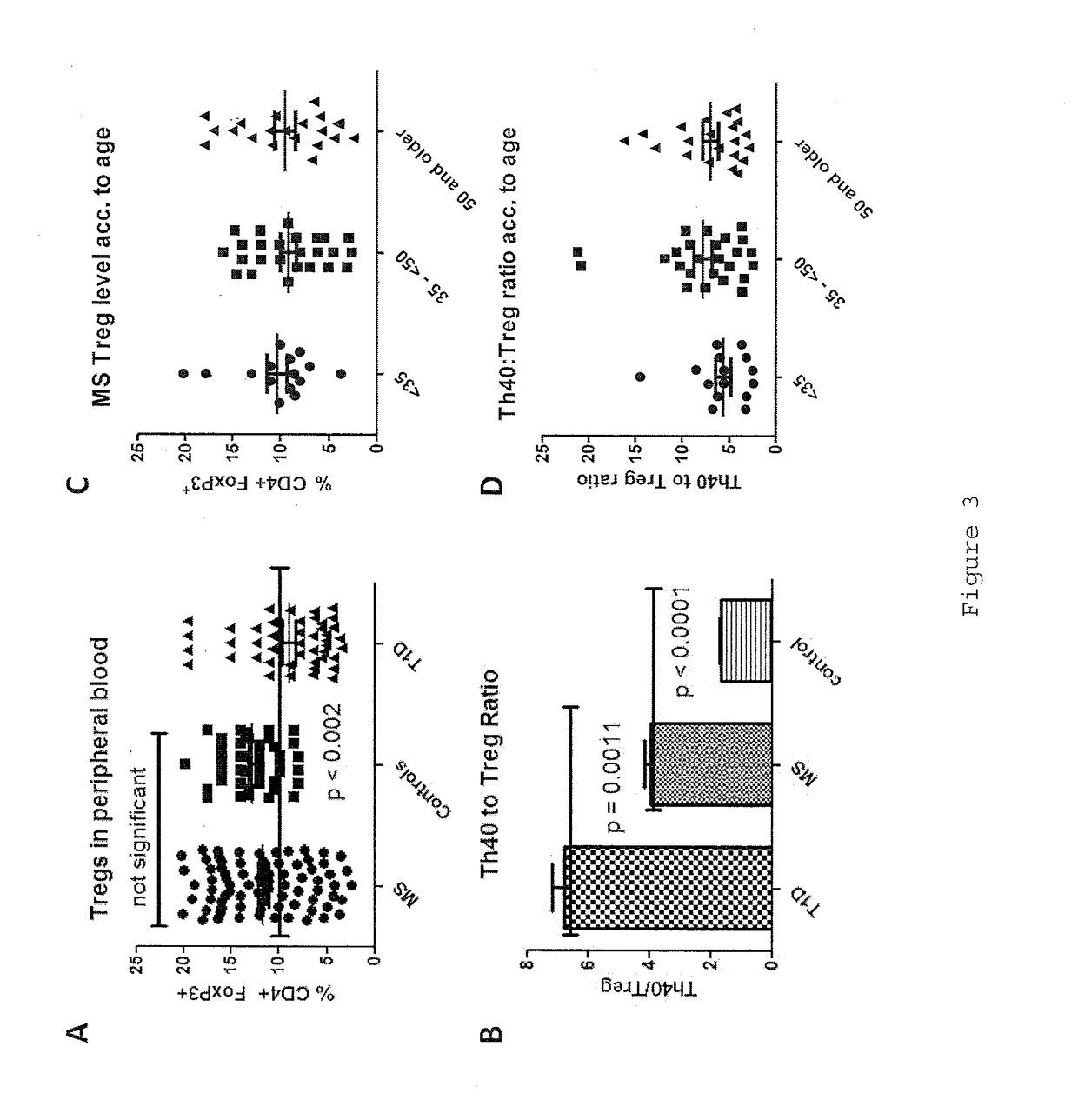
InactiveUS20170108514A1Decrease the percentage of Th40 cells in the patient's bloodPeptide/protein ingredientsBiological particle analysisAnimal subjectDisease progression
Cellular markers useful for methods of diagnosing multiple sclerosis (MS), relapse of MS patients and disease progression in MS patients, as well as identifying treatments for and monitoring treatment of patients with multiple sclerosis (MS). Methods of differential diagnosis of patients presenting with clinically isolated syndrome (CIS) suggestive of MS and / or Radiologically Isolated Syndrome (RIS) for presence of MS or relapse of MS, or lack thereof. Methods of treating patients having multiple sclerosis.
Owner:UNIV OF COLORADO THE REGENTS OF
Treatment of multiple sclerosis with combination of laquinimod and fingolimod
Owner:TEVA PHARMA IND LTD
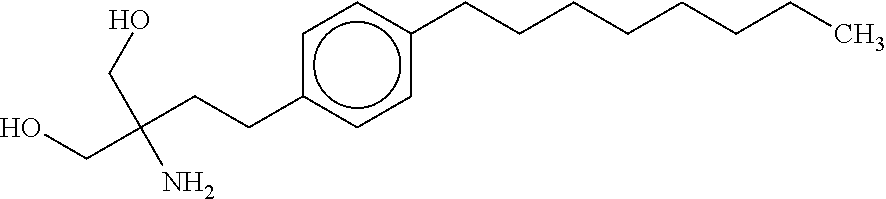
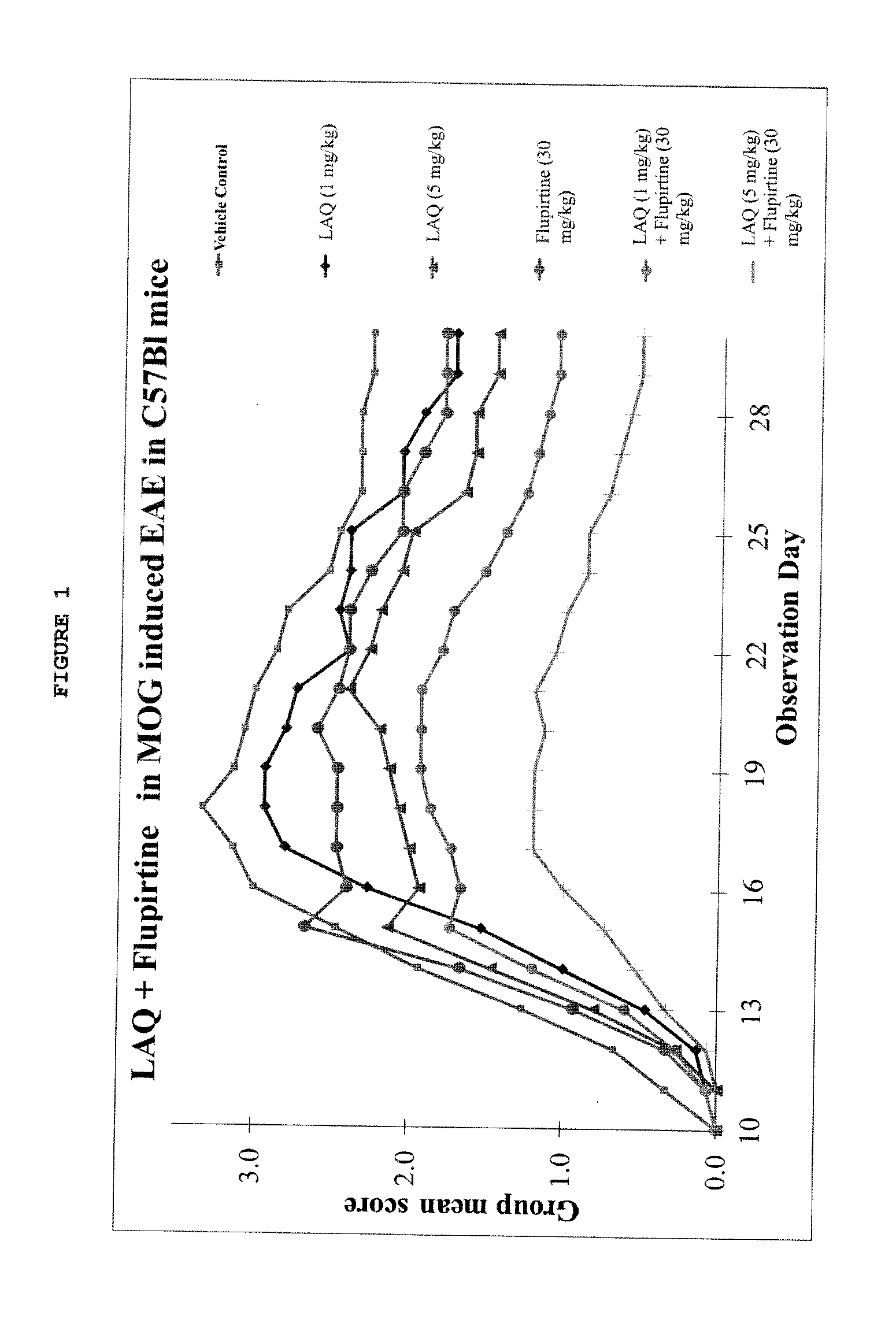
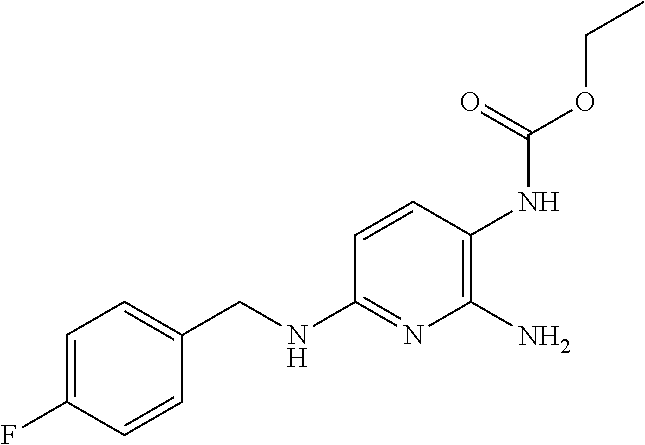
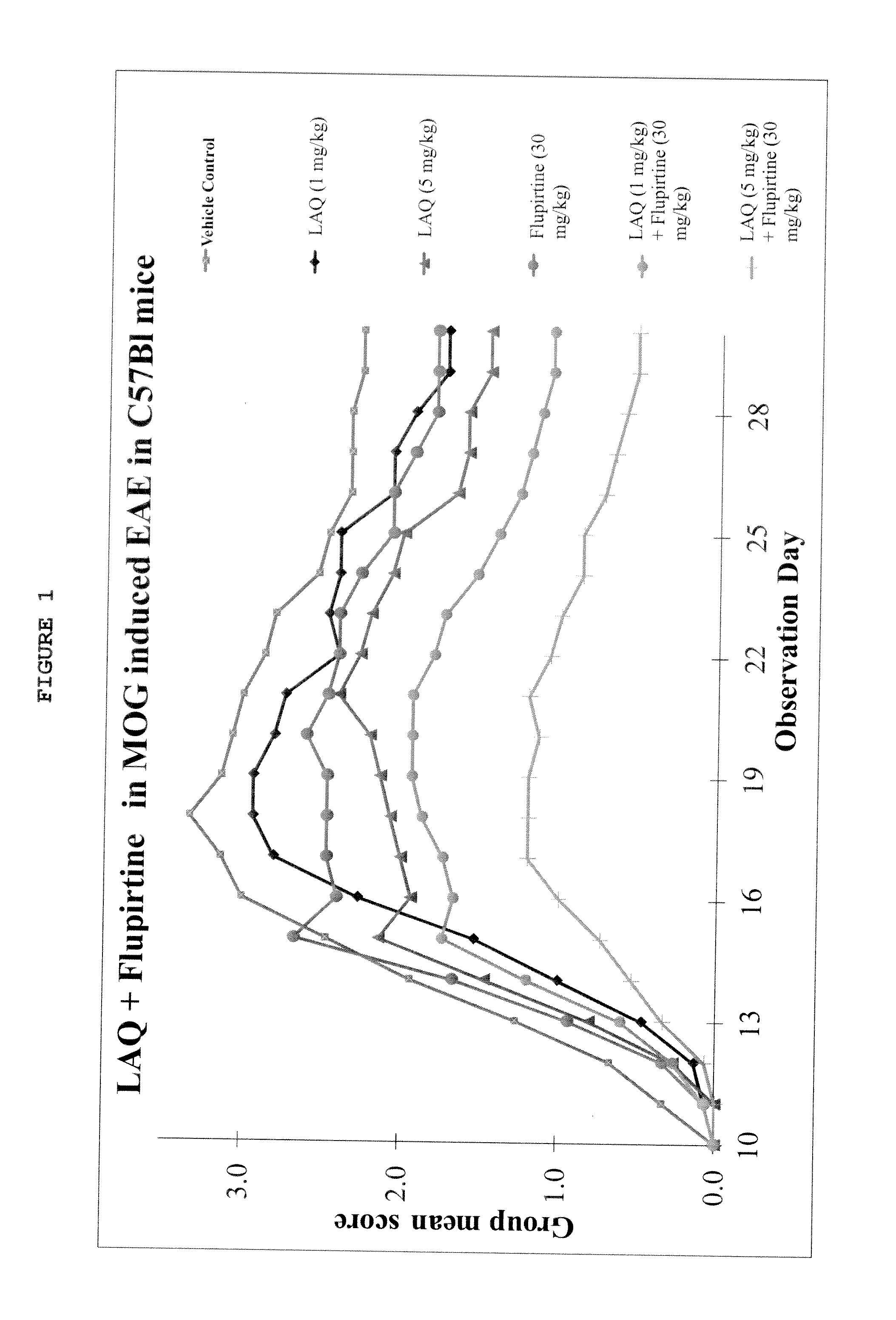
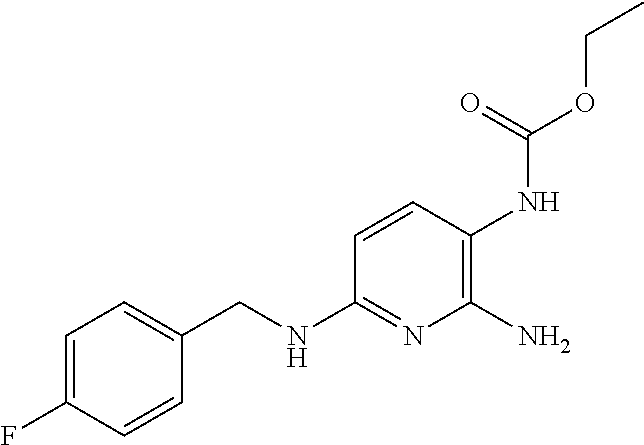
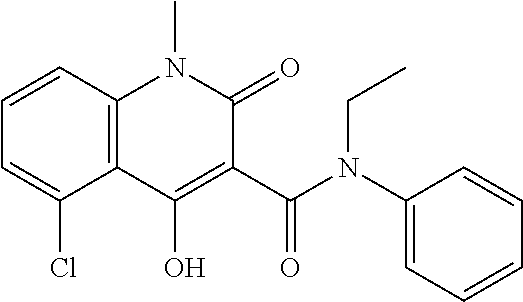
Treatment of multiple sclerosis with combination of laquinimod and flupirtine
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with flupirtine. This invention also provides a package and a pharmaceutical composition comprising laquinimod and flupirtine for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with flupirtine in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and flupirtine in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Biomarkers for multiple sclerosis
InactiveUS20130184173A1Reduce concentrationMicrobiological testing/measurementLibrary screeningPediatric patientBiomarker (petroleum)
Biomarkers that can be used for the diagnosis and prognosis of multiple sclerosis in pediatric patients presenting with clinically isolated syndrome or acquired demyelination syndrome are described.
Owner:MCGILL UNIV
Method for Evaluating Risk in Multiple Sclerosis
InactiveUS20100203569A1Long been problemMicrobiological testing/measurementDisease diagnosisDiseaseAnti-Glycan Antibody
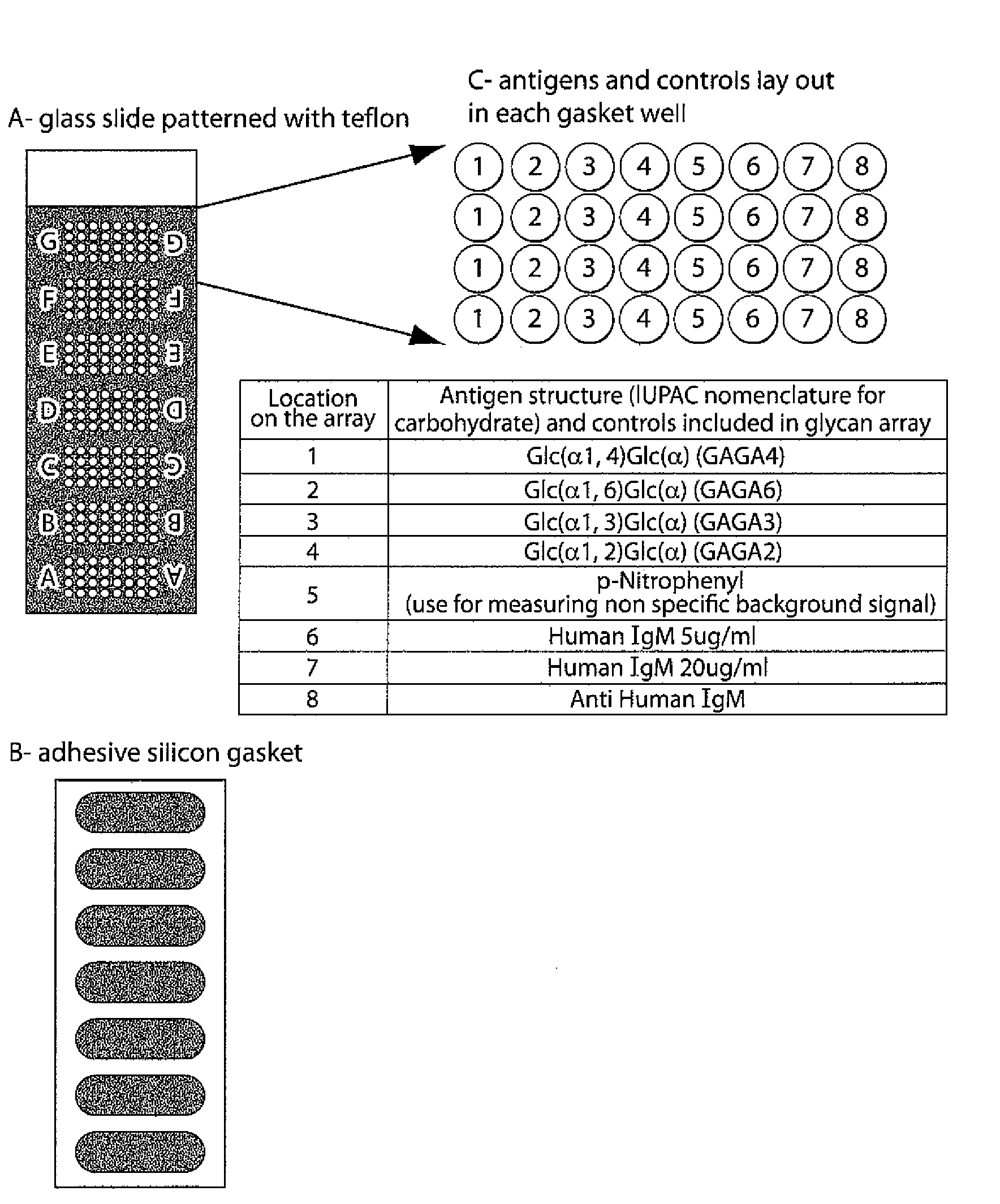
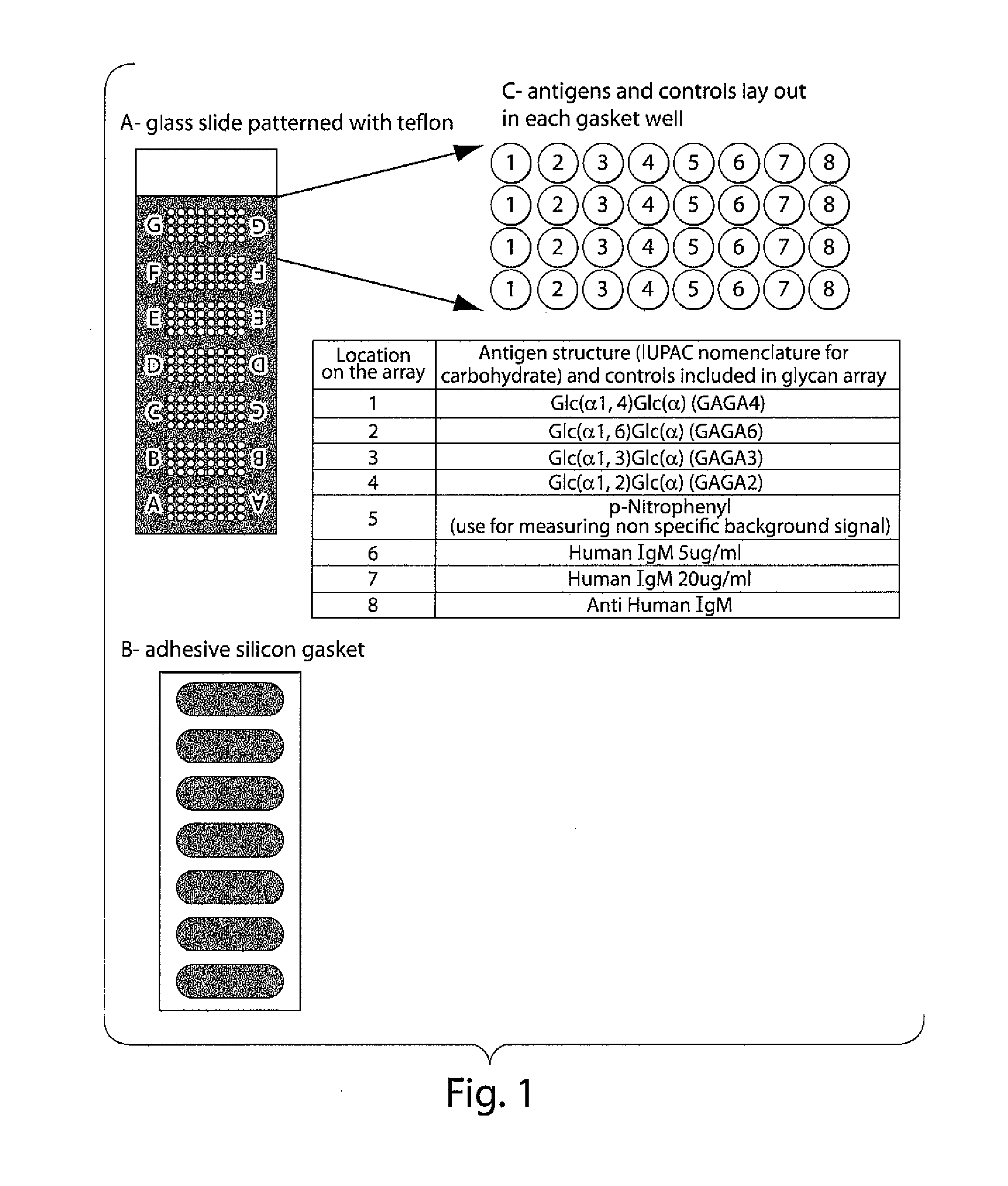
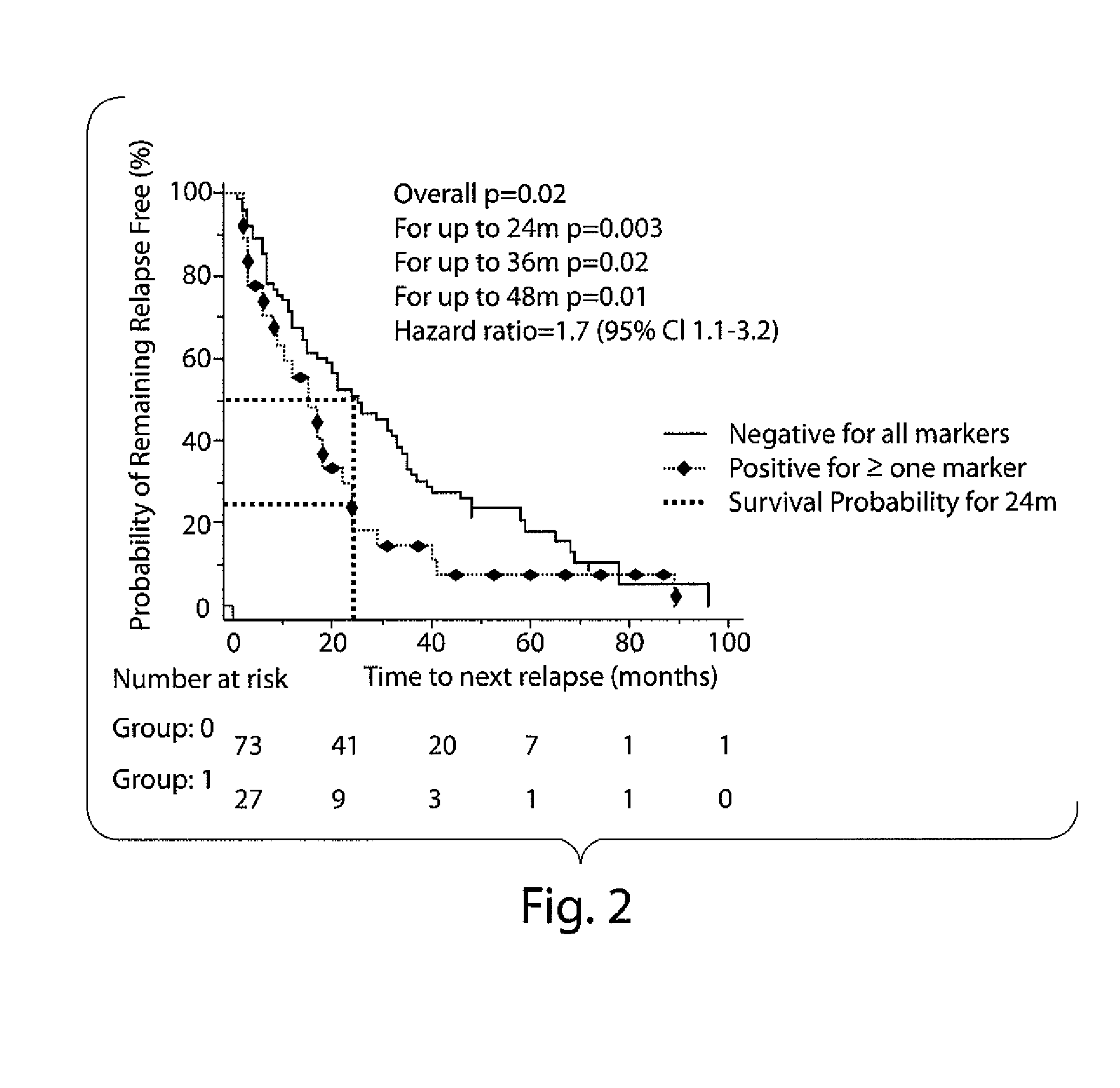
The invention relates to methods and reagents for diagnosing and assessing the prognosis of multiple sclerosis. The present invention is based, in part, upon the discovery that anti-glycan antibodies are useful in evaluating the risk of whether clinically isolated syndrome (CIS) patients suggestive of Multiple sclerosis (MS) will have a clinical relapse within, e.g., 24 months. The invention is also based upon the discovery that anti-glycan antibodies are useful for evaluating the risk of CIS patients suggestive of MS to have a rapid disease progression and accumulate disabilities, e.g., permanent disability, within a certain time frame, e.g., 5 years.
Owner:GLYCOMINKS LTD
Rituximab induction therapy followed by glatiramer acetate therapy
The present invention provides a method of treating a subject afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome comprising periodic administration of an amount of an anti-CD20 antibody at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject, wherein the amounts are effective to treat the subject.
Owner:TEVA PHARMA IND LTD
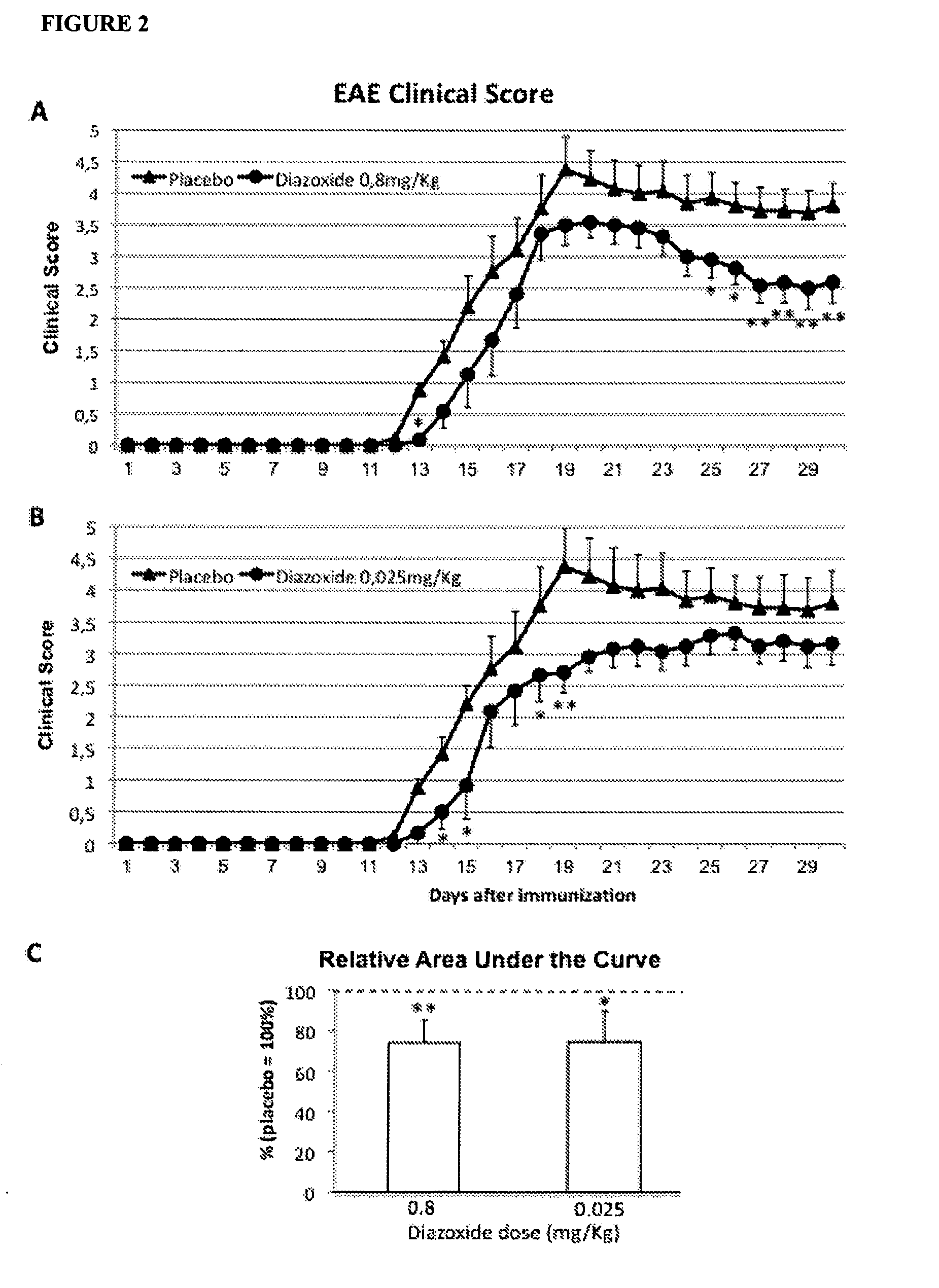
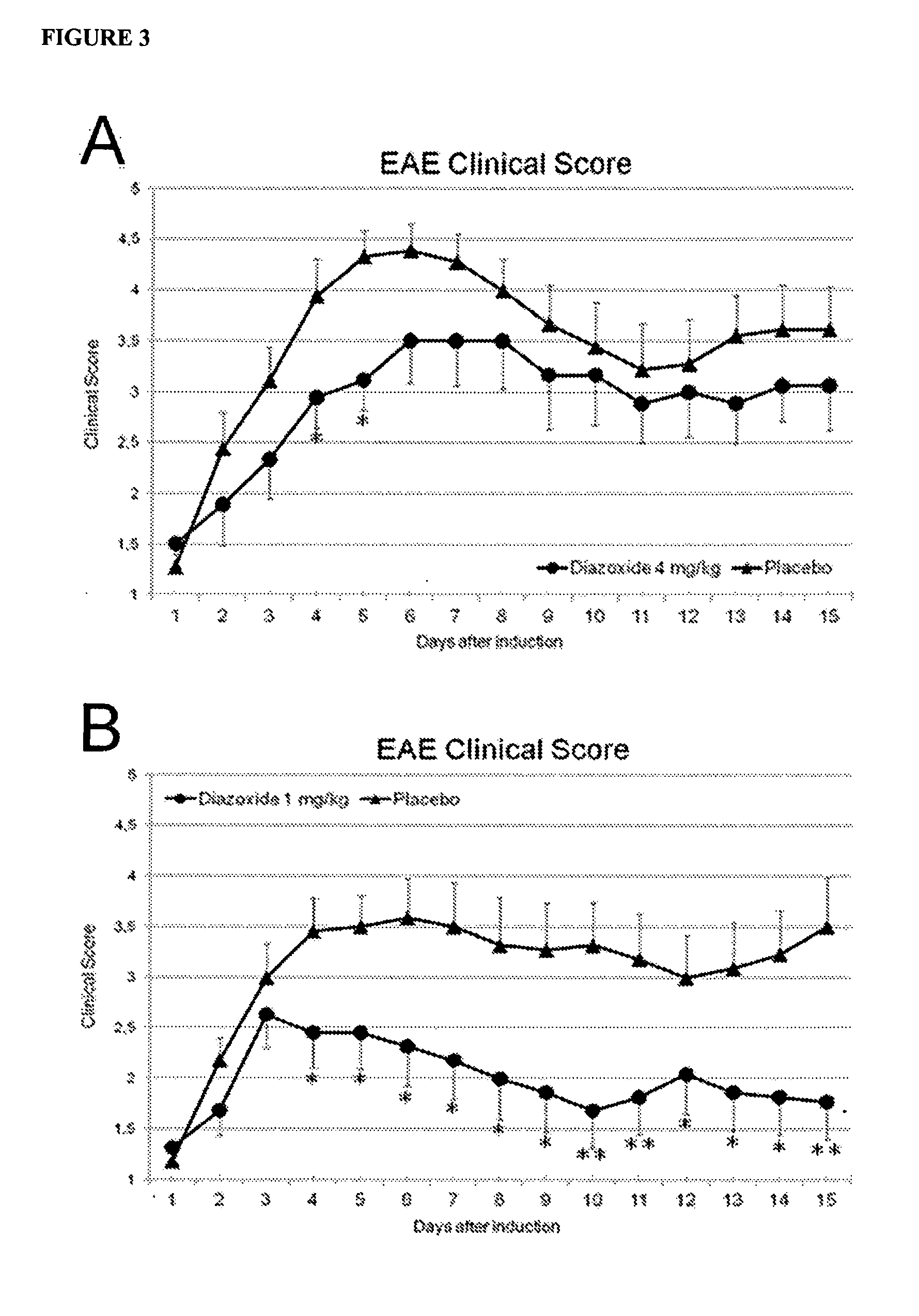
Diazoxide For Use In The Treatment Or Prevention Of A Central Nervous System (CNS) Autoimmune Demyelinating Disease
InactiveUS20130039905A1Improving clinical manifestationNervous disorderAntibody ingredientsNervous systemAutoimmunity
The invention relates to the use of diazoxide or a pharmaceutically acceptable salt thereof at low doses to treat a CNS autoimmune demyelinating disease selected from selected from multiple sclerosis (MS), clinically isolated syndrome (CIS), tumefactive (tumor-like) M S, Marburg's acute M S, Balós's concentric sclerosis, acute disseminated encephalomyelitis (ADEM), post-vaccinal encephalitis (PVE), post-infectious encephalomyelitis (PIE) and neuromyelitis optica (NMO).
Owner:NEUROTEC PHARMA
Use of high dose laquinimod for treating multiple sclerosis
InactiveUS20160000775A1Reducing brain atrophyExtension of timeBiocideNervous disorderAtrophyHigh doses
Disclosed herein are methods of treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome, methods for treating a human subject by providing neuroprotection to the human subject, and methods of treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome by increasing the time to confirmed disease progression, increasing the time to confirmed relapse or reducing brain atrophy in the human patient, comprising orally administering to the human patient or subject a daily dose of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof. The subject invention also provides a pharmaceutical oral unit dosage form of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier for use in treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome, for use in treating a human subject by providing neuroprotection to the human subject, or for use treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome by increasing the time to confirmed disease progression, increasing the time to confirmed relapse or reducing brain atrophy in the human patient.
Owner:TEVA PHARMA IND LTD
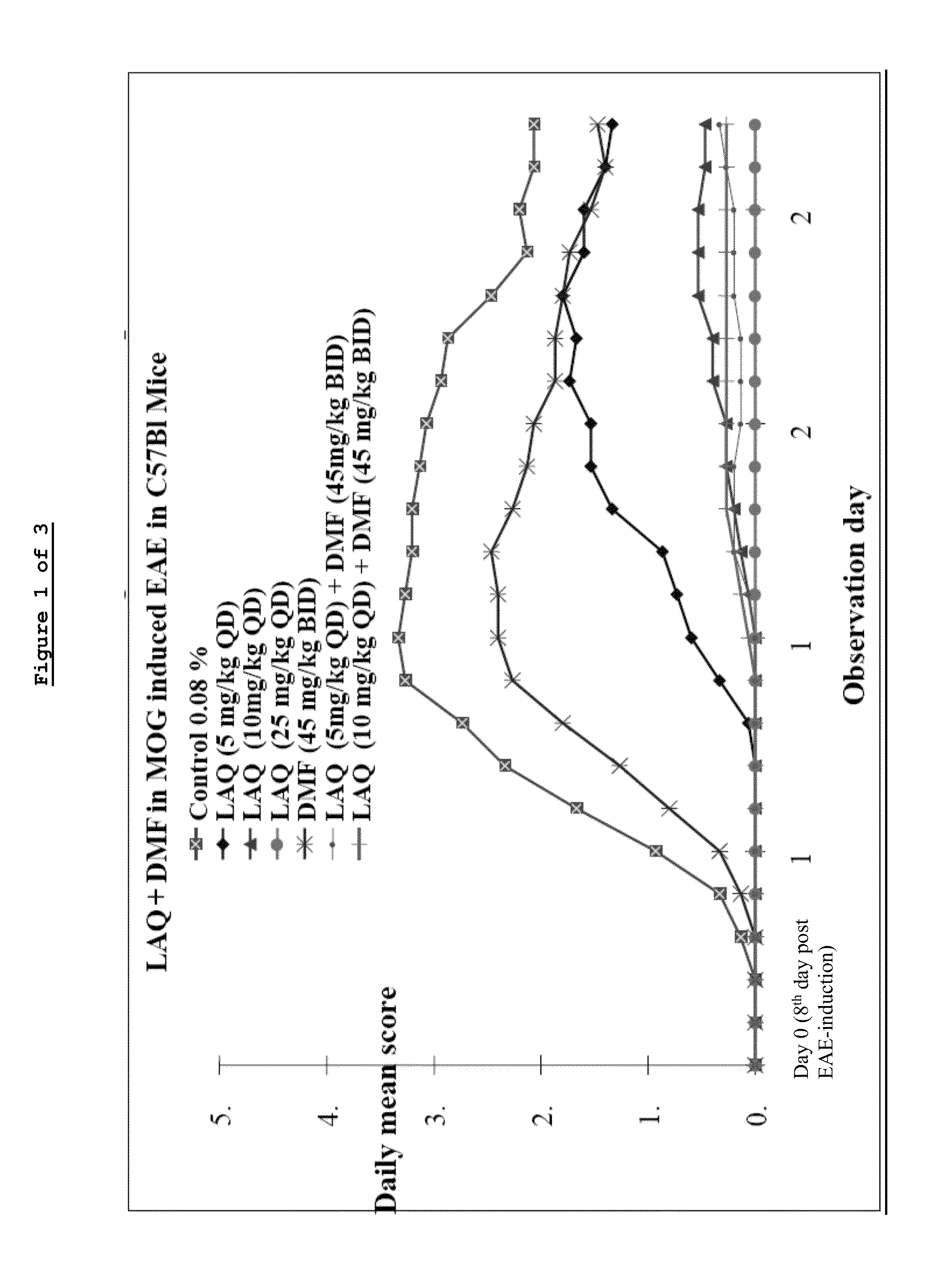
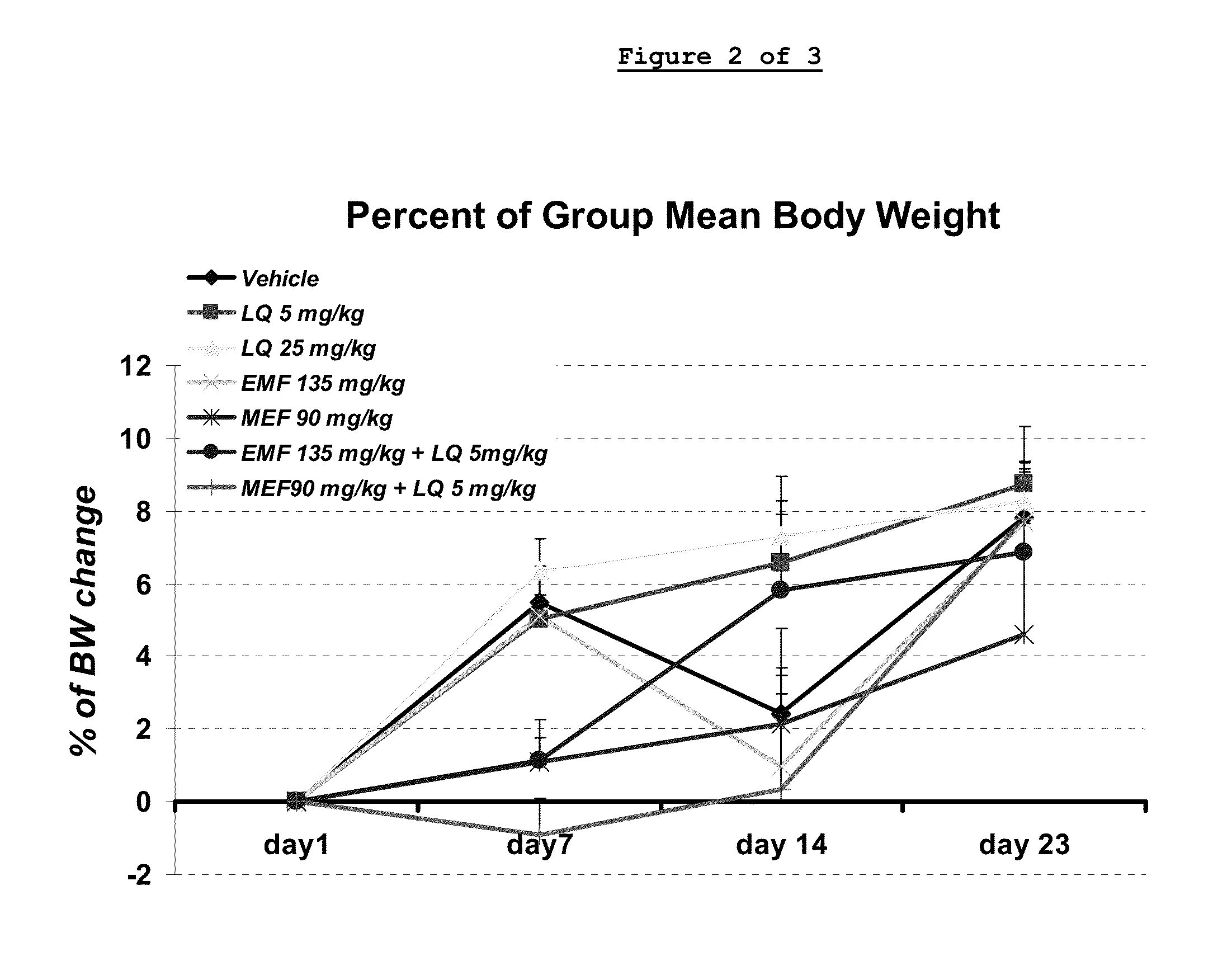
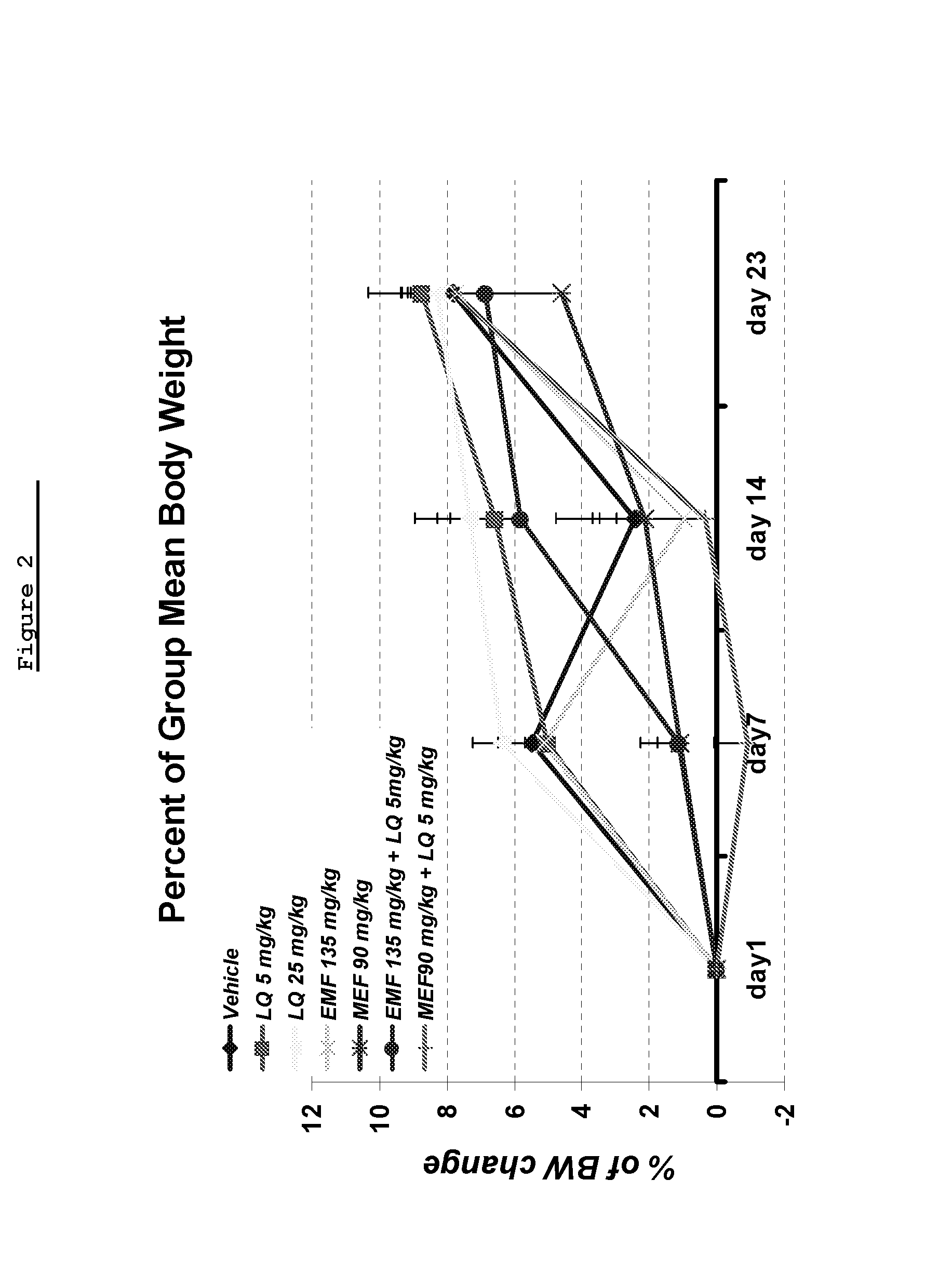
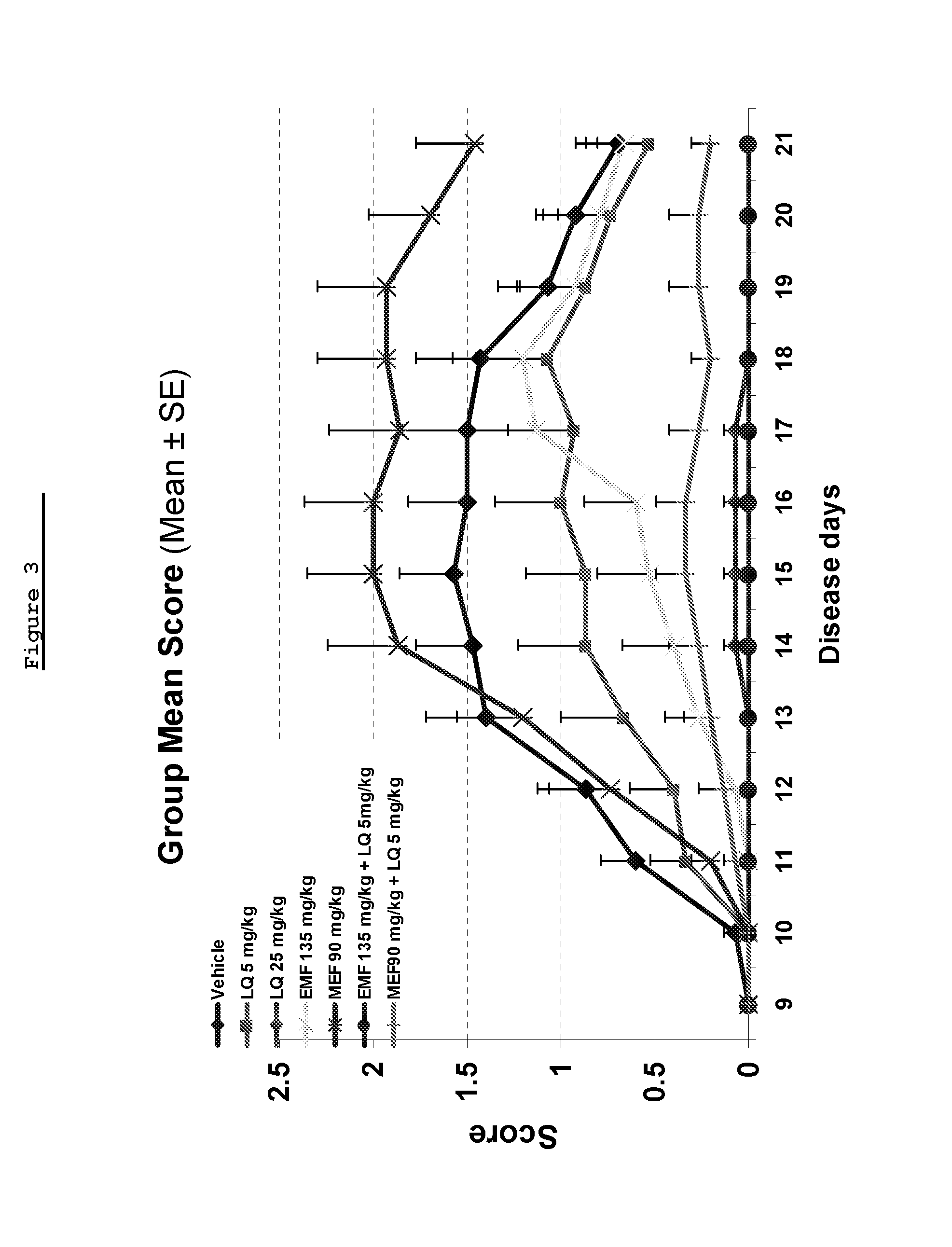
Laquinimod Combination Therapy For Treatment Of Multiple Sclerosis
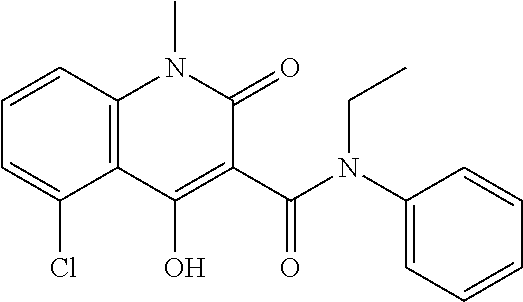
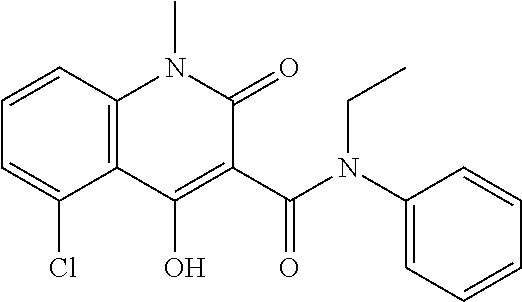
The subject invention provides a method for treating a subject afflicted with a form of multiple sclerosis (MS) or presenting a clinically isolated syndrome (CIS) comprising periodically administering to the subject an amount of laquinimod and an amount of a compound of formula (I):as described herein. The subject invention also provides packages and pharmaceutical compositions comprising laquinimod and a compound of formula (I) as described herein. The subject invention further provides uses of said compounds, pharmaceutical compositions and packages in treating a subject afflicted with a form of MS or presenting a CIS.
Owner:TEVA PHARMA IND LTD
Treatment of Multiple Sclerosis With Combination of Laquinimod and Glatiramer Acetate
InactiveUS20160038532A1Effective treatmentPowder deliverySenses disorderLaquinimodGlatiramer acetate
This invention provides a method of treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the patient laquinimod an add-on therapy to or in combination with glatiramer acetate. This invention also provides a package and a pharmaceutical composition comprising laquinimod and glatiramer acetate for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with glatiramer acetate in treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and glatiramer acetate in the preparation of a combination for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Treatment of Multiple Sclerosis With Combination of Laquinimod and Dimethyl Fumarate
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with DMF. This invention also provides a package comprising laquinimod and DMF for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with DMF in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides a pharmaceutical composition comprising laquinimod and DMF for use in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and DMF in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Laquinimod for reducing thalamic damage in multiple sclerosis
This invention provides methods for inhibiting or reducing thalamic damage in a subject comprising administering to the subject an amount of laquinimod, wherein the subject is a human patient afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome who has been determined to have thalamic damage at baseline, a subject afflicted with a disease or disorder other than a form of multiple sclerosis or a clinically isolated syndrome, or a subject not afflicted with a form of multiple sclerosis or a presenting clinically isolated syndrome, and laquinimod and laquinimod pharmaceutical compositions for use thereof. This invention also provides methods for inhibiting or reducing tremor or spasticity in a subject afflicted by tremor or spasticity, comprising administering to the subject an amount of laquinimod, and laquinimod and laquinimod pharmaceutical compositions for use thereof.
Owner:TEVA PHARMA IND LTD
Diazoxide for use in the treatment of a central nervous system (CNS) autoimmune demyelinating disease
InactiveCN102770142AShow fullOrganic active ingredientsNervous disorderAcute disseminated encephalomyelitisNervous system
The invention relates to the use of diazoxide or a pharmaceutically acceptable salt thereof at low doses to treat a CNS autoimmune demyelinating disease selected from selected from multiple sclerosis (MS), clinically isolated syndrome (CIS), tumefactive (tumor-like) MS, Marburg's acute MS, Bal?s's concentric sclerosis, acute disseminated encephalomyelitis (ADEM), post-vaccinal encephalitis (PVE), post-infectious encephalomyelitis (PIE) and neuromyelitis optica (NMO).
Owner:NEUROTEC PHARMA
Treatment of Multiple Sclerosis With Combination of Laquinimod and Interferon-Beta
This invention provides a method of treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the patient laquinimod as an add-on therapy to or in combination with interferon-β. This invention also provides a package and a pharmaceutical composition comprising laquinimod and interferon-β for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with interferon-β in treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and interferon-β in the preparation of a combination for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Treatment of multiple sclerosis by alemtuzumab induction followed by laquinimod therapy
This invention provides a method of treating a subject afflicted with multiple sclerosis (MS) or presenting clinically isolated syndrome (CIS) which comprises a) administering to the subject an amount of an anti-CD52 antibody, followed by b) periodically administering to the subject an amount of laquinimod. This invention also provides packages comprising pharmaceutical compositions of laquinimod or an anti-CD52 antibody for treating such a subject wherein laquinimod is to be administered as a maintenance therapy in such a subject who has received an anti-CD52 antibody induction therapy.
Owner:TEVA PHARMA IND LTD
Laquinimod Combination Therapy For Treatment Of Multiple Sclerosis
Owner:TEVA PHARMA IND LTD
Treatment of multiple sclerosis with combination of laquinimod and flupirtine
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with flupirtine. This invention also provides a package and a pharmaceutical composition comprising laquinimod and flupirtine for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with flupirtine in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and flupirtine in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Use of high dose laquinimod for treating multiple sclerosis
InactiveUS20150265592A1Reducing brain atrophyExtension of timeBiocideNervous disorderAtrophyHigh doses
Owner:TEVA PHARMA IND LTD
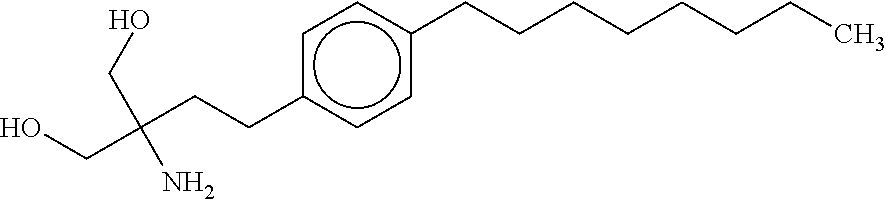
Tri-substituted glycerol compounds for use in the treatment of clinically isolated syndrome and/or multiple sclerosis
InactiveUS20150157651A1Reduce in quantityReduce penetrationBiocideNervous disorderInterferon therapyMedicine
The present invention relates to tri-substituted glycerol compounds according to formula (I) as described herein for use in the treatment of clinically isolated syndrome, relapsing remitting multiple sclerosis (RR-MS) and / or secondary progressive multiple sclerosis (SP-MS). The tri-substituted glycerol compounds according to formula (I) as described herein may also be used for the treatment of multiple sclerosis patients not adequately responding to interferon therapy. The present invention also relates to a tri-substituted glycerol compound as described herein, for use as a medicament, wherein the tri-substituted glycerol compound is administered in combination with at least one further pharmaceutically active compound.
Owner:ALPHAPTOSE +1
Laquinimod for reducing thalamic damage in multiple sclerosis
InactiveUS20160296511A1Inhibiting and reducing thalamic damageReduce harmOrganic active ingredientsNervous disorderDiseaseLaquinimod
This invention provides methods for inhibiting or reducing thalamic damage in a subject comprising administering to the subject an amount of laquinimod, wherein the subject is a human patient afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome who has been determined to have thalamic damage at baseline, a subject afflicted with a disease or disorder other than a form of multiple sclerosis or a clinically isolated syndrome, or a subject not afflicted with a form of multiple sclerosis or a presenting clinically isolated syndrome, and laquinimod and laquinimod pharmaceutical compositions for use thereof. This invention also provides methods for inhibiting or reducing tremor or spasticity in a subject afflicted by tremor or spasticity, comprising administering to the subject an amount of laquinimod, and laquinimod and laquinimod pharmaceutical compositions for use thereof.
Owner:TEVA PHARMA IND LTD
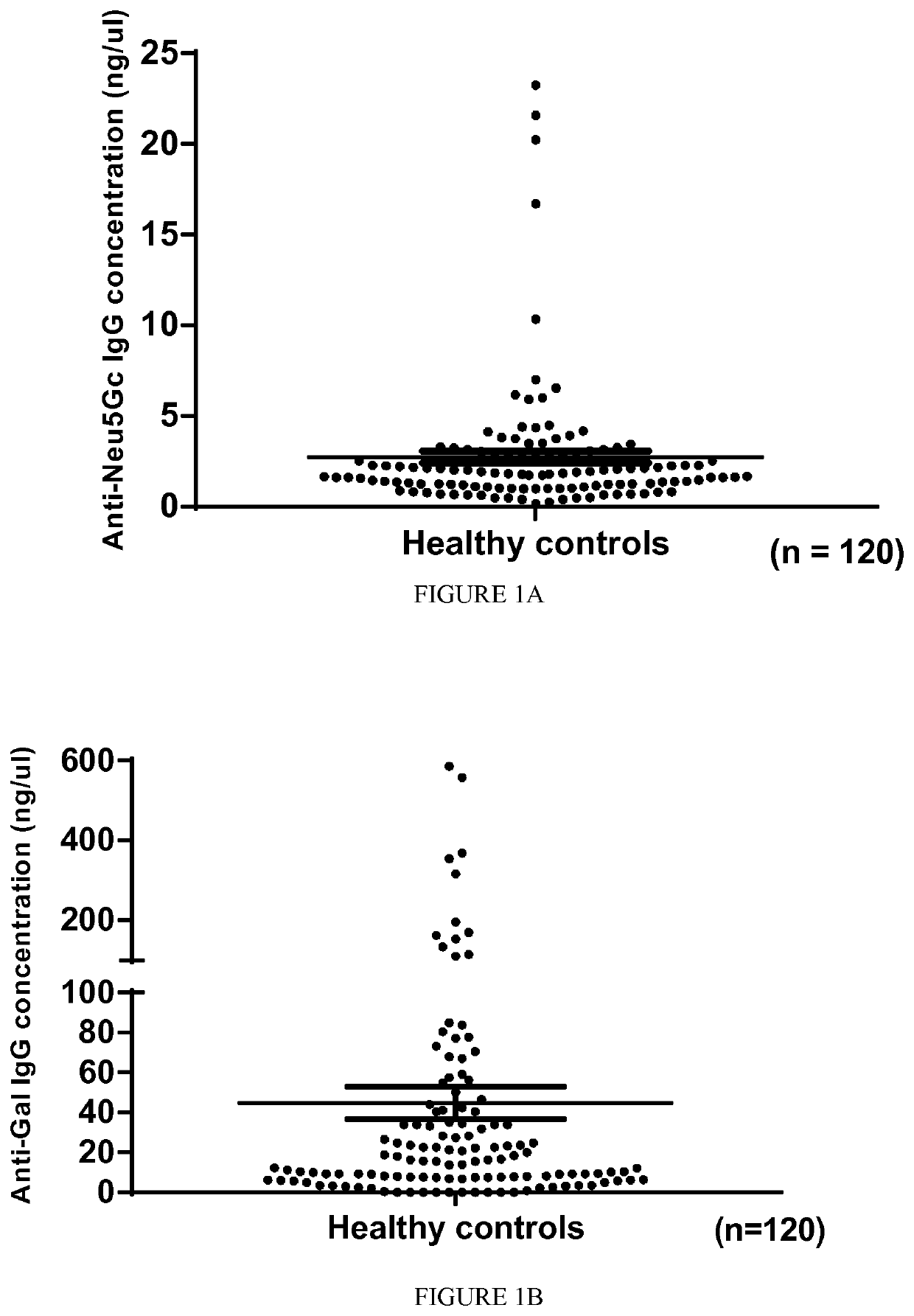
Diagnosis, prevention and treatment of demyelinating disorders of the central nervous system
PendingUS20210138001A1Peptide/protein ingredientsUnknown materialsDemyelinating DisorderAntiendomysial antibodies
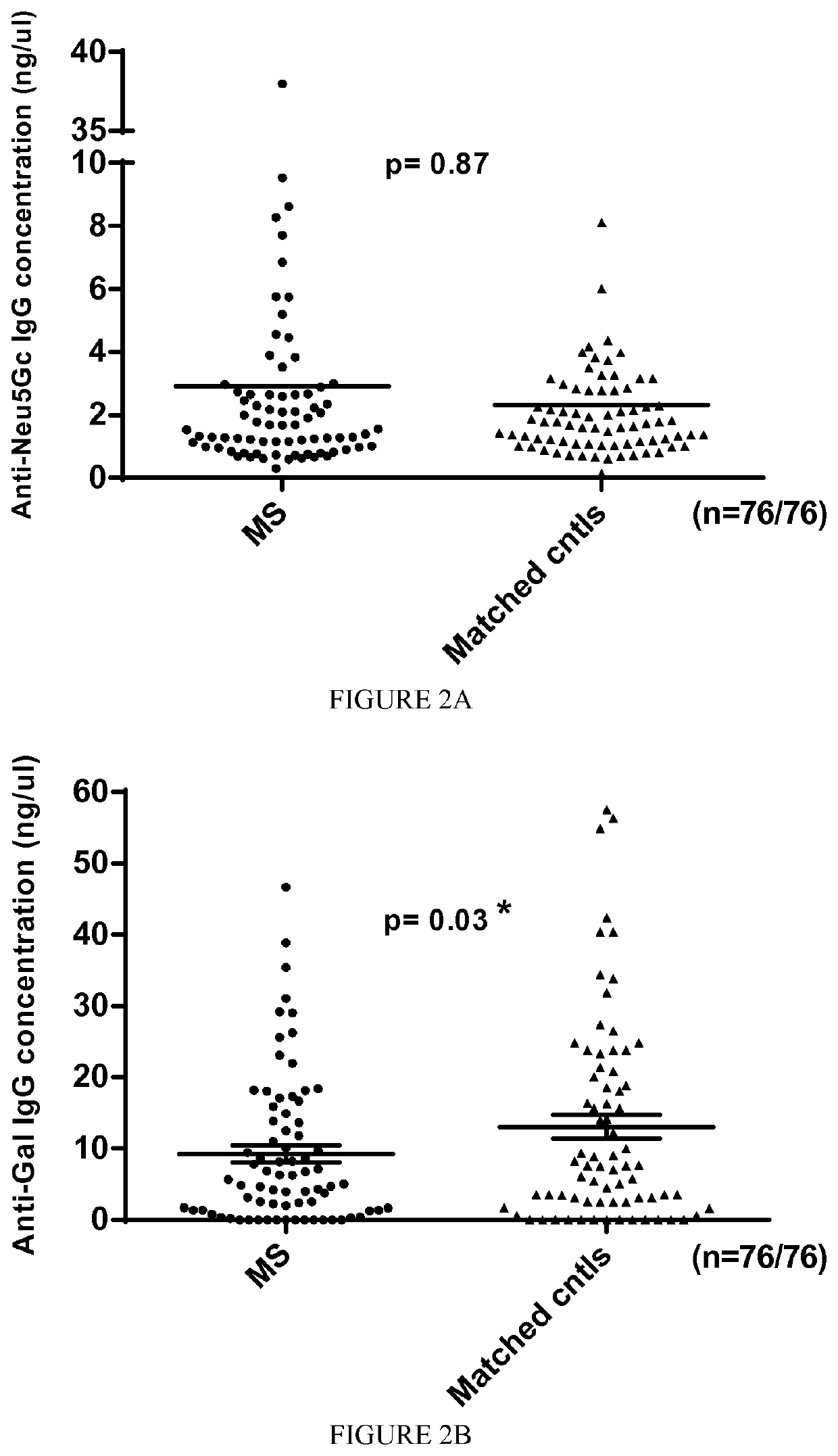
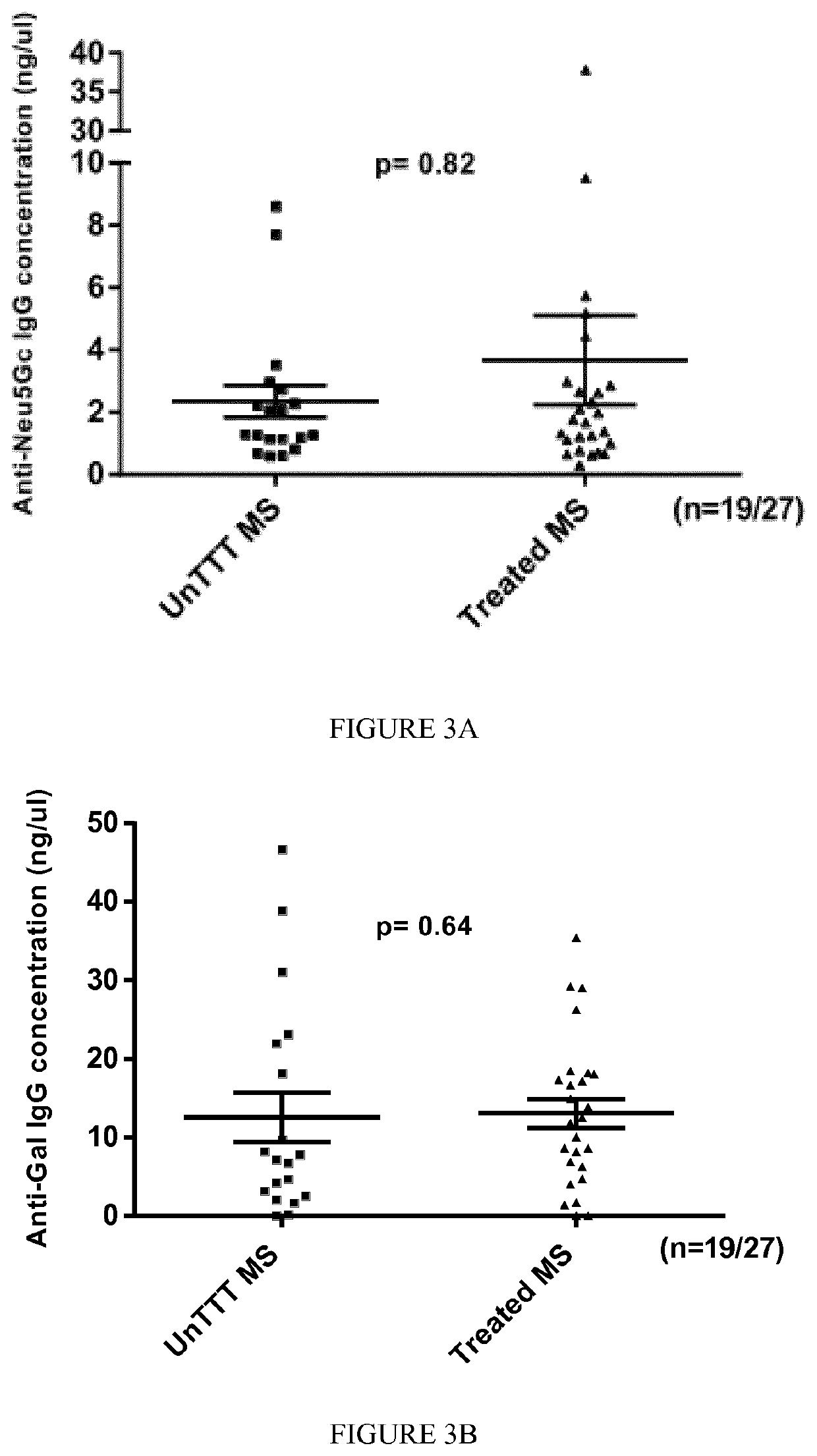
The present invention relates to the diagnosis, the prevention and the treatment of demyelinating disorders of the central nervous system (CNS), in particular disorders exhibiting altered levels of antibodies directed against α1-3-galactose, such as multiple sclerosis (MS) and clinically isolated syndrome (CIS). The diagnosis of MS and CIS still suffers from a lack of reliable biomarkers and there is cure for these diseases, except medication acting on the immune component of the overt disease, with the usual side effects of the immunosuppression or anti inflammation intended to relieve the associated symptoms. The inventors showed that there is a significant decrease in the levels of antibodies directed against α1-3-galactose in individuals having a MS or a CIS. In addition, the present invention relates to the prevention and / or the treatment of a demyelinating disorder of the CNS in an individual, comprising a step of administering a compound suitable for generating an immune reaction directed against α1-3-galactose in the said individual. Large cohorts of human individuals having MS or CIS have been studied and compared to matched cohorts on the basis of age and gender.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com